Introduction
Extant crocodiles and alligators are characterized by their adaptation of the aquatic realm and much of their evolutionary history traditionally has been interpreted within the context of their adaptation to this mode of life. The most notable example of this view is the posterior shift of the choanal opening, traditionally interpreted as an adaptation to aquatic habits (Langston, Reference Langston, Gans and Parsons1973; Clark and Norell, Reference Clark and Norell1992; Busbey, Reference Busbey and Thomason1995). The fossil record of Crocodyliformes, however, shows that their past ecological diversity was much larger than today, including species interpreted as fully terrestrial or fully marine (Gasparini, Reference Gasparini1984; Pol and Gasparini, Reference Pol and Gasparini2009; Herrera et al., Reference Herrera, Gasparini and Fernandez2013; Kellner et al., Reference Kellner, Pinheiro and Campos2014). One of the extinct groups that has the highest ecological variability is Notosuchia, a clade that radiated during the Cretaceous and includes terrestrial taxa with adaptations to carnivorous (Carvalho et al., Reference Carvalho, Campos and Nobre2005; Sereno and Larsson, Reference Sereno and Larsson2009; Montefeltro et al., Reference Montefeltro, Larsson and Langer2011; Godoy et al., Reference Godoy, Montefeltro, Norell and Langer2014), omnivorous (Clark et al., Reference Clark, Jacobs and Downs1989; Fiorelli and Calvo, Reference Fiorelli and Calvo2008; Lecuona and Pol, Reference Lecuona and Pol2008; Ösi, Reference Ösi2013), and possibly herbivorous diets (Wu et al., Reference Wu, Sues and Sun1995; Wu and Sues, Reference Wu and Sues1996; Melstrom and Irmis, Reference Melstrom and Irmis2019).
Although most notosuchians lived during the Cretaceous, one of its lineages, Sebecidae, survived the mass extinction event at the end Cretaceous. Sebecidae is a diverse clade of carnivorous taxa characterized by their narrow and high rostrum with ziphodont (i.e., serrated, theropod-like) teeth that thrived in terrestrial ecosystems during the Paleocene–Miocene of South America (Gasparini, Reference Gasparini1984; Kellner et al., Reference Kellner, Pinheiro and Campos2014). Most members of Sebecidae have been interpreted as large terrestrial carnivores, but the ecological diversity of this group may have been larger due to the semiaquatic habits inferred for a basal member of the group (Lorosuchus nodosus Pol and Powell, Reference Pol and Powell2011). Sebecids, therefore, present an interesting case of evolution due to their unique anatomy, ecological diversity, and their survival through the K-Pg extinction event.
There are currently nine South American sebecids species, with the highest diversity recorded in the Paleogene of northwestern Argentina. Similarly, the distribution of the clade was likely larger, as shown by a recently described species from the Late Cretaceous of Spain (Ogresuchus furatus Sellés et al., Reference Sellés, Blanco, Vila, Marmi, López-Soriano, Llácer, Frigola, Canals and Galobart2020), which has been interpreted as a sebecid (Sellés et al., Reference Sellés, Blanco, Vila, Marmi, López-Soriano, Llácer, Frigola, Canals and Galobart2020), and the close affinities of Iberosuchus Antunes, Reference Antunes1975, and Eremosuchus Buffetaut, Reference Buffetaut1989, from the Paleogene of Europe and Africa, respectively (Ortega et al., Reference Ortega, Gasparini, Buscalioni and Calvo2000; Pol et al., Reference Pol, Leardi, Lecuona and Krause2012). The evolutionary origins of Sebecidae remain unclear because this clade has been retrieved as the sister group of different notosuchians from the Cretaceous of South America. Traditional phylogenetic hypotheses grouped Sebecidae together with Baurusuchidae within the group Sebecosuchia (Colbert, Reference Colbert1946; Gasparini, Reference Gasparini1972, Reference Gasparini1984; Buffetaut and Ingavat, Reference Buffetaut and Ingavat1980), the monophyly of which has been retrieved in several phylogenetic analyses (e.g., Ortega et al., Reference Ortega, Gasparini, Buscalioni and Calvo2000; Pol, Reference Pol2003; Pol and Gasparini, Reference Pol, Gasparini, Gasparini, Coria and Salgado2007; Pol and Powell, Reference Pol and Powell2011; Pol et al., Reference Pol, Leardi, Lecuona and Krause2012, Reference Pol, Nascimiento, Carvhalo, Riccomini, Pires-Domingues and Zaher2014). However, some studies have proposed an alternative position for Sebecidae, allying them to Peirosauridae, forming a clade named Sebecia (Larsson and Sues, Reference Larsson and Sues2007; Godoy et al., Reference Godoy, Bronzati, Eltink, Marsola, Cidade, Langer and Montefeltro2016), which also includes in some cases the group Mahajangasuchidae (Pinheiro et al., Reference Pinheiro, Pereira, Souza, Brum, Lopes, Machado, Bergqvist and Simbras2018, Reference Pinheiro, Souza, Bandeira, Brum, Pereira, Castro, Ramos and Simbras2021). Current knowledge on the anatomy of sebecids is largely based on cranial morphology, in which the rostral region concentrates many of the distinctive features among taxa (Paolillo and Linares, Reference Paolillo and Linares2007; Pol et al., Reference Pol, Leardi, Lecuona and Krause2012; Bravo et al., Reference Bravo, Pol and García-López2021), but a persistent problem in understanding the evolution of the group is the current lack of detailed anatomical studies of many of its constituent species.
Sebecus icaeorhinus Simpson, Reference Simpson1937, is the best-known species of the group and is mainly known from different specimens consisting of disarticulated skull and postcranial remains. The holotype (AMNH 3160) consists of a disarticulated skull described in detail by Colbert (Reference Colbert1946) in conjunction with a second fragmentary specimen (AMNH 3159). Gasparini (Reference Gasparini1972) reviewed the taxonomic diversity of the sebecosuchians of Argentina and reported new data on the choanal region based on a new specimen (MMP 235), which was not preserved in the holotype. More recently, Pol et al. (Reference Pol, Leardi, Lecuona and Krause2012) described postcranial remains of Sebecus icaeorhinus based on new specimens (MPEF-PV 1776, MPEF-PV 3970–3972), which provided support to previous inferences (based on skull anatomy) on the terrestrial habits of this taxon. Here, we expand the original description provided by Gasparini (Reference Gasparini1972) employing computed tomography scan of MMP 235. This noninvasive technique allows us to provide new anatomical information on the palate and choanal region of Sebecus icaeorhinus, which was poorly known in previously described specimens. This region is of particular interest given that it has been shown to be highly variable within Sebecidae. All sebecids have a mesosuchian-type choana (delimited by palatines and pterygoids), but there is large variation within the group and some forms have a posteriorly located choana that resembles the condition of aquatic crocodylians (Turner and Buckley, Reference Turner and Buckley2008; Bravo et al., Reference Bravo, Pol and García-López2021). This is an unexpected pattern of variation among terrestrial crocodyliforms, and previous studies have not analyzed in detail the available morphological variation or assessed the role of this anatomical region in the evolution of the group. Here we analyze the variability of the palatine morphology, a key central element of the palate, through a morphogeometrical analysis within the context of terrestrial crocodyliforms, including other sebecids and representatives of the major notosuchian clades.
