Introduction
Obsessive–compulsive disorder (OCD) is a chronic and disabling psychiatric condition. It is characterized by persistent, intrusive and distressing thoughts, known as obsessions, and the urge to perform repetitive or ritualistic behaviours, known as compulsions, that aim to reduce the anxiety and distress caused by obsessions (McGuire et al. Reference McGuire, Lewin, Horng, Murphy and Storch2012).
Some aspects of memory are notably impaired in individuals who are suffering from OCD (Tallis, Reference Tallis1997; Cuttler & Graf, Reference Cuttler and Graf2009a ). Although working and declarative verbal memory could be considered to be normal in OCD, neuropsychological studies have consistently reported impairments in visuospatial working memory (Woods et al. Reference Woods, Vevea, Chambless and Bayen2002; Kuelz et al. Reference Kuelz, Hohagen and Voderholzer2004; Fontenelle et al. Reference Fontenelle, Mendlowicz, Mattos and Versiani2006; Martinez-Gonzalez & Piqueras-Rodriguez, Reference Martinez-Gonzalez and Piqueras-Rodriguez2008; Morein-Zamir et al. Reference Morein-Zamir, Craiq, Ersche, Abbott, Muller, Fineberg, Bullmore, Sahakian and Robbins2010). Indeed, deficits are observed in both immediate (Zielinski et al. Reference Zielinski, Taylor and Juzwin1991; Zitterl et al. Reference Zitterl, Urban, Linzmayer, Aigner, Demal, Semler and Zitterl-Eqlseer2001; Boldrini et al. Reference Boldrini, De Pace, Placidi, Keilp, Ellis, Signori, Placidi and Cappa2005) and long-term visuospatial working memory (Kuelz et al. Reference Kuelz, Hohagen and Voderholzer2004; Fontenelle et al. Reference Fontenelle, Mendlowicz, Mattos and Versiani2006; Martinez-Gonzalez & Piqueras-Rodriguez, Reference Martinez-Gonzalez and Piqueras-Rodriguez2008). These deficits are even more important when patients have checking compulsions (Woods et al. Reference Woods, Vevea, Chambless and Bayen2002), suggesting a putative relationship between deficits in visuospatial working memory and the psychological correlate of checking compulsions, namely uncertainty.
Recently, many studies have placed new emphasis on the psychological and neural correlates of checking behaviours (Tallis, Reference Tallis1997; Tallis et al. Reference Tallis, Pratt and Jamani1999), the most frequent form of compulsive behaviours observed in OCD patients (Rasmussen & Eisen, Reference Rasmussen and Eisen1992; Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a , Reference Rotge, Langbour, Dilharreguy, Bordessoulles, Guehl, Bioulac, Martin-Guehl, Jaafari, Aouizerate, Allard and Burbaud2012; Kim et al. Reference Kim, Kim, Cha, Park, Rosenthal, Kim, Han, Kim and Kim2010; Toffolo et al. Reference Toffolo, Van den Hout, Hooge, Engelhard and Cath2013). To assess these behaviours, the checking task, a delayed matching-to-sample task with the unrestricted possibility of checking a decision, appears particularly relevant because it shares similar features with checking compulsions, which OCD patients experience daily (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ). Indeed, checking behaviours are repeated in OCD patients, especially when they suffer from checking compulsions, when compared with healthy controls. Distressing uncertainty appears to trigger checking behaviours, which is enhanced in OCD patients when compared with healthy controls. Furthermore, checking behaviours increase throughout the task in the OCD patients, but not in the healthy controls, as a coping strategy to reduce the cumulative distress caused by uncertainty (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ; Jaafari et al. Reference Jaafari, Aouizerate, Tignol, El-Hage, Wassouf, Guehl, Bioulac, Daniel, Lacoste, Gil, Burbaud and Rotge2011). However, one major issue in the assessment of checking behaviours lies in the putative role of working memory deficits in checking behaviours and their psychological correlates (Harkin & Kessler, Reference Harkin and Kessler2011).
