Published online by Cambridge University Press: 21 January 2005
Repair of complex malformations that necessitate restoration of continuity between the right ventricle and the pulmonary arteries can now safely be performed with low morbidity and mortality. Major concerns still remain on the long-term outlook for these patients, and about the durability of the different prostheses used to restore that continuity, whether during initial correction or at the time of reintervention for failure of the conduit or pulmonary regurgitation. In this review, we discuss the salient morphologic features of the right ventricular outflow tract, and then focus on the indications for early and late intervention, current therapeutic options, and outcomes.
Before considering the indications and the manner of surgical reconstruction of the right ventricular outflow tract, let us first consider its normal morphology. It is of note that this seemingly simple task has, over the years, been unnecessarily complicated through the indiscriminate and inconsistent use of terminology. Thus, although most clinicians will understand the equivalency of terms such as “conus” and “infundibulum”, there may be confusion for other portions of the outflow tract. In our review, while primarily using our preferred terminology, we will seek to acknowledge the recognised alternatives (Fig. 1).1
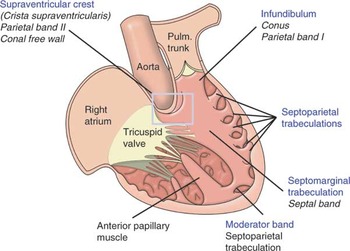
Figure 1. A schematic illustration of the arrangement and principal features of the right side of the normal heart. It also demonstrates the alternative terms for the supraventricular crest, infundibulum, septomarginal trabeculation, and the moderator band.
The right ventricular outflow tract possesses a unique morphologic feature that influences its surgical reconstruction, namely that it contains the only cardiac valve that is supported in its entirety by freestanding musculature. Observed in surgical orientation, this muscular support lifts the outflow tract, and the valve contained within, into the top left hand corner of the operating field, sitting atop the ventricular mass (Figs 2 and 3). This singular arrangement also accounts for the separation of the inlet and outlet components of the morphologically right ventricle (Fig. 4), in contrast to the left heart, where the two portions are in close working proximity. As will be seen, the nature of this muscular support, together with its relationship to surrounding structures, is the key to understanding most surgical manipulations of the outflow tract. When the epicardial layer of the right ventricular outflow tract is stripped away at the sternocostal margin of the ventricular mass, it can be seen that the myocardial fibres are obliquely oriented (Fig. 3). If this dissection is continued behind the pulmonary valve, in front of the aortic root, it then becomes evident that these fibres encircle the base of the pulmonary valve and distal outflow tract, forming a cuff of supporting musculature (Fig. 5). This sleeve is variously termed the infundibulum, meaning “little funnel”, or conus.1 It is fed by arterial twigs from the proximal right coronary artery (Fig. 6).
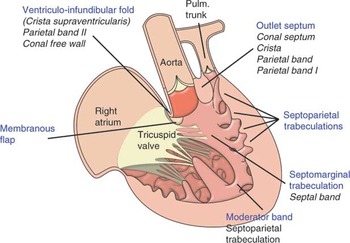
Figure 2. This illustrates the arrangement of the right heart in a case of tetralogy of Fallot, including the narrowed right ventricular outflow tract and the muscular outlet septum, which is deviated in the anterocephalad direction. It is this combination, together with hypertrophy of the septoparietal trabeculations, that accounts for narrowed outflow tract.

Figure 3. This close view of the right ventricular outflow tract, with the epicardium stripped away, demonstrates the circumferential nature of the myocardial fibres at the sub-pulmonary infundibulum.
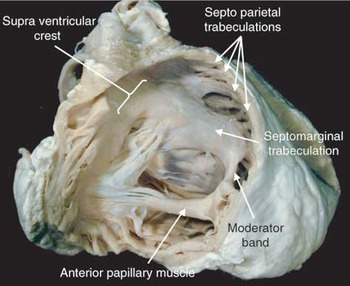
Figure 4. This is a photograph of the normal heart, analogous to Figure 1, demonstrating the main features of the right ventricular outflow. Note the connection of the base of the anterior papillary muscle to the septomarginal trabeculation by way of the so-called moderator band.

Figure 5. This view from above demonstrates the tissue plane between the two arterial valves. Note their orientation relative to one another. LCS: left coronary sinus; RCS: right coronary sinus.