Material and methods
Specimen MMP 235, which is described below, was referred to Sebecus icaeorhinus by Gasparini (Reference Gasparini1972). It consists of partial remains of the skull and jaws that were unfortunately included in a plaster reconstruction soon after it was found, following the skull reconstruction illustrated by Colbert (Reference Colbert1946). In this context, the bones of MMP 235 have been covered with plaster since its initial preparation, making it difficult to distinguish the original elements from those that were reconstructed.
The skull and lower jaw of specimen MMP 235 were CT-scanned at the Centro de Imágenes Médicas del Instituto del Diagnóstico del Este de Chubut S.R.L. (Trelew, Argentina). The specimen was scanned in the transverse plane, resulting in a total of 1,114 slices, with an interslice spacing of 0.5 mm and a pixel resolution of 512 x 512 pixels. The original transverse slices were then resliced in both the frontal and sagittal planes. The obtained CT images were examined directly using 3D-Slicer software (Pieper et al., Reference Pieper, Halle and Kikinis2004). In addition, these images were segmented to create a three-dimensional model of the bones preserved in this specimen (Fig. 1).
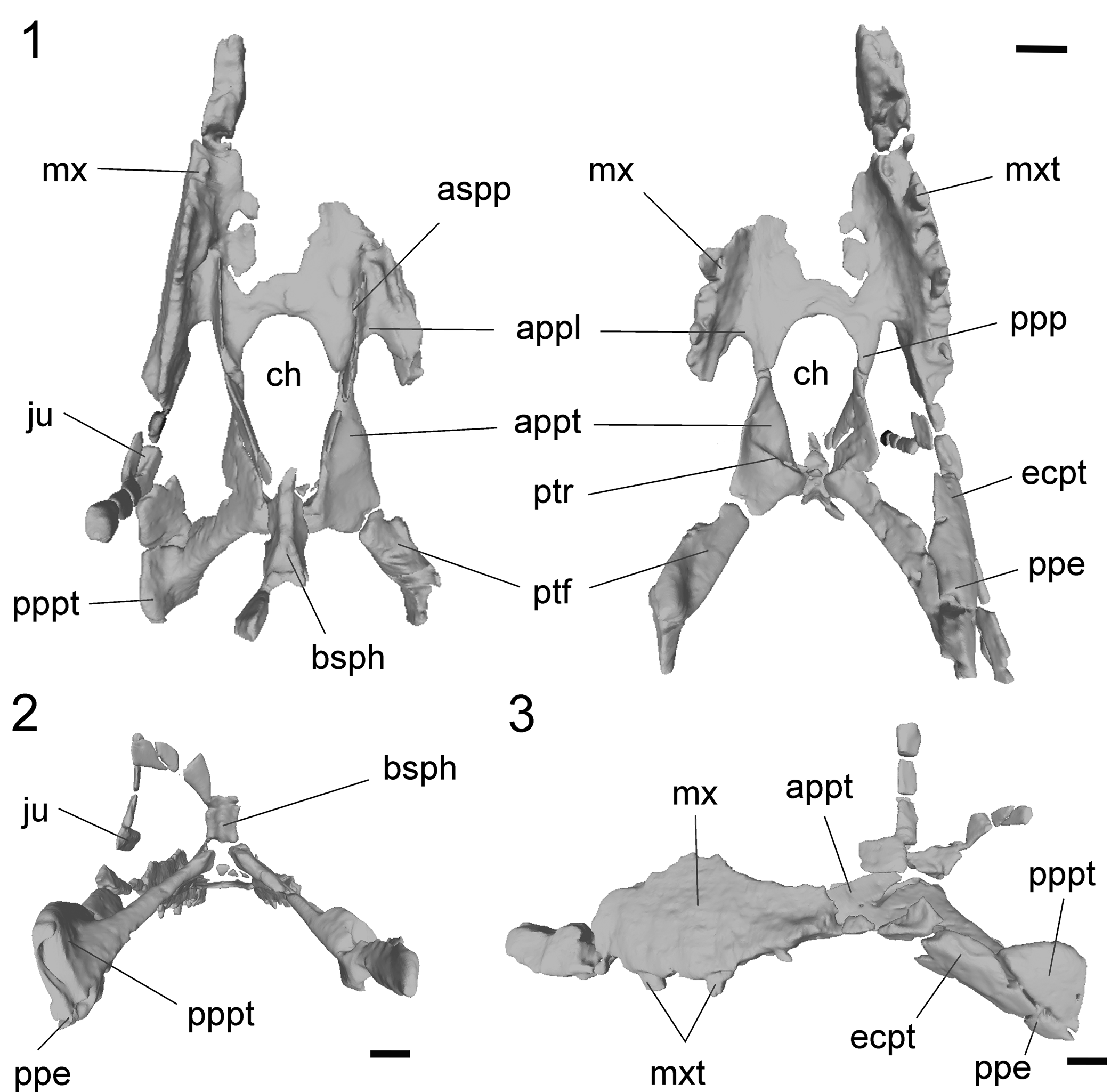
Figure 1. Sebecus icaeorhinus (MMP 235) from the Casamayor Formation, Cañadón Vaca locality, Chubut Province. Tridimensional model of the skull in different views. (1) Dorsal (left) and ventral (right) views; (2) distal view; (3) left lateral view. Abbreviations: appl, anterior process of the palatine; appt, anterior process of the pterygoid; aspp, ascending processes of the palatine; bsph, basisphenoid; ch, choana; ecpt, ectopterygoid; ju, jugal; mx, maxilla; mxt, maxilar teeth; ppe, posterior process of the ectopterygoid; ppp, posterior process of the palatine; pppt, posterior process of the pterygoid; ptf, pterygoid flange; ptr, pterygoid ridge. Scale bars = 2 cm.
The referred skull and jaw fragments include some elements that are absent in the holotype of Sebecus icaeorhinus, providing new anatomical information. Among these are the complete pterygoids, the choanal opening, and the sutural details between the bones that form this region (Fig. 1). The description is mostly focused on the new anatomical data as well as on features that justify the taxonomic assignment.
Geometric morphometric two-dimensional analysis
The palatines are a major component of the secondary palate of mesoeucrocodylians. We focused on the palatal surface of the palatine of sebecids (and more generally those of notosuchians) that show a high morphological variability. Shape variation of the ventral surface was assessed using 2D geometric morphometric analyses. We analyzed all sebecids specimens from which a complete palatine has been preserved and representatives of each major clade of Notosuchia from which palatine information was readily available. To avoid morphological differences related to ontogeny, we included only information from presumably adult specimens.
Following the above-mentioned criteria, 20 specimens were included in the analyses of the palatines (Table 1). To standardize the 2D images, each sample was positioned in the same orientation and a scale was included to account for the size. To describe the shape, we built a design of six anatomical landmarks and 36 equidistant semilandmarks between them (Fig. 3; Table 2). Landmarks and semilandmark digitalization, generalized Procrustes analyses (GPA; i.e., removal of the differences related to scaling, translation, and rotation), and principal components analyses (PCA) were carried out using the software tps series (Rohlf, Reference Rohlf2013). Semilandmarks were slid using the minimum bending energy criterion (Mitteroecker and Gunz, Reference Mitteroecker and Gunz2009). Resulting principal components represent different aspects of the geometric variation present in the set of studied configurations (MacLeod, Reference MacLeod2012). Only principal components representing at least 10% of the original variation were retained. Scores of specimens on principal components were used as the input variables for all subsequent statistical analyses.