According to an inference-based approach (O'Connor et al. Reference O'Connor, Aardema and Pelissier2005; Wu et al. Reference Wu, Aardema and O'Connor2009), uncertainty could be viewed as an intermediate state between a perceived reality and a given possibility with an overestimated likelihood based on subjective processes. Checking behaviour, as the behavioural output of uncertainty, could be defined as the renewal of an action that might be mistaken and responsible for negative consequences. The behaviour allows the subject to obtain more information and provides relief concerning the accuracy of an action and, therefore, concerning the level of uncertainty. Checking behaviour may therefore be viewed as the behavioural output of a perceived uncertainty, a coping strategy to compensate for visuospatial memory deficits or both. Furthermore, the repeated loss of accuracy, possibly related to memory deficits, may decrease confidence in memory and increase the likelihood of the occurrence of intrusive thoughts (Harkin & Kessler, Reference Harkin and Kessler2011; Harkin et al. Reference Harkin, Miellet and Kessler2012). However, these results were observed in subclinical samples and need to be replicated in patients suffering from OCD (Harkin et al. Reference Harkin, Miellet and Kessler2012). Furthermore, the core of OCD symptoms could reasonably be independent of memory deficits, and those memory deficits could be secondary to the pathological processes observed in OCD patients. Conversely, poor working memory abilities may contribute to an enhanced likelihood of feeling uncertainty in individuals without OCD. In other words, one could propose that checking behaviour is the behavioural output of uncertainty in OCD whereas it represents a strategy to compensate for visuospatial failures in healthy subjects. The relationship and the direction of the relationship between memory deficits and the psychological correlates of checking behaviour remain unclear.
Two inconsistent studies using a checking task have attempted to challenge the role of visuospatial memory deficits in repetitive checking behaviours in patients with OCD. First, Jaafari et al. (Reference Jaafari, Frasca, Rigalleau, Rachid, Gil, Olie, Guehl, Burbaud, Aouizerate, Rotge and Vibert2013) reported a correlation between memory deficits and checking behaviours. However, in this study, checking behaviours consisted of gaze moves for comparing two pictures that were presented simultaneously. Furthermore, the direction of the relationship was not fully assessed. Indeed, it remains unclear whether pathological uncertainty affects working memory abilities, whether memory deficits increase the level of uncertainty or both. Second, Clair et al. (Reference Clair, N'Diaye, Baroukh, Pochon, Morgieve, Hantouche, Falissard, Pelissolo and Mallet2013) showed that repetitive checking behaviours are independent of visuospatial working memory performance. However, visuospatial working memory abilities were measured with a two-alternative, forced-choice, delayed matching-to-sample task without checking opportunity. Therefore, it was not possible to discern working memory performance from uncertainty processes. Finally, figurative pictures without explicit assessment regarding memory load or difficulties were used in both studies. In this context, the level of memory processes required for completing the task is neither controlled nor constant throughout the task.
Therefore, the relationship between working memory deficits and uncertainty, as the main psychological correlate of checking behaviours, remains to be refined in both OCD patients and healthy subjects. To address this issue, we developed a delayed matching-to-sample task with the explicit possibility of expressing a lack of knowledge during decision making. The task was designed to explicitly discriminate decision making related to certainty from either uncertainty about one possible option or complete ignorance of the correct answer. Furthermore, to control for and to make constant the level of memory processes engaged in the task, we used a battery of checkerboards as visual stimuli that allowed us to control the level of difficulty through the proportion of white and black squares under identical psychophysical conditions.
The aim of the present study was to assess the relationship between visuospatial working memory abilities and uncertainty. We hypothesized that (i) OCD patients would exhibit visuospatial working memory deficits when compared with healthy controls; (ii) visuospatial working memory abilities would not contribute to the propensity toward uncertainty in OCD patients but would in healthy controls; and (iii) uncertainty would contribute to decreased working memory skills in OCD patients, but not in healthy controls. To address these hypotheses, we used neuropsychological tests to objectively assess baseline working memory abilities and the present experimental task to assess the putative relationship between visuospatial working memory abilities and uncertainty in OCD patients and healthy controls.
Method
Participants and clinical assessments
A total of 25 adult patients with a primary diagnosis of OCD and 25 age-, sex- and handedness-matched healthy volunteers were recruited at two specialized French university hospitals (Centre Hospitalier Henri-Laborit, Poitiers, and Centre Hospitalier Charles Perrens, Bordeaux) and from the local community by advertisements and word of mouth. All participants gave their written informed consent to participate in the experiment, and the protocol was approved by the local medical ethical review committee.
OCD and other Axis-I Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) diagnoses were assessed using the Mini International Neuropsychiatric Interview (version 5.0.0) (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Herqueta, Baker and Dunbar1998). OCD patients were not included if they were currently suffering from an episode of mood disorders. Healthy controls were not included if they had a history of psychiatric illness or if they were taking psychotropic medications. The severity of OCD symptoms was assessed with the Yale–Brown Obsessive–Compulsive Scale (YBOCS; Goodman et al. Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989; Storch et al. Reference Storch, Larson, Price, Rasmussen, Murphy and Goodman2010a , Reference Storch, Rasmussen, Price, Larson, Murphy and Goodman b ). To be included, OCD patients had to have a YBOCS score >16, indicating the presence of clinically significant symptoms. The YBOCS Symptom Checklist identified past and current obsessions and compulsions. Target symptom lists for obsessions and compulsions were then generated by listing symptoms in order of severity and time consumption, allowing us to identify OCD patients with (n = 13) or without (n = 12) checking compulsions (Goodman et al. Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989; Mataix-Cols et al. Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004). Levels of anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS) and the Hamilton Depression Rating Scale (HAMD; Hamilton, Reference Hamilton1960). Subjects with a score of >10 on the HADS depression subscore or >7 on the HAMD were not included in the present study to eliminate the impact of depressive symptoms on visuospatial working memory.