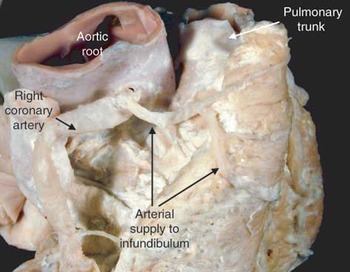
Figure 6. This shows the arterial supply to the sub-pulmonary infundibulum via branches of the right coronary artery that cross the atrio-ventricular groove.
The principal, and fortuitous, surgical consequence of this cuffed arrangement to the right ventricular outflow tract is to create a discrete fibro-fatty tissue plane between the distal right ventricular outflow tract and the root of the aorta. It is this plane that permits easy separation of the two components during the Ross procedure (Figs 5 and 7). The arrangement, nevertheless, is still a mixed blessing for the surgeon, who has to negotiate a number of potential hazards when separating the outflow tract from the rest of the ventricular mass. Foremost is the first septal perforating artery, which arises as the first branch of the superior interventricular coronary artery, often described inappropriately as the anterior descending artery, and supplies the antero-superior part of the conduction system.2 In order to reach the ventricular septum, the perforating artery passes obliquely from the interventricular groove towards the medial papillary muscle, and in doing so comes into close proximity to the sub-pulmonary infundibulum (Fig. 8). Thus, the muscular infundibulum has to be dissected in a manner that takes account of the angled course of this vessel.

Figure 7. This view of the normal heart emphasises the clear fibro-fatty tissue plane between the sub-pulmonary infundibulum and the root of the aorta. It also shows the muscular sleeve that is the freestanding sub-pulmonary infundibulum. Note the close proximity of the right coronary artery and its branches to the outflow tract.

Figure 8. This view of the normal heart shows the close relationship of the left coronary artery to the right ventricular outflow tract, and in particular the position of the first septal perforating artery which takes an oblique course deep to the sub-pulmonary infundibulum.
While the anatomic arrangement so far described explains some of the peculiarities to the dissection in the operating room, it also begs the question of why it is even necessary to explant the pulmonary valve along with its muscular infundibulum? The answer lies in the structure of the pulmonary valve itself, specifically the relationship of its leaflets to the junction between arterial wall and myocardium. At the distal junction of the sub-pulmonary infundibulum, or conus, the myocardium gives way to a thin collagenous layer that “glues” the arterial wall of the pulmonary trunk to the myocardium. This junction between the myocardial and arterial walls forms the scaffold forms the circular anatomic ventriculo-arterial junction, which is positioned virtually in the coronal plane, anterior and to the left of the aortic root (Fig. 9).

Figure 9. The morphologist’s view of the pulmonary valve, which has been opened out, and laid flat. The anatomic myocardial–arterial junction is marked by the red stars. As a consequence of the coronal attachment of the pulmonary leaflets, they straddle the myocardial–arterial junction, so that the tips of the three interleaflet triangles (green triangles) are arterial, and not muscular as might be expected.
When seen in the closed position, the pulmonary valve takes the form of a crown, or coronet, with well-formed sinusal scoops on its arterial side, which intertwine with intervening fibrous triangles on its ventricular aspect. Rather than being entirely within the arterial segment of the outflow tract, the leaflets of the valve itself sit astride the anatomic junction between myocardium and arterial wall. Consequently, the basal part of each of the three sinuses of the pulmonary valve is composed not of arterial wall but of myocardium. Similarly, the tips of the three interleaflet triangle are not myocardial, as might be expected, but are arterial (Fig. 9).3 This anatomic point may be difficult to appreciate by the surgeon, who relies on the somewhat limited view in the operating room, but is readily seen by morphologist, who has the advantage of being able to open the valve and lay it flat! Be that as it may, it explains anatomically the practice of having to remove a greater mass of tissue than merely the pulmonary sinuses when performing the Ross procedure.4
Normally, the right ventricular trabeculations extend into the outflow tract, usually being restricted to the lateral walls of the sub-pulmonary infundibulum (Fig. 4). We name these structures, which extend from the septum to the free wall of the right ventricle throughout the length of the outflow tract, the septoparietal trabeculations. Others prefer to restrict this term to the apical trabeculation that extends from the septum to the anterior wall of the right ventricle. We call this the moderator band, and it is but one of the series of septoparietal trabeculations.