Table 1. List of taxa used for morphogeometric analysis, together with their ages, clades, and the size of the skull. The category “skull size” is defined according to length intervals, so for the small, medium, and large sizes, the length intervals in centimeters are equal to 0–14 cm, 15–29 cm, and 30–60 cm, respectively.

Table 2. Landmark and semilandmark design used for palatine digitalization.

Morphological groups within the dataset were explored using the gap statistic method (Tibshirani et al., Reference Tibshirani, Walther and Hastie2001; Charrad et al., Reference Charrad, Ghazzali, Boiteau and Niknafs2014), which is a standard technique for estimating the optimal number of clusters (i.e., groups) in a data set by comparing the within-cluster dispersion to the expectation under a null reference distribution with no obvious clustering structure. Euclidean distances were selected as a dissimilarity measure. Twenty-seven different clustering algorithms were used to determine the optimal number of groups, and the best number of clusters was selected according to the majority rule (Charrad et al., Reference Charrad, Ghazzali, Boiteau and Niknafs2014).
Classification of specimens in the obtained number of clusters was determined using partitioning around medoids (PAM), a non-hierarchical clustering method that is insensitive to both noise and outliers (Singh and Chauhan, Reference Singh and Chauhan2011). Moreover, the medoids are robust representations of the cluster centers, which is particularly important in the common context that many elements do not fit well within any cluster (Van der Laan et al., Reference Van der Laan, Pollard and Bryan2003). This classification was used as the input categories for linear discriminant analysis (LDA) with leave-one-out cross-validation to generate the canonical axes, identify the shape variables involved in-group distinction, and produce a robust reclassification of the specimens (prior probabilities were set to be equal).
Finally, to compare the morphology of the palatine of sebecid specimens, a dissimilarity matrix (describes pairwise distinction between taxa) was computed using Euclidean distances based on PCA scores. Dissimilarity is lower for more similar pairs of specimens.
The above-mentioned analyses were performed in the R environment (R Core Team, 2020, v. 3.6.3) using the packages “cluster” (Maechler et al., Reference Maechler, Rousseeuw, Struyf, Hubert and Hornik2014), “NbClust” (Charrad et al., Reference Charrad, Ghazzali, Boiteau and Niknafs2014), “ggplot2” (Wickham, Reference Wickham2016), “rgl” (Adler et al., Reference Adler, Murdoch, Nenadic, Urbanek and Chen2021), and “MASS” (Venables and Ripley, Reference Venables and Ripley2002).
Repositories and institutional abbreviations
AMNH, American Museum of Natural History; New York, United States of America; FC-DPV, Facultad de Ciencias, Colección de Vertebrados Fósiles, Montevideo, Uruguay; LPRP, Laboratorio de Paleontologia, Universidade de Sao Paulo, Sao Paulo, Brazil; MACN, Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina; MCT, Museu de Ciencias da Terra, Companhia de Pesquisas de Recursos Minerais (CPRM), Rio de Janeiro, Brazil; MMP, Museo de Historia Natural “Galileo Scaglia,” Mar del Plata, Argentina; MNHN, Museum National d'Histoire Naturelle, Paris, Francia; MNK-PAL, Museo Noel Kempff Mercado, Santa Cruz de la Sierra, Bolivia. MNN, Muséum National du Niger, Niamey, République de Niger; MPEF, Museo Paleontológico Egidio Feruglio, Trelew, Argentina; MPMA, Museu de Paleontologia de Monte Alto, Monte Alto, Brazil; MZSP-PV, Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil; PVL, Paleontología de Vertebrados Lillo, Tucumán, Argentina; ROM, Royal Ontario Museum, Toronto, Canada; URC, Universidade Estadual Paulista, Rio Claro, Brazil.
Systematic paleontology
Superorder Crocodylomorpha Walker, Reference Walker1970
Order Crocodyliformes Hay, Reference Hay1930 (sensu Clark, Reference Clark1986)
Suborder Mesoeucrocodylia Whetstone and Whybrow, Reference Whetstone and Whybrow1983
Infraorder Sebecosuchia Gasparini, Reference Gasparini1972
Family Sebecidae Simpson, Reference Simpson1937
Genus Sebecus Simpson, Reference Simpson1937
Type species
Sebecus icaeorhinus Simpson, Reference Simpson1937.
Sebecus icaeorhinus Simpson, Reference Simpson1937
Figures 1, 2, S1–S4 [Supplemental Data 1]
Holotype
AMNH 3160, disarticulated skull and mandible.
Emended diagnosis
Mesoeucrocodylian crocodyliform diagnosed by the following unique combination of characters: rostrum mediolaterally compressed and dorsoventrally deep; elliptical choanal opening, remarkably large, representing 15% of the total length and 25% of the maximum width of the skull; anterior margin of the choana positioned at level of the anterior margin of the suborbital fenestra** (see Remarks, below); quadratojugal-surangular forming accessory craniomandibular articulation; distal body of quadrate bearing sharp ridge on posterior surface; tabular shaped pterygoid flanges that are lateromedially elongated and anteroposteriorly short, with flat and slightly concave ventral surface* (see Remarks, below); basisphenoid virtually or completely excluded from the ventral surface of the skull; four premaxillary, nine maxillary, and 13 dentary teeth; posterior teeth ziphodont and highly compressed mediolaterally; shallow notch at premaxillary-maxillary contact for reception of enlarged fourth dentary tooth; markedly deep prespinal fossa in mid to posterior cervical vertebrae, facing dorsally and well separated from anterior margin of neural arch; hypapophysis present in all cervicals and extending posteriorly to dorsal 6; coracoid shaft subcylindrical in cross-section; low deltopectoral crest that deflects medially along distal half; horizontal shelf above humeral condyles on anterior surface of humerus; articular surface for ulna on radiale mediolaterally narrow and dorsoventrally long; postacetabular process of ilium elongated, horizontal, and tapering posteriorly; posterior half of postacetabular process of ilium free of sacral rib attachment; iliac antitrochanter higher than anteroposteriorly long; shallow and smooth insertion area for M. puboischiofemoralis internus 1, and M. caudifemoralis longus anterior to fourth trochanter; absence of anterior ridge limiting calcaneal socket; absence of dorsolateral ridge on calcaneal tuber.
Occurrence
All the specimens come from the Eocene of Chubut, Argentina. AMNH 3160 was found in the “Bird Clay” locality of Cañadón Hondo. AMNH 3159 and MMP 235 were found in Cañadón Vaca. MPEF-PV 1776 and MPEF-PV 3970–3972 were found in the “Cerro Verde” locality, on the western margin of Cañadón Hondo.
Material
MMP 235, fragmentary skull and mandible.
Remarks
In the diagnosis, the incorporation of a new autapomorphy is noted with two asterisks (**), and the other diagnostic character is noted with a single asterisk (*). These characters so far are unique for Sebecus icaeorhinus, although given the lack of anatomical information for the group, these could be diagnostic of other sebecids.
Results
Descriptions
Both maxillae are partially preserved (Fig. 1). The left maxilla has preserved the complete alveolar margin with fragments of tooth roots (Fig. 2.2) This specimen bears nine alveoli, which fits the tooth count given in the reconstruction of the skull of the holotype by Molnar (Reference Molnar2010). The maxilla is narrow and vertically oriented, as in the holotype, but it does not provide more information than the more complete type specimen described by Colbert (Reference Colbert1946).