None of the 25 normal volunteers was taking medication at the time of study, whereas 24 OCD patients were receiving the maximum tolerated doses of antidepressants with serotonin reuptake-inhibiting properties (n = 22) alone or combined with low doses of atypical psychotics (n = 10) or buspirone (n = 4).
Visuospatial working memory
Spatial working memory function was measured by the Corsi block-tapping test (Milner, Reference Milner1971; Wechsler, Reference Wechsler1987). The material consisted of 10 square blocks that were spatially distributed on a board numbered from 1 to 10 on the side hidden from the participant but visible to the experimenter. To measure working memory in the subjects, the experimenter would point to a sequence of blocks, and participants were required to recall in the correct order, then in the reverse order, a sequence of block positions that had just been indicated on the board by the experimenter. The test measured both the number of correct sequences and the longest sequence remembered. In the ‘forward test’, the subject was asked to repeat back sequences of block spatial positions of increasing length in original order, indicated by the experimenter, allowing a measure of immediate memory. In the ‘backward test’, the subject was required to repeat the sequences in reverse order, with increasing length of sequences, thus measuring spatial working memory and assessing the ability to manipulate information in visuospatial working memory. The total, forward and backward Corsi scores were individually adjusted for age (Wechsler, Reference Wechsler1997).
Task design
Participants were tested individually. Dedicated software (E-Prime2®; Psychology Software Tools, USA) was used to prepare and present stimuli. A personal computer-compatible laptop was used to control the presentation of stimuli and to record behavioural responses. The screen displaying stimuli was placed at a fixed viewing distance (65 ± 5 cm). All participants had normal or corrected-to-normal vision, and before running the experiment, it was made sure that participants could comfortably view stimuli. The experimental task is described in Fig. 1. Participants were required to examine a black and white checkerboard pattern of 25 black and 75 white squares, hold it in their memory and then compare it with a similar pattern that was presented consecutively. Participants were then asked to decide whether the two pictures were identical. To answer, they responded by pressing a left, central or right button corresponding to ‘yes’, ‘I don't know’ or ‘no’, respectively. Participants were instructed to answer ‘yes’ or ‘no’ only if they were sure of the accuracy of their decision (certain trials) and to answer ‘I don't know’ in other cases. When pressing the left or right buttons (‘yes’ or ‘no’), participants were informed of the accuracy of their decision (‘right’ or ‘wrong’). When pressing the central button (‘I don't know’), an additional screen appeared with two options: ‘uncertainty’ (uncertain trials) or ‘not a clue’ (ignorant trials). In order to better discriminate uncertainty, as this ‘intermediate state’ between two possibilities that we described earlier, and ignorance, as a state without any reasonable option, participants were instructed to choose ‘uncertainty’ if they felt uncertain about one response to give and to choose ‘not a clue’ if they had absolutely no idea whether the two pictures were identical. Afterward, participants were given feedback on the correct answer. At the end of each trial, the cumulative percentage of accurate decisions was presented to remind the subject of the necessity of being sure of the response during the decision-making phase. At the end of an uncertain or ignorant trial, the cumulative percentage of accuracy remained unchanged in order not to punish or reward these trials. To ensure that subjects were familiar with the procedure, the task was explained, and 10 trials were performed for training purpose. Each participant completed six runs of 60 randomized trials (30 with matched and 30 with unmatched trials) in approximately 1 h. Unmatched trials consisted of the removal of one black square during the matching phase.

Fig. 1. Experimental task. The sample, a single image consisting of a checkerboard, was displayed, followed by the presentation of the second image after a 1.5-s delay. Then, participants were required to answer the question ‘are both pictures identical?’ The subject could press the left, central or right button, corresponding to the choices ‘yes’ (Y), ‘I do not know’ or ‘no’ (N), respectively. Participants were explicitly asked to answer ‘yes’ or ‘no’ only when they were certain of their answer (certain trials). In these cases, feedback was given. In the other case (‘I do not know’), an additional screen appeared with two options: ‘uncertainty’ (uncertain trials) or ‘not a clue’ (ignorant trials). Participants were explicitly asked to choose ‘uncertainty’ if they felt uncertain about the response to give and to choose ‘not a clue’ if they had absolutely no idea whether the two pictures were matched or unmatched. Afterward, participants were given feedback on the correct answer. At the end of each trial, the cumulative percentage of accurate decisions was presented to remind the subject of the necessity of being certain of the response to give. After 10 training trials, the participants completed 360 randomized trials.