Because of the normally wide individual variability in the distribution of these muscular bands, it is easy to see how the right ventricular outflow tract may, in some cases, contain the substrate for sub-pulmonary obstruction should there be hypertrophy of one or more of the septoparietal trabeculations. In the setting of tetralogy of Fallot, for example,5, 6 it is the pernicious combination of hypertrophy of these trabeculations, together with malattachment and rotation of the muscular outlet septum in anterocephalad direction, that leads to sub-pulmonary obstruction.
Most of these septoparietal trabeculations originate from the sides of a particularly prominent muscular band that reinforces the right side of the ventricular septum. This structure, which we call the septomarginal trabeculation, and others describe as the septal band, is a tree-like structure which arborises to give rise to many coarse right ventricular trabeculations. At the base of its body, extending to the right ventricular apex, the septomarginal trabeculation almost always gives rise to a discrete muscular band, which traverses the right ventricular cavity to its anterior free wall, and joins the base of the anterior papillary muscle. This is the moderator band discussed above (Fig. 4). Although it appears to have no functional significance in moderating the function of the tricuspid valve (its presumed initial role), histologic analysis shows that it contains embedded within it the distal extension of the right bundle branch. If the moderator band is large, and emerges from the body of the septomarginal trabeculation rather than its root, as can occur as a variation in the otherwise normally structured heart, it can itself be a cause right ventricular outflow tract obstruction. Some describe this variant as one form of “double chambered right ventricle”.
When traced towards the ventricular base, the septomarginal trabeculation has two discrete arms. One points superiorly towards the facing leaflets of the pulmonary valve, overlying the course of the first septal perforating artery. The other is directed inferiorly towards the inner curvature of the heart and the central fibrous body. The medial papillary muscle usually springs from this inferior limb. Since the conduction system runs from the central fibrous body towards the septal insertion of the medial papillary muscle, the inferior limb of the septomarginal trabeculation has special significance for the surgeon. Beyond this, the body of the septomarginal trabeculation, with its embedded right bundle branch is another potentially hazardous area. Since this portion of the conduction system is encased in a fibrous sheath, damage to the body of the septomarginal trabeculation can lead to disturbances of conduction if blood or oedema tracks retrogradely along the fibrous sheath towards the atrio-ventricular node.
The two arms of the septomarginal trabeculation embrace the musculature of the supraventricular crest. The normal crest itself is a composite structure produced, during development, from the correct alignment and union of the inner heart curvature, the proximal outflow cushions and crest of the ventricular septum. Concomitant with closure of the interventricular foramen, this process of union also walls the aorta into the left ventricle.3
Since the various components are indistinguishable from one another in the formed heart, we prefer to group them together under the umbrella of the supraventricular crest (Fig. 10).7 In tetralogy of Fallot, however, these building blocks remain discrete entities, and can be recognised as such.5, 6 We see no problem in naming the individual components in this setting. Indeed, this is preferable for the surgeon, since these structures are markedly different. Thus, in terms of muscular structures, we can differentiate a ventriculo-infundibular fold separating the leaflets of the atrio-ventricular valve and arterial valves, a discrete outlet septum separating the sub-arterial outflow tracts, the septomarginal trabeculation, and the crest of the ventricular septum (Fig. 2). Any interventricular communication, whether perimembranous or possessing a muscular inferior rim, is typically cradled between the two limbs of the septomarginal trabeculation. This feature is also typical for other surgical conditions involving reconstruction of the right ventricular outflow tract, such as double outlet right ventricle and the common arterial trunk.
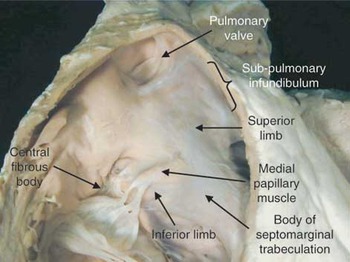
Figure 10. This is a close-up view of the internal features of the right ventricular outflow tract, emphasising the relationships of the septomarginal trabeculation. The body of this structure has two distinct limbs. The superior points to the facing leaflets of the pulmonary valve, and the inferior arm directed towards the inner curvature of the heart and central fibrous body. It is from this latter arm that the medial papillary muscle takes origin.
From the anatomical stance, therefore, it is clear that the right ventricular outflow tract is a complex structure, both in terms of its external relationships and from within. Both aspects of this freestanding mass have to be considered by the surgeon during dissection and subsequent reconstruction, providing a formidable set of morphologic challenges in each case.