The palatines are partially preserved in MMP 235. The right palatine is almost completely preserved, however, both elements are missing parts of the anterior and medial regions (Fig. 1). The palatine has two horizontally oriented palatal processes (the anterior and posterior processes) and a vertically oriented ascending process (Fig. 2.2, 2.3), as in other sebecids (e.g., Sebecus querejazus Buffetaut and Marshall, Reference Buffetaut and Marshall1991, Sahitisuchus fluminensis Kellner, Pinheiro, and Campos, Reference Kellner, Pinheiro and Campos2014), baurusuchids (e.g., Pissarrachampsa sera Montefeltro, Larsson, and Langer, Reference Montefeltro, Larsson and Langer2011, Stratiotosuchus maxhechti Campos et al., Reference Campos, Suarez, Riff and Kellner2001), peirosaurids (e.g., Montealtosuchus Carvalho, Vasconcellos, and Tavares, Reference Carvalho, Vasconcellos and Tavares2007, Lomasuchus Gasparini, Chiappe, and Fernandez, Reference Gasparini, Chiappe and Fernandez1991), and other notosuchians (e.g., Uruguaysuchus Rusconi, Reference Rusconi1933, Sphagesaurus Price, Reference Price1950). The anterior region is short and has a lateral acuminated and recurved end that contacts the palatal process of the maxilla at the anterior margin of the suborbital fenestra. The palatine does not contact the ectopterygoid at the anterior end of the suborbital fenestra. These features are shared by most notosuchians, except for Yacarerani Novas et al., Reference Novas, Pais, Pol, Carvalho, Scanferla, Mones and Riglos2009, and Mariliasuchus Carvalho and Bertini, Reference Carvalho and Bertini1999, which have a palatine-ectopterygoid contact in this area. The posterior process is long and acuminated and is obliquely oriented with respect to the sagittal plane. This process forms the anterolateral margin of the choanal opening, contacts the anterior region of the pterygoid, and forms the medial margin of the suborbital fenestra (Fig. 1). The anatomy of this region differs markedly from the highly modified palatine anatomy of Baurusuchidae, which has the anterior process less developed (Stratiotosuchus maxhechti and Aplestosuchus sordidus Godoy et al., Reference Godoy, Montefeltro, Norell and Langer2014), or even absent (Pissarrachampsa sera, Baurusuchus pachecoi Price, Reference Price1945, and Baurusuchus salgadoensis Carvalho, Campos, and Nobre, Reference Carvalho, Campos and Nobre2005), and the posterior process is shorter and wider.
The ascending process of Sebecus icaeorhinus arises from the dorsal surface of the palatine and extends dorsomedially towards the pterygoid contact (on the dorsolateral margin of this bone). This process forms part of the lateral walls of the nasopharyngeal passage (Fig. 2.2). In both MMP 235 and AMNH 3160, the ascending processes of the palatine are fractured and it is not possible to determine whether the margins were in contact dorsally, completely closing the internal nasal passage. According to Colbert (Reference Colbert1946), due to the manner in which this process thins, the internal nasal passage was bounded by cartilaginous walls. Unlike the holotype of Sebecus icaeorhinus (AMNH 3160), the specimen MMP 235 has preserved the palatine-pterygoid contact (Fig. 2.3). Posterior to ascending process, the palatine contacts the lateral margin of the dorsolateral portion of the anterior process of the pterygoid, forming an oblique (anterodorsally directed) suture that extends along the lateral walls of the choana (Fig. S3.3). The palatine is set dorsolaterally to the pterygoids in this region and, in ventral view, the suture between these bones is linear and oriented obliquely, forming an angle of ~45° with the longitudinal axis (Fig. S2.3, S2.4). As in other elements of the skull, the palatine of Sebecus icaeorhinus contains small pneumatic cavities along most of its length (Fig. 2.3).
Only two small fragments of the pterygoids have been preserved in AMNH 3159 (Colbert, Reference Colbert1946). The pterygoid is fortunately well preserved and almost complete in MMP 235. The central portion of the pterygoid has not been preserved in any of the specimens so we cannot confirm if it was a single element, although phylogenetically it would be more parsimonious to assume Sebecus icaeorhinus had a single fused pterygoid (Colbert, Reference Colbert1946). The pterygoid is obliquely oriented forming an angle of ~45° with the longitudinal plane. This orientation is present along the anterior (choanal) region and the posterior (flanges) region (Fig. 2.4–2.8).
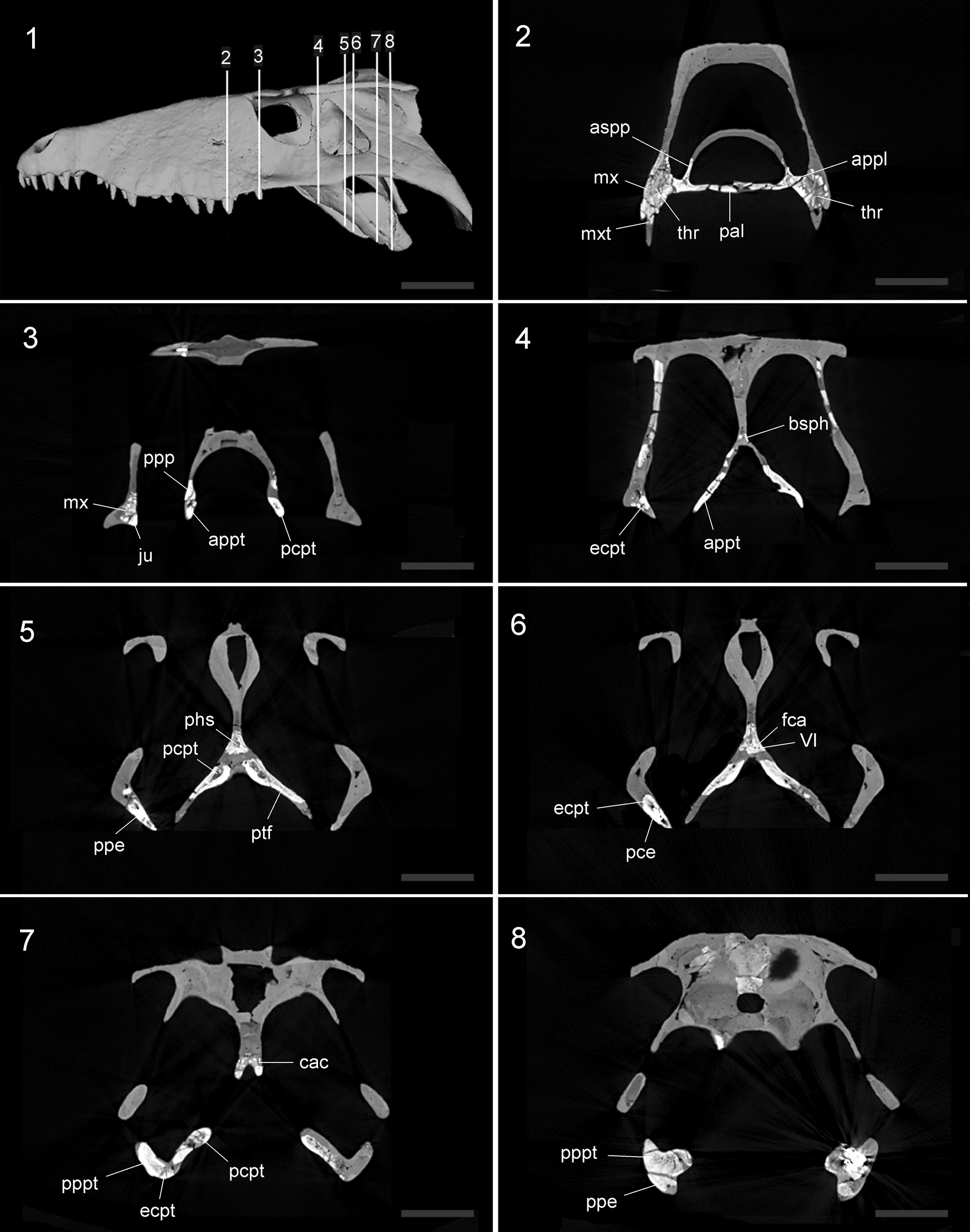
Figure 2. Serial coronal slices through the skull of the MMP 235 specimen of Sebecus icaeorhinus. The upper left panel (1) shows the three-dimensional reconstruction with the position of each sagittal slice (2–8) along the horizontal axis of the skull. Abbreviations: appl, anterior process of the palatine; appt, anterior process of the pterygoid; aspp, ascending process of the palatine; bsph, basisphenoid; cac, carotid canal; ecpt, ectopterygoid; fca, foramen carotid anterior; VI, foramen of the abducens nerve; ju, jugal; mx, maxilla; mxt, maxilar teeth; pal, palatine; phs, pharyngeal sinus; pce, pneumatic cavities into the ectopterygoid; pcpt, pneumatic cavities into the pterygoid; ppe, posterior process of the ectopterygoid; ppp, posterior process of the palatine; pppt, posterior process of the pterygoid; ptf, pterygoid flange; thr, teeth root. Scale bars = 10 cm.