We monitored the number of successful trials; the number of certain, uncertain and ignorant responses; and the corresponding response time for decision making (defined as the time elapsing from the appearance of the matching phase to the time the response button was pressed) during the 360 consecutive trials.
Data analysis
Sociodemographic and clinical characteristics of both the patient and control groups were compared using χ 2 tests and t tests for independent samples.
The measured behavioural variables (accuracy, numbers of certain, uncertain and ignorant trials and the corresponding response times for decision making) were considered to be independent variables. Certain, uncertain and ignorant trials were considered to be different experimental conditions (condition factor) to be compared. Based on prior research highlighting the behavioural effects of the trial type, i.e. matched versus unmatched trials, a trial factor was included in the statistical model (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a , Reference Rotge, Langbour, Dilharreguy, Bordessoulles, Guehl, Bioulac, Martin-Guehl, Jaafari, Aouizerate, Allard and Burbaud2012). The diagnosis status was used as the between-group factor. Because the levels of education were different between the OCD subjects and the healthy comparison controls, this variable was entered as a statistical covariate to control for its putative effect on behavioural measures. Therefore, variables were entered into multivariate analyses of covariance with three different factors (trial, condition and group) and with the level of education as a control variable.
To test whether baseline working memory abilities predicted the propensity to be uncertain during the task, a regression model was created with working memory scores as independent variables and the number of uncertain trials as dependent variables. The level of education was included in this model as a control variable.
To test whether uncertainty may affect visuospatial discrimination ability, we compared the response accuracy of two different conditions, i.e. unmatched trials following and not following uncertain trials, in an analysis of variance with the diagnosis status as the between-group factor. The level of education was included in this model as a control variable.
All analyses were two-tailed, and the α risk was set at 0.05 as the threshold for significance. Statistical analyses were performed with SPSS software (release 16.0; SPSS, Inc., USA).
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of both OCD patients and healthy comparison subjects are presented in Table 1. According to the inclusion criteria, all patients had a YBOCS score >16 and HAMD and HADS scores of <8 and <11, respectively. The OCD and control comparison groups did not differ with respect to age, sex or handedness. However, the groups were not matched for educational level and there was a statistical trend for age (p = 0.09). OCD patients demonstrated worse visuospatial working memory performances than healthy comparison subjects. This difference remained significant after controlling for educational level and age (Table 1).
Table 1. The demographic and clinical characteristics of OCD patients and healthy comparison subjects

OCD, Obsessive–compulsive disorder; s.d., standard deviation; n.a., not available; YBOCS, Yale–Brown Obsessive–Compulsive Scale; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Rating Scale.
a The statistics consisted of χ 2 tests or t tests for independent samples.
b Statistics remained significant after the introduction of the level of education or HAMD scores as covariates.
Behavioural measures
Each participant performed 360 trials, among which certain [OCD: 223.4 (s.d. = 75, range 49–340), controls: 244.8 (s.d. = 69, range 106–341)], uncertain [OCD: 87.4 (s.d. = 86, range 9–307), controls: 96.7 (s.d. = 63, range 10–224)] and ignorant trials [OCD: 5.8 (s.d. = 10, range 0–41), controls: 10.5 (s.d. = 16, range 0–47)] were determined post-hoc. Neither significant main effects nor significant interactions were found regarding the response accuracy; the number of certain, uncertain and ignorant trials; or the response times for the different conditions between groups. The statistics are fully described in Table 2. To account for symptom dimensions, analyses were repeated with a group factor that discriminated among OCD patients with checking compulsions (n = 13), OCD patients without checking compulsions (n = 12) and healthy comparison controls (n = 25). All p values were over the threshold of significance.
Table 2. The behavioural performances in OCD patients and healthy comparison subjects

Data are given as mean (standard deviation).
OCD, Obsessive–compulsive disorder; MANCOVA, multivariate analyses of covariance.
a The statistics consisted of MANCOVA with the level of education as a control variable. The inclusion of age as an additional control variable in the statistical models did not influence the present results. MANCOVA without the level of education as a control variable did not reveal any statistical significance.
Working memory abilities and the propensity to be uncertain
In OCD patients, regression analyses revealed that the baseline standardized backward scores (F = 0.49, R = 0.14, p = 0.49) and the standardized forward scores (F = 0.99, R = 0.20, p = 0.33) did not predict the number of uncertain trials during the task. However, the baseline standardized backward scores (F = 5.55, R = 0.44, p < 0.05), but not the standardized forward scores (F = 3.51, R = 0.36, p = 0.07), predicted the numbers of uncertain trials in healthy controls. These observations differed statistically regarding interaction terms for both backward scores (F = 5.84, p < 0.05) and forward scores (F = 4.68, p = 0.041).
Uncertainty and the ability to discriminate visuospatial differences
In this analysis, the response accuracy during trials with unmatched pairs of pictures was assumed to estimate the ability to discriminate visuospatial differences throughout the task. To assess the effect of uncertain trials on this ability, the response accuracy was compared between unmatched trials following [OCD: 43.5 (s.d. = 42, range 8–149), controls: 50.4 (s.d. = 32, range 15–122)] and not following uncertain trials [OCD: 116.2 (s.d. = 2, range 44–172), controls: 128.1 (s.d. = 31, range 59–179)] for both OCD and healthy comparison groups. The response accuracy decreased after an uncertain trial, especially in OCD patients (group: F = 6.69, p = 0.013; condition: F = 12.12, p < 0.001; group × condition: F = 4.52, p = 0.039). Following uncertainty, visuospatial abilities dropped to 65% of usual performance in OCD patients whereas they remained quite stable at 96% in healthy comparison controls (Fig. 2).

Fig. 2. Percentage of variation in response accuracy during unmatched trials not following versus following uncertain trials in the obsessive–compulsive disorder (OCD) and healthy groups. OCD patients exhibited a decrease in their ability to discriminate visuospatial differences after uncertain trials when compared with certain trials, whereas memory performance remained stable in healthy controls.
OCD characteristics and behavioural measures
No significant correlation was found between clinical characteristics of OCD (onset age, duration of OCD, YBOCS, HADS and HAMD scores) and the behavioural effects observed in the present study (the number of uncertain trials, the response accuracy during unmatched trials following or not uncertain trials) (all p values > 0.05). Concerning visuospatial abilities and consequences on uncertainty, no significant differences were observed between patients with OCD with and without checking compulsions.
Discussion
In the present study, we used neuropsychological assessments and an experimental task that allows the explicit recognition of uncertainty, the psychological correlate of checking behaviours, to explore the temporal direction of the relationship between uncertainty and working memory abilities. As hypothesized, we found a specific relationship between working memory deficits and uncertainty in OCD patients; this relationship could be distinguished from the relationship observed in healthy subjects. Specifically, our findings show that the baseline performance in visuospatial working memory did not influence the occurrence of uncertainty in patients with OCD, whereas this baseline performance promoted the propensity to feel uncertainty in healthy subjects. Furthermore, uncertainty prevented the correct discrimination of visuospatial information in patients with OCD, suggesting that uncertainty interferes with the ability to hold and manipulate environmental information, which could contribute to maintaining uncertainty and the repetition of its behavioural consequence, namely checking behaviours. The present findings support and extend the memory deficit theory and the meta-memory deficit theory of compulsive checking (Cuttler & Graf, Reference Cuttler and Graf2009a ).
Visuospatial working memory deficits did not predict the number of uncertain trials in OCD patients whereas they did in the healthy group. This finding revealed that the pathological uncertainty observed in patients with OCD was not induced by memory deficits whereas poor memory performance contributed to the propensity to normal uncertainty in healthy subjects. Our study is in accordance with a previous study that showed that pathological uncertainty is an independent phenomenon in OCD; specifically, uncertainty did not depend on information related to the environmental context (Rotge et al. Reference Rotge, Langbour, Dilharreguy, Bordessoulles, Guehl, Bioulac, Martin-Guehl, Jaafari, Aouizerate, Allard and Burbaud2012). Therefore, the ability to hold and manipulate environmental information, as allowed by visuospatial working memory, does not mediate pathological uncertainty. This assumption may appear as contradictory to the data of Jaafari et al. (Reference Jaafari, Frasca, Rigalleau, Rachid, Gil, Olie, Guehl, Burbaud, Aouizerate, Rotge and Vibert2013). Indeed, the authors used an original version of Rotge's task (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ) and they reported strong relationships between baseline working memory performances and the number of gazes between two drawings presented simultaneously. Two different hypotheses may explain the apparent discrepancy between the results of Jaafari et al. (Reference Jaafari, Frasca, Rigalleau, Rachid, Gil, Olie, Guehl, Burbaud, Aouizerate, Rotge and Vibert2013) and the present results. First, the relationship between working memory performances and gaze moves was also observed in healthy controls, suggesting that gaze moves could represent a behavioural marker of a sum of effects including uncertainty, but also working memory skills. Second, in the present study, although relationships between working memory performances and the propensity to be uncertain differed between the OCD and control groups, we did not assess the level of uncertainty throughout the task. Indeed, participants were asked if they were uncertain, but uncertainty was not scored. Therefore, we could not formally exclude that the number of gaze moves were related to the level of uncertainty that was not captured in the present study. When considering uncertainty as the main psychological correlate leading to checking behaviour, our results argue against the claim of the memory deficit theory, i.e. memory deficits contribute to the compulsion of checking in OCD patients, whereas they support it in healthy controls. From a phenomenological point of view, we propose that poor visuospatial working memory enhances the propensity to be uncertain, thus leading to an increased likelihood to check a past action, which may therefore be viewed as an adaptive process to palliate the lack of information resulting from difficulties in holding and manipulating visuospatial information. However, we did not assess checking in the present study and this interpretation should be taken cautiously and should be properly tested.