The anterior region of the pterygoid has a subtriangular shape and the palatal surface is smooth (Fig. 1). The anterior end of this region is sutured to the posterior process of the palatines, and forms two-thirds of the lateral margins of the choanal opening. Given the oblique orientation of the anterior process of the pterygoid, its ventrolateral margin forms the lateral border of the choanal opening. In ventral view, the choanal margin extends posterolaterally, broadening the choanal opening along the level of the medial third of the suborbital fenestra (Fig. S2.4, S2.5). The palatal surface of the pterygoids forms an oblique dorsal roof of the choanal opening. The pterygoid has a posterior choanal ridge that runs obliquely (anterolaterally-posteromedially) and is located approximately on the central part of the anterior pterygoid region (Fig. 1). In this region there are small pneumatic cavities within the pterygoid (Fig. 2.3–2.8). The lateral surface of the anterior region of the pterygoid delimits the suborbital fenestra, forming the posterior two-thirds of its medial margin. The posterior end of the anterior pterygoid region has an ascending portion that usually sutures to the ventral margin of the basisphenoid, although this suture is not preserved in MMP 235.
The pterygoid flanges are tabular shaped, narrow, and elongated (Fig. 1). This is a derived condition shared with other sebecids such as Bretesuchus bonapartei Gasparini, Fernandez, and Powell, Reference Gasparini, Fernandez and Powell1993, Lorosuchus nodosus, the Lumbrera form (unpublished taxon; Pol and Powell, Reference Pol and Powell2011), and the sebecid close-relative Iberosuchus. In contrast to this trait, the pterygoid flanges in Sebecus querejazus are laminar shaped and have an extensive flat surface posterior to the choanal opening that projects posteriorly beyond the level of the basisphenoid and basioccipital. An extensive surface posterior to the choana is found in other notosuchians such as baurusuchids (e.g., Baurusuchus salgadoensis and Aplestosuchus sordidus), peirosaurids (e.g., Montealtosucuhs and Hamadasuchus Buffetaut, Reference Buffetaut1994), and uruguaysuchids (e.g., Araripesuchus Price, Reference Price1959, and Uruguaysuchus). This is a generalized condition also found in some neosuchians (e.g., Pholidosaurus Meyer, Reference Meyer1841, and Shamosuchus Mook, Reference Mook1924), with broad, laminar flanges that are ventrolaterally directed.
Colbert (Reference Colbert1946) correctly inferred the long, downward sweep of the ectopterygoid showed that the pterygoid wing was very long posteriorly (Fig. 1). However, the pterygoid flanges of MMP 235 are not anteroposteriorly broad as in other crocodyliforms. The elongated pterygoid flanges in Sebecus icaeorhinus are directed posterolaterally forming an angle of ~45° with the longitudinal plane, and, in palatal view, they have an acuminated posterior end (Fig. 2.5–2.7). The ventral surface is slightly concave, as in the Lumbrera form and Iberosuchus. The condition of Sebecus icaeorhinus, however, differs from the deeply concave ventral surface of the pterygoid flanges of Bretesuchus bonapartei. A concave ventral surface of the pterygoid flanges is also present in baurusuchids, although these taxa have a flange that is much broader anteroposteriorly and frequently has deep pterygoid parachoanal fossae. The anterolateral margin of the pterygoid flanges does not have the laterally open notch or fossa (parachoanal fossa, sensu Andrade and Bertini, Reference Andrade and Bertini2008) that has been described for other Sebecidae (Sebecus querejazus, Bretesuchus bonapartei, Sahitisuchus fluminensis, and Lorosuchus nodosus). Towards the posterolateral end, close to contact with the ectopterygoid, the palatal surface of the pterygoid flanges widens slightly. The pterygoid flange has a well-developed and dorsoventrally expanded pterygoid buttress (sensu Holliday and Witmer, Reference Holliday and Witmer2007), which is the osseous correlate of the placement of the cartilago trasiliens. This process is anteroposteriorly long, extends to approximately the level of the posterior end of the jugal, and is visible in lateral and posterior views (Fig. 1). It contacts the ectopterygoid ventrally, and the lineal suture among these bones runs along the entire dorsal surface of the posterior process of the ectopterygoid.
The specimen MMP 235 has preserved an almost complete left ectopterygoid. This bone is long and acuminated and is oriented obliquely to the longitudinal axis, forming an angle of ~55° (Fig. 1). This orientation is the generalized condition of notosuchians (e.g., sebecosuchians, peirosaurids, and uruguaysuchids), except for some advanced notosuchians (Yacarerani, Caipirasuchus Iori and Carvalho, Reference Iori and Carvalho2011, and Mariliasuchus) in which the anterior region is almost horizontally oriented. The anterior region of the ectopterygoid of the Sebecus icaeorhinus is flat and subrectangular in cross section. Dorsally, the ectopterygoid contacts the posteroventral end of the palatal process of the maxilla along a transversely oriented suture and its ventromedial contacts the jugal. The posterior process is slightly curved and is subtriangular in cross section (Fig. 2.5–2.8).