Uncertain trials induced a drop in performance in visuospatial working memory in OCD patients whereas memory performance was not affected in healthy controls, suggesting that pathological uncertainty contributes to decreases in visuospatial working memory abilities in patients with OCD. These findings may be prima facie viewed as contradictory to the meta-memory deficit theory, which claims that diminished confidence in memory abilities contribute to compulsive checking. Indeed, prior findings have consistently described the ‘ironic effects’ of repeated checking behaviours (Giele et al. Reference Giele, van den Hout, Engelhard, Dek and de Wit2013) that reduce confidence in memory and increase uncertainty (Van den Hout & Kindt, Reference Van den Hout and Kindt2003a , Reference Van den Hout and Kindt b ; Coles et al. Reference Coles, Radomsky and Horng2006; Radomsky et al. Reference Radomsky, Gilchrist and Dussault2006; Ashbaugh & Radomsky, Reference Ashbaugh and Radomsky2007; Boschen & Vuksanovic, Reference Boschen and Vuksanovic2007; Cuttler & Graf, Reference Cuttler and Graf2009a ; Dek et al. Reference Dek, van den Hout, Giele and Engelhard2010; Giele et al. Reference Giele, van den Hout, Engelhard, Dek and de Wit2013; Toffolo et al. Reference Toffolo, Van den Hout, Hooge, Engelhard and Cath2013), whereas we described a diminished ability to detect a visuospatial difference in the present study. Furthermore, like healthy subjects, the OCD patients were asked to answer only if they were certain of their answer, i.e. they felt confident in their answer. Although we did not assess confidence in memory, the data clearly showed this drop in visuospatial working memory performance. The loss of confidence in memory has been mainly described in ‘subclinical’ samples of students, whereas we included patients who had suffered from OCD for more than 20 years in the present study (Cuttler & Graf, 2009b). It seems reasonable to hypothesize that a long disease duration could contribute not only to decrements in working memory abilities but also to increased sensitivity to the balance between uncertainty and working memory, whereas individuals with a shorter disease duration could benefit from more adaptive processes concerning their working memory abilities. Finally, the uncertainty-induced decrease in working memory performance could contribute to the impaired monitoring of forward action outcomes or promote stimulus–response habit formation, both of which have been proposed as critical in OCD pathophysiology (Gentsch et al. Reference Gentsch, Schütz-Bosbach, Endrass and Kathmann2012; Gillan et al. Reference Gillan, Morein-Zamir, Kaser, Fineberg, Sule, Sahakian, Cardinal and Robbins2013a , Reference Gillan, Morein-Zamir, Urcelay, Sule, Voon, Apergis-Schoute, Fineberg, Sahakian and Robbins b ). This idea is in accordance with the security-motivation theory of OCD, which proposes that compulsive behaviours should be considered as dysfunctions of termination, i.e. behaviours that are unable to terminate the motivation evoked by potential danger (Szechtman & Woody, Reference Szechtman and Woody2004; Hinds et al. Reference Hinds, Woody, Van Ameringen, Schmidt and Szechtman2012). The inability of a behavioural response to influence the distressing thoughts associated with OCD may account for deficits in the top-down control of the affective drive associated with uncertainty, as recently proposed (Rotge et al. Reference Rotge, Langbour, Dilharreguy, Bordessoulles, Guehl, Bioulac, Martin-Guehl, Jaafari, Aouizerate, Allard and Burbaud2012). The present findings invite us to consider this decrease in working memory performance to be a possible marker of these deficits in top-down processes. Such hypotheses should be tested in further studies.
Regarding the number of uncertain trials, no difference was found between OCD patients and controls in the present study. Many experimental factors could contribute to the present result. Measuring the intensity of uncertainty for each trial could be helpful for assessing a possible dissociation between the occurrence and the intensity of uncertainty between OCD patients and controls. Furthermore, due to the characteristics of the task, uncertainty was not allowed to be expressed during long periods, especially in a repetitive manner contrary to Rotge's task (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ). Therefore, it appears possible that the dynamic of the task ended putative intrusive and repetitive thoughts regarding the accuracy of the response to give without negative consequences since uncertain trials did not make an impact on accuracy scores. Moreover, no difference was found regarding the number of uncertainty when the two pictures were identical or different, contrary to previous studies (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ; Jaafari et al. Reference Jaafari, Frasca, Rigalleau, Rachid, Gil, Olie, Guehl, Burbaud, Aouizerate, Rotge and Vibert2013). This discrepancy could be explained by the nature of the pictures used in these studies. Indeed, some standardized pictures with possible subtle difference, as revealed by the level of accuracy, were used in the present study whereas figurative pictures with some great differences for some trials were used in prior studies. The importance of differences within some couples of pictures may contribute to extinguish possible uncertainty and lead to some great differences between matched and unmatched trials.
In light of prior research, our findings extend phenomenological views of OCD, which may be of clinical importance, as shown in Fig. 3. First, although the baseline performance in visuospatial working memory is altered in patients with OCD, this performance does not induce OCD symptoms. Second, uncertainty and its intolerability would result in a reduction in memory confidence, which in turn enhances the level of uncertainty, thereby contributing to the maintenance of high levels of uncertainty and the repetition of checking behaviours, as counterproductive attempts to control uncertainty and its intolerability. Daily repetitions of this vicious cycle over years may lead to a loss of cognitive adaptability with progressive worsening of abilities to manipulate environmental information to control uncertainty. Finally, at the neurobiological level, it is interesting to note the overlap of the brain regions that are functionally involved in OCD (Rotge et al. Reference Rotge, Guehl, Dilharreguy, Cuny, Tignol, Bioulac, Allard, Burbaud and Aouizerate2008b ) and the developmental change of the brain network involved in visuospatial working memory (Scherf et al. Reference Scherf, Sweeney and Luna2006), especially in the frontal, parietal and temporal regions. Uncertainty processes engaged in the pathophysiology of OCD may disrupt networks involved in executive function. Furthermore, when OCD begins at puberty or earlier (Stein, Reference Stein2002; Anholt et al. Reference Anholt, Aderka, van Balkom, Smit, Schruers, van der Wee, Eikelenboom, De Luca and van Oppen2013), it could be hypothesized that the brain memory network has not been fully diffused and thus remains impaired in patients with adolescent-onset OCD. Obviously, such a hypothesis should be properly tested.
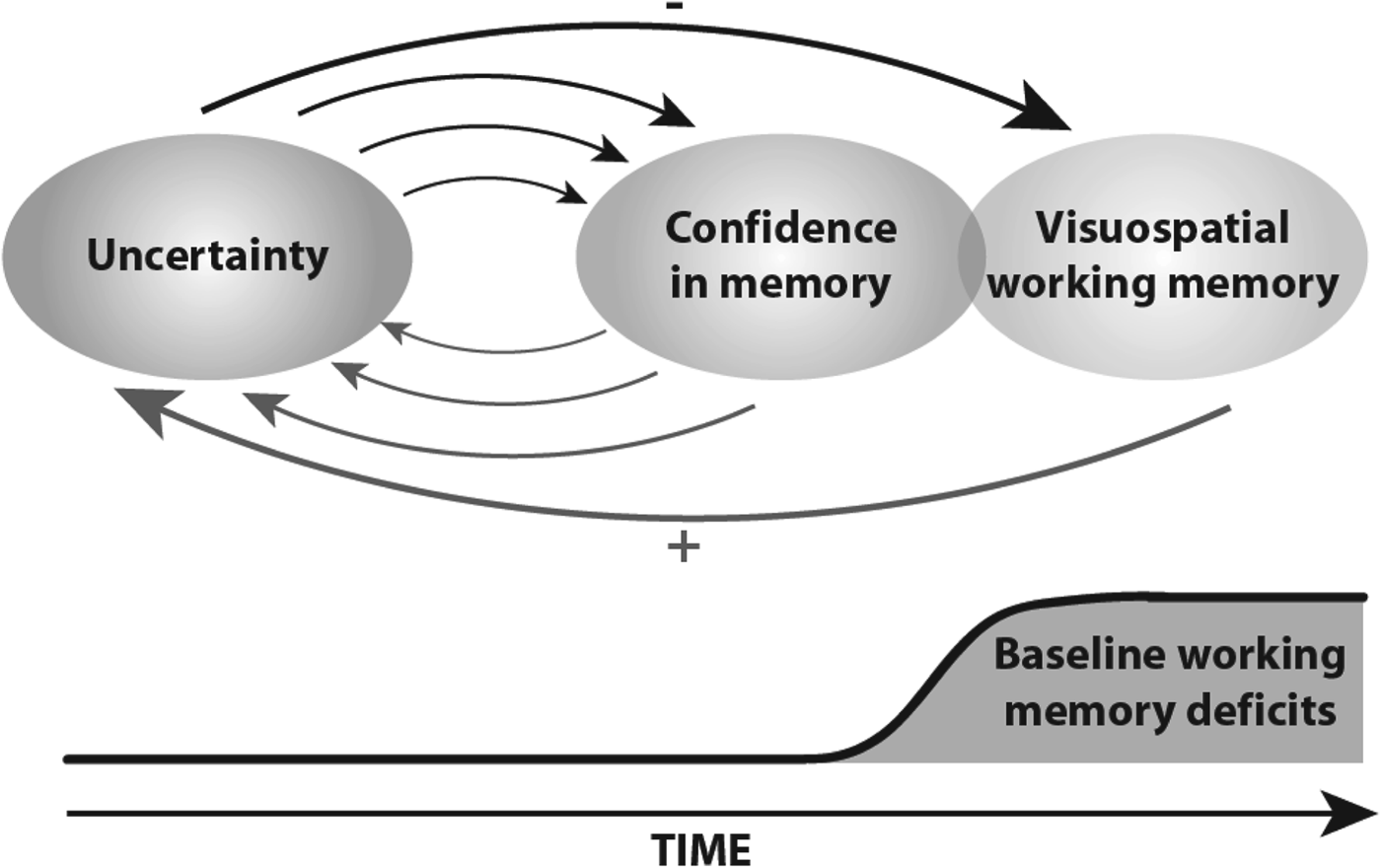
Fig. 3. A proposal regarding the relationships between uncertainty, meta-memory processes and working memory deficits in obsessive–compulsive disorder.