The basisphenoid is not completely preserved, being only exposed in occipital view, because its posteroventral region is missing (Fig. 1). The overall orientation of the bones in this region resembles the verticalized braincase of eusuchians (Tarsitano, Reference Tarsitano1985). A similar trait is present in other sebecids (e.g., Sebecus querejazus) and only some peirosaurids (e.g., Hamadasuchus). In most notosuchians, however, the basisphenoid is visible in ventral view owing to the strong anteroventral inclination of the occipital surface (e.g., Montealtosuchus, Araripesuchus Price, Reference Price1959, Malawisuchus Gomani, Reference Gomani1997, Mariliasuchus, Notosuchus Woodward, Reference Woodward1896, Baurusuchus Price, Reference Price1945). The contact with the dorsal surface of the pterygoid is not preserved. The posterior surface (occipital) of the basisphenoid faces posteriorly and is mediolaterally concave, a feature shared with Sebecus querejazus and the Lumbrera form (Fig. S2.5). This surface is less concave in other sebecids, such as Sahitisuchus fluminensis, Bretesuchus bonapartei, and Lorosuchus nodosus. In cross section, the basisphenoid has a posterior pneumatic cavity that likely corresponds to the median pharyngeal sinus and/or part of the pharyngotympanic sinus (sensu Dufeau and Witmer, Reference Dufeau and Witmer2015) (Fig. 2.5). In turn, there are paired ducts that would have occupied the abducens (VI) nerve that cross the basisphenoid downwards, lateral, and paralleling the path of the canal for the internal carotid artery (Lessner and Holliday, Reference Lessner and Holliday2020) (Fig. 2.6).
The choana of Sebecus icaeorhinus is anteroposteriorly longer than lateromedially wide, being approximately twice as long as its width at the level of the posterior palatine processes. This characteristic is similar to that of other sebecids, such as Lorosuchus nodosus, Sahitisuchus fluminensis, Barinasuchus arveloi Paolillo and Linares, Reference Paolillo and Linares2007, and the Lumbrera form. The length/width relationship of the choana, however, is inverse in Sebecus querejazus and Bretesuchus bonapartei. The presence of a septum cannot be determined in Sebecus icaeorhinus, although other sebecids (e.g., Sahitisuchus fluminensis, Sebecus querejazus, Lorosuchus nodosus, Lumbrera form) have a low and laminar choanal septum. The anterior margin of the choana of Sebecus icaeorhinus is located at the level of the anterior margin of the suborbital fenestra and forms a broad curvature anteriorly to the straight lateral margins. This feature differs from that of other sebecids, in which the choana is located at different levels with respect to the suborbital fenestra. The position of the choanal anterior margin in Sebecus icaeorhinus is more similar to that of non-sebecid notosuchians (e.g., Araripesuchus, Notosuchus, Uruguaysuchus, Sphagesaurus, Lomasuchus; Bravo et al., Reference Bravo, Pol and García-López2021). The new information on the pterygoid of MMP 235 shows that the posterior margin of the choanal opening is slightly wider than the anterior region so that the entire choanal opening is bell-shaped (i.e., closed posteriorly by a medial ridge of the pterygoids).
The mandibular rami of MMP 235 are partially preserved, with the left ramus more complete (Fig. S4). In contrast to the holotype of Sebecus icaeorhinus, the anterior end and the articular of MMP 235 have not been preserved; however, the angular and surangular are complete, and fragments of dentary and splenial also are preserved (Gasparini, Reference Gasparini1972). As with the maxilla, they do not provide more anatomical information than what is known for the species so far.
Morphometric analysis of palatines
Nine algorithms found that the best number of clusters in the sample is eight (according to the majority rule; Fig. 4.1; Table S1 [Supplemental Data 2]). Among these clusters, henceforth referred to as morphotypes (Fig. 4.2), four were clearly distinguished by the first two LD axes (Fig. 4.1). Following the PAM and LDA, the Kaprosuchus Morphotype (positive extreme scores on LD1; Fig. 4.1, 4.2) is only composed of Kaprosuchus Sereno and Larsson, Reference Sereno and Larsson2009, which is characterized by a tabular palatine that extends anteroposteriorly and laterally, but does not reach the suborbital fenestra as in other notosuchians. The Baurusuchid Morphotype (positive extreme scores on LD2; Fig. 4.1, 4.2) includes all the baurusuchid taxa in this study (i.e., Aplestosuchus sordidus, Pissarrachampsa sera, and Stratiotosuchus maxhechti) and is characterized by a narrow and tabular-shaped palatine that is approximately three times as long as wide. The advanced notosuchian Morphotype (negative extreme scores on LD1; Fig. 4.1, 4.2) contains the genera grouped in the advanced notosuchians clade (sensu Pol et al., Reference Pol, Nascimiento, Carvhalo, Riccomini, Pires-Domingues and Zaher2014) included in this study (i.e., Caipirasuchus paulistanus Iori and Carvalho, Reference Iori and Carvalho2011, Caipirasuchus montealtensis Andrade and Bertini, Reference Andrade and Bertini2008, Notosuchus, Mariliasuchus, and Yacarerani). The palatine of these forms is characterized mainly by a small palatal surface with a long and narrow posterior process that widens at its posterolateral end. The Sebecus icaeorhinus Morphotype (negative extreme scores on LD2; Fig. 4.1) is characterized by an arrow-shaped palatine, with the anterior margin extended more posteriorly than in other sebecids. Among the extreme forms noted above, there are morphotypes that include representatives of diverse clades such as Sebecidae, Peirosauridae, and Uruguaysuchidae.
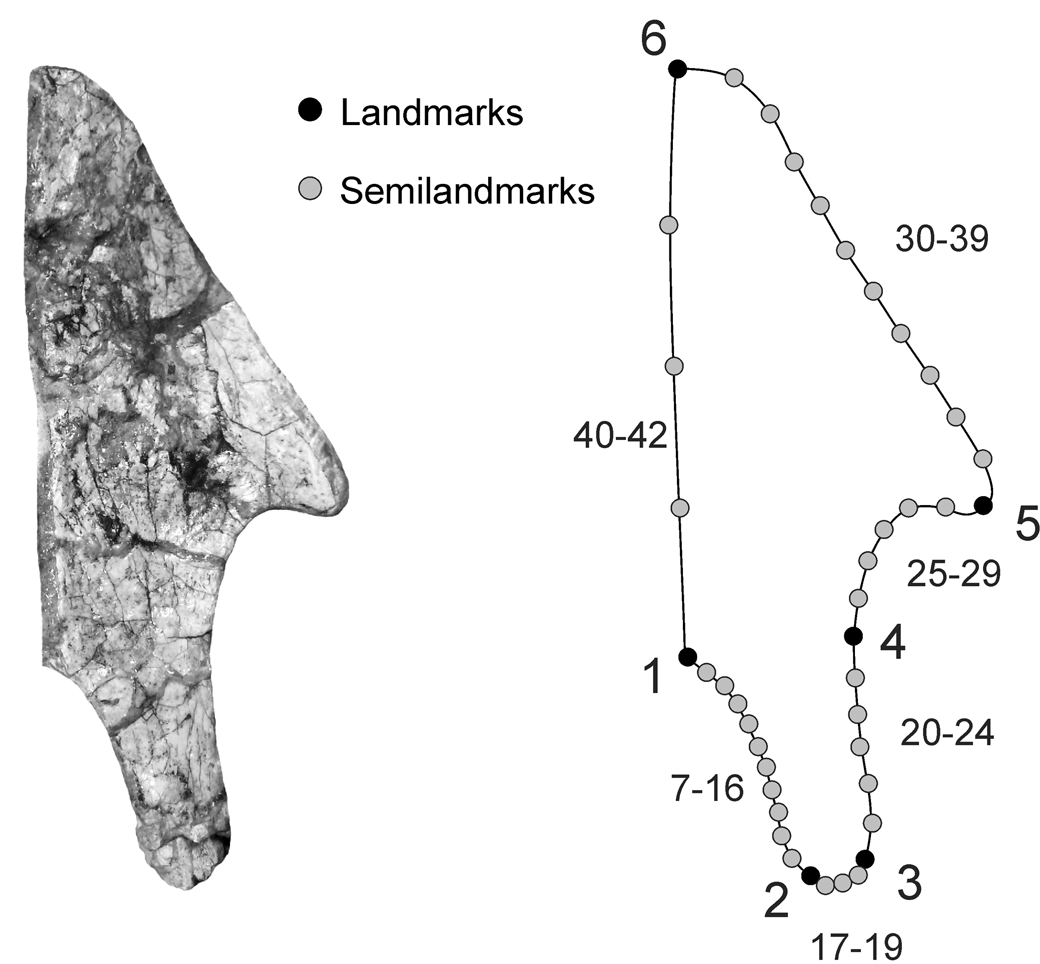
Figure 3. Landmarks and semilandmarks used for geometric morphometric analyses of the ventral surface of the palatine of the notosuchians. The process of the palatine is indicated. Image not to scale.