The present study had some limitations. First, the large majority of OCD patients included in the present study were taking antidepressants with inhibitor of serotonin reuptake properties, which affect frontal functions, although their specific role in visuospatial working memory remains obscure (Morrens et al. Reference Morrens, Wezenberg, Verkes, Hulstijn, Ruigt and Sabbe2007). These psychotropic medications were not discontinued for obvious ethical reasons; therefore, we cannot rule out the influence of these medications on the present findings. However, this limitation should be tempered, as all patients exhibited clinically significant OCD symptoms, as illustrated by YBOCS scores >16. Second, confidence in memory was not directly assessed, and our interpretations of the present results must be considered in the light of this deficiency. However, this point did not rule out our main findings. Indeed, participants were asked to give an answer only when they were certain of their decision, and they systematically had the opportunity to answer ‘I do not know’. Third, the task was designed according to our hypotheses, and we did not implement the possibility of checking behaviours, as previously described (Rotge et al. Reference Rotge, Clair, Jaafari, Hantouche, Pelissolo, Goillandeau, Pochon, Guehl, Bioulac, Burbaud, Tignol, Mallet and Aouizerate2008a ). Although many studies have reported the relationship between uncertainty and checking behaviours, we cannot formally extend our results to checking compulsions (Rotge et al. Reference Rotge, Langbour, Dilharreguy, Bordessoulles, Guehl, Bioulac, Martin-Guehl, Jaafari, Aouizerate, Allard and Burbaud2012; Toffolo et al. Reference Toffolo, Van den Hout, Hooge, Engelhard and Cath2013). Fourth, there was a statistical trend regarding possible difference in age between the two groups. However, statistics were repeated with age as a covariate and no marked difference in our results was observed; therefore, it seemed reasonable to eliminate this possible bias in the present study. Furthermore, although multivariate analyses of covariance allowed us to assess multiple variables that need to be taken into account, the relatively low number of subjects included in the present study may contribute to limit the statistical power of our analysis regarding behavioural measures, and also when regarding the possible differences between OCD patients with and without checking compulsions. Finally, although we attempted to control the difficulty and memory load throughout the task by using standardized images composed of black and white squares, it remained possible that the perception of the removal of one black square could be easier on some trials, depending on the variability of the spatial distribution of the initial 25 black squares.
In conclusion, our study supports and extends previous findings by showing that baseline abilities in visuospatial working memory contribute to the propensity to be uncertain in healthy subjects whereas they do not directly contribute to pathological uncertainty in patients with OCD. In contrast, pathological uncertainty directly decreases working memory performance in patients with OCD whereas it has no memory effect in healthy subjects. These data argue for different, and even opposite, psychological correlates of checking behaviours in patients with OCD and healthy subjects, which could be important when considering working memory performance in cognitive therapy.
Acknowledgements
The present study received no financial support.
Declaration of Interest
None.