Figure 4. Results of the geometric morphometric analysis. (1) First two axes of the linear discriminant analysis (LDA) of the ventral surface of the palatine in notosuchians and the location of representatives of different clades of Notosuchia; Baurusuchid Morphotype = open squares; Advanced notosuchian Morphotype = diamonds; Sebecid Morphotype = triangles; Generalized notosuchian Morphotype = circles; Uruguaysuchus = plus sign; Hamadasuchus = cross; Kaprosuchus = asterisk; Sebecus icaeorhinus = filled square. (2), Deformation grids of different clusters (morphotypes). Images not to scale.
A better distinction among morphotypes was reached by analyzing simultaneously the three LDA axes (Fig. S5 [Supplemental Data 2]). On the one hand, the Sebecid Morphotype (negative scores on all axes; Figs. 4, S5) include only representatives sebecids: S. ayrampu Bravo et al., Reference Bravo, Pol and García-López2021, Sebecus querejazus, Bretesuchus bonapartei, and the Lumbrera form. On the other hand, the Generalized Notosuchian Morphotype (around the origin point for the LD1, 2, and 3 axes; Figs. 4.1, S5) includes the sebecids Lorosuchus nodosus and Sahitisuchus fluminensis, the uruguaysuchid Araripesuchus wegeneri Buffetaut, Reference Buffetaut1981, and the peirosaurid Montealtosuchus. Both morphotypes are characterized by a set of intermediate features relative to the other morphotypes (e.g., expansion of the palatine body, intermediate length of the posterior process, the presence or absence of a constriction at the lateral margin of the palatine). Finally, the morphotypes of Uruguaysuchus and of Hamadasuchus (negative and positive scores on LD1, respectively; Fig. 4.1) differ from the others in the degree of the concavity of the lateral margin of the palatine, and the width of the posterior process (being wider in Hamadasuchus).
Discussion
Morphological disparity in the palate of terrestrial notosuchians
The choana of Crocodyliformes has been cited as an exemplar case of progressive evolutionary change (Huxley, Reference Huxley1875; Langston, Reference Langston, Gans and Parsons1973), traditionally interpreted as showing a gradual posterior displacement and change in architecture of different palatal bones along the evolutionary history of the clade leading to extant species. The adaptive interpretation of this evolutionary change focused on the need for separation of the oral cavity from the nasopharyngeal passage (Huxley, Reference Huxley1875), as required in the case of extant crocodylians, which mostly capture prey and feed in the water. Recent studies, however, have shown that evolution of the choanal region has followed a more complex pattern among many different lineages of Crocodyliformes (Turner and Buckley, Reference Turner and Buckley2008; Dollman et al., Reference Dollman, Clark, Norell, Xing and Choiniere2018). The large variability of morphologies present in different groups cannot be explained by a linear evolutionary trend of correlated progression. This complex variation occurs even within the aquatic clade Neosuchia (Pol et al., Reference Pol, Turner and Norell2009), and the pattern of morphological variation is even more complex in other non-aquatic clades (Dollman et al., Reference Dollman, Clark, Norell, Xing and Choiniere2018). Within this context, patterns of morphological variation in non-aquatic clades of Crocodyliformes are particularly interesting because they provide independent cases of palatal evolution in the absence of selective pressures enforced by the aquatic feeding behavior of neosuchian lineages.
Notosuchia contains the largest diversity of terrestrial crocodyliforms and the palatal region is highly variable (Andrade et al., Reference Andrade, Bertini and Pinheiro2006; Turner and Buckley, Reference Turner and Buckley2008), but the pattern of morphological change of the palate within the clade has not been studied in detail. This region is particularly variable within Sebecidae, in terms of position and shape of the anterior margin of the choana, as well as the shape of the suborbital fenestra that laterally bounds the posterior region of the nasopharyngeal passage (Bravo et al., Reference Bravo, Pol and García-López2021). Our morphometric analysis of the palatine in Notosuchia shows that the morphological disparity in the palatine of Sebecidae is greater than in other notosuchian groups (e.g., advanced notosuchians, baurusuchids), as evinced in the results of the cluster analysis. In fact, our analysis indicates three distinct morphotypes of palatine shape within Sebecidae. The palatine of Lorosuchus nodosus and Sahitisuchus fluminensis belongs to a morphotype that represents the plesiomorphic condition of notosuchians (e.g., the condition in the basal clades of uruguaysuchids and peirosaurids, exemplified by taxa such as Montealtosuchus and Araripesuchus wegeneri). The palatines of these taxa form a U-shaped concavity along the anterior margin of the choana, which results in a narrower and shorter anterior margin than in other sebecids.
A second morphotype is represented by the palatines of Bretesuchus bonapartei, Sebecus querejazus, S. ayrampu, and the Lumbrera form. This is interpreted as a derived condition that is recorded only in these Paleogene notosuchians. In these taxa, the posterior process of the palatine extends posterolaterally at a broad angle, delimiting a broad choana. The two morphotypes mentioned above are present in Paleogene taxa recorded at mid latitudes of South America, and share the presence of the anterior margin of the choana, which is located slightly posteriorly to the anterior margin of the suborbital fenestrae (with the exception of Sebecus querejazus and Bretesuchus bonapartei, which have a much more posteriorly positioned choana; Bravo et al., Reference Bravo, Pol and García-López2021).
The choanal region of Sebecus icaeorhinus described in this contribution is highlighted as remarkably different from that of all other sebecids and, consequently, its palatine represents a distinct morphotype in our morphometric analysis. The shape of the palatal surface of the palatine in this taxon alters the position of the choana with respect to the suborbital fenestrae. Sebecus icaeorhinus is the only sebecid in which the anterior margin of the choana and the anterior margin of the suborbital fenestrae are anteroposteriorly leveled. The anterior margin of the palatine is longer than in other sebecids, while the lateral margin is anteroposteriorly shorter. These differences in the palatine of Sebecus icaeorhinus and the deeply nested phylogenetic position of this taxon from the Eocene of Patagonia imply evolutionary changes that go in the opposite direction to the traditional trend of posterior migration of the choana cited above. Sebecus icaeorhinus displays an apomorphic change towards an anterior shift of the choana and a relative posterior displacement of the suborbital fenestra in comparison with other sebecids (including all other members of Sebecus clade). This shows that the evolutionary history of palatal changes in terrestrial crocodyliforms not only involves independent events of posterior displacements of the choana (e.g., Turner and Buckley, Reference Turner and Buckley2008), but also cases of reversals in this evolutionary trend, as evinced in the case of Sebecus icaeorhinus.
Palatal types and ecological diversity in Notosuchia
During the last two decades, new records of notosuchians along with phylogenetic analyses have reinforced the presence of multiple clades within this group, such as uruguaysuchids, peirosaurids, advanced notosuchians, baurusuchids, and sebecids (Ortega et al., Reference Ortega, Gasparini, Buscalioni and Calvo2000; Pol, Reference Pol2003; Turner and Sertich, Reference Turner and Sertich2010; Montefeltro et al., Reference Montefeltro, Larsson and Langer2011; Pol and Powell, Reference Pol and Powell2011; Pol et al., Reference Pol, Leardi, Lecuona and Krause2012, Reference Pol, Nascimiento, Carvhalo, Riccomini, Pires-Domingues and Zaher2014; Bravo et al., Reference Bravo, Pol and García-López2021; Ruiz et al., Reference Ruiz, Bronzati, Ferreira, Martins, Queiroz, Langer and Montefeltro2021). Each of these groups has been noted to differ in important ecological aspects, including drastic variation in body size, and various dietary habits, as inferred from their dental and mandibular anatomy (Wu and Sues, Reference Wu and Sues1996; Ösi, Reference Ösi2013; Stubbs et al., Reference Stubbs, Pierce, Rayfield and Anderson2013, Reference Stubbs, Pierce, Elsler, Anderson, Rayfield and Benton2021; Melstrom and Irmis, Reference Melstrom and Irmis2019; Godoy, Reference Godoy2020).
Our discriminant analyses on the palatine shape cluster members of most of these ecologically distinct clades (i.e., advanced notosuchians, Baurusuchidae, and some sebecids), revealing an interesting link between palatal types and inferred ecology. Among the most distinctive notosuchian subgroups in terms of the shape of the palatine are the advanced notosuchians, a group with inferred terrestrial habits, medium to small body size, heterodont dentition, and possibly omnivorous/herbivorous feeding habits (Fiorelli and Calvo, Reference Fiorelli and Calvo2008; Lecuona and Pol, Reference Lecuona and Pol2008; Ösi, Reference Ösi2013; Melstrom and Irmis., Reference Melstrom and Irmis2019). The other group with a highly distinct palatine shape is Baurusuchidae, which includes large terrestrial hypercarnivores with a reduced number of ziphodont teeth and robust skull bones. Both clades mainly comprise taxa from the Late Cretaceous of South America. The morphology of the palatine places sebecids and other notosuchians (peirosaurids and uruguaysuchids) in an intermediate position between the two above-mentioned groups, possibly representing a more generalized and plesiomorphic condition of Notosuchia. This morphology is present in species recorded along an extensive temporal range (Early Cretaceous–late Eocene), and includes both terrestrial carnivores (Bretesuchus) and possibly omnivores (e.g., Araripesuchus wegeneri) of medium to large body sizes.
Our results show that the palatine, which is a key element of the secondary palate, is highly variable within Notosuchia. Our results also reinforce the idea that the palatal and choanal region represent a potential source of valuable phylogenetic information in this clade, especially using morphogeometric approaches that capture subtle variations in the shape of palatal structures.
As mentioned above, changes in the palatine shape and posterior extension have also been recorded in other clades of non-eusuchian crocodyliforms and interpreted as having been acquired independently (Turner and Buckley, Reference Turner and Buckley2008; Dollman et al., Reference Dollman, Clark, Norell, Xing and Choiniere2018, Bravo et al., Reference Bravo, Pol and García-López2021). Our results distinguishing palatal anatomy of notosuchian clades with distinct ecological and morphofunctional skull traits suggest the palate played a role in different craniomandibular functions in addition to the construction of the nasopharyngeal passage. Previous adaptive interpretations of the evolution of the secondary palate of Crocodyliformes have invoked biomechanical explanations related to both feeding and breathing. These inferences were mainly based on neosuchian lineages and commonly focused on eusuchians (McHenry et al., Reference McHenry, Clausen, Daniel, Meers and Pendharkar2006; Gignac and O'Brien, Reference Gignac and O'Brien2016; McCurry et al., Reference McCurry, Evans, Fitzgerald, Adams, Clausen and McHenry2017; Ballell et al., Reference Ballell, Moon, Porro, Benton and Rayfield2019; Gignac et al., Reference Gignac, O'Brien, Turner, Erickson, Bels and Whishaw2019). The high variability of this region in multiple non-aquatic clades of Crocodyliformes with different feeding ecologies likely reflects the presence of diverse selective forces that may have played a role in the evolution of the secondary palate and choana in Crocodyliformes. These exceeded those involved in the adaptation of an aquatic lifestyle (Huxley, Reference Huxley1875; Busbey, Reference Busbey and Thomason1995; Rayfield and Milner, Reference Rayfield and Milner2008), and therefore future efforts are necessary to explore the relationship of these palatal changes to the biomechanical performance and/or functional morphology in non-eusuchian taxa (such as notosuchians or shartegosuchids) to acquire a deeper understanding of the evolution of these traits.
Conclusions
Specimen MMP 235 provides new information on the anatomy of Sebecus icaeorhinus. In particular, it allows a complete description of the posterior palatal region, characterized by a large, anteroposteriorly extensive, choana. The palatines have two short posterior processes that delimit the anterolateral margins of the choana and the suborbital fenestra. In ventral view, these margins are located at the same anteroposterior level on the coronal plane of the palate. The pterygoids of this specimen have a sub-triangular anterior region that delimited the posterior half of a bell-shaped choana. However, the pterygoid flanges are different from other taxa in being tabular and anteroposteriorly narrow along most of their length.
Our geometric morphometric analysis of the palatine allows us to distinguish eight clusters and indicates that the variability of this bone within Sebecidae is greater than in other clades of Notosuchia. This is clearly reflected in the morphological disparity of the palatine of Sebecidae, which can be divided into three distinct morphotypes. One morphotype represents a large percentage of the members of the family, whereas a second morphotype (present in Lorosuchus nodosus and Sahitisuchus fluminensis) resembles the plesiomorphic condition of the clade Notosuchia. The palatine of Sebecus icaeorhinus represents the third recognized morphotype and is different from all other members of Sebecidae. This is distinguished by a more extensive (or long) anterolateral margin, and a narrower posterior process. These features of Sebecus icaeorhinus help to better diagnose this taxon and broaden knowledge of the morphological variability of Sebecidae, as well as to identify a case of anterior migration of the anterior margin of the choana within this clade, following an inverse direction from the evolutionary trend of posterior migration of the choana previously recognized for Crocodyliformes.
Acknowledgments
We thank Museo Municipal de Ciencias Naturales L. Scaglia for allowing us to access material under their care. We also thank Centro de Imágenes Médicas del Instituto del Diagnóstico del Este de Chubut S.R.L., and the technician C. Ríos. The manuscript greatly benefitted from valuable comments from the reviewers, and from editor H.-D. Sues.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fj6q573xh.