Several congenital cardiac malformations require restoration of continuity between the right ventricle and the pulmonary arteries, or surgical relief of obstruction within the right ventricular outflow tract. Tetralogy of Fallot, and its variants, is the most common malformation to represent in adulthood after intra-cardiac repair during childhood. Most of the literature regarding the late fate of reconstructed right ventricular outflow tracts, therefore, concerns patients with tetralogy of Fallot. Other pathologies that require reconstruction as part of the initial corrective procedure are common arterial trunk, pulmonary atresia with and without ventricular septal defect, various forms of discordant ventriculo-arterial connections with obstruction to the left ventricular outflow tract, some forms of double outlet right ventricle, and congenital aortic valvar disease treated by means of the Ross procedure.
During recent years, the trend has been to repair such malformations in early infancy, rather than proceeding to complete correction after initial palliation. In particular, most authors now advocate correction of tetralogy of Fallot within the first 6–18 months of life. In contemporary series, 10-year survival has been reported for nine-tenths of the patients undergoing operation, with no difference in outcome between repairs performed as a single stage in infancy, and initial palliation followed by definitive repair.8 Proponents of early repair point out the benefits of early relief of obstruction and cyanosis, as well as the potential for growth and avoidance of late ventricular fibrosis and its consequences.9
Several centres have pushed this trend even further, proposing neonatal repair of tetralogy of Fallot with and without pulmonary atresia, stressing the advantage of avoiding extensive muscular resection, as well as the potential decrease in incidence of late ventricular dysfunction and arrhythmias.10 On the other hand, neonatal intervention may necessitate reconstruction of the right ventricular outflow tract with a valved conduit that will likely require early replacement, or relief of stenosis with a transjunctional patch. Although the transjunctional patch is most frequently described as “transannular”, as we have discussed above, it is the circular ventriculo-arterial junction that is the only true ring within the outflow tract. “Transjunctional”, therefore, is the more accurate anatomic term. When compared to infants and children undergoing intra-cardiac repair, transjunctional patching is more frequently used in neonatal correction of tetralogy, in the proportions of four-fifths as opposed to one-third of cases.11
With regard to both operative and late survival, results of correction of the anomalies listed above are excellent. This is true for both staged palliation followed by correction, and for complete repair in the neonatal period or early infancy.10, 12 When reviewing outcomes of single-stage repair in neonates, results in the intermediate term are encouraging, with survival of up to 93% at 1 and 5 years. The need for surgical reintervention in neonates, and on the right ventricular outflow tract in particular, nevertheless, is higher than in older children, with only two-thirds of the latter group being free from reintervention at 1 and 5 years in the reported experience of the University of Michigan.11 The risk for late reintervention on the right ventricular outflow tract appears to be higher when postoperative ratio between right and left systolic ventricular pressures is >0.75, the gradient across the right ventricular outflow tract exceeds 40 millimetres of mercury at discharge, or when the gradient across the left pulmonary artery is higher than 15 millimetres of mercury. The latter finding underscores the importance of extending patching into the left pulmonary artery, where insertion and ligation of the arterial duct may be responsible for angulation and late stenosis.12, 13
Important considerations in late outcome after complete correction are the extent of the ventriculotomy, and of the infundibular resection performed at the time of initial repair. In addition to failure of the conduit, right ventricular aneurysm (Fig. 11) and significant pulmonary regurgitation (Fig. 12) are common anatomical substrates for disturbances of ventricular rhythm, and underscore the need for late reintervention on the right ventricular outflow tract. They often result from extensive transjunctional patching, along with excessive ventricular resections widely used in the early experience with complete repair for right-sided obstructive lesions.14, 15 Particularly in patients with tetralogy, transatrial closure of the ventricular septal defect, avoidance of excessive resection within the right ventricular outflow tract, and use of a minimal right ventriculotomy should yield improved late systolic function, exercise endurance, and decreased incidence of late ventricular arrhythmias. Such a modified approach, with less aggressive resection and a limited ventriculotomy, has also resulted in lower rates of reoperation and comparable residual gradients after 12 years of follow-up.12, 16–18

Figure 11. Magnetic resonance phase contrast flow images through the pulmonary artery: (a) systole – forward flow shown as white; (b) disastole – reverse flow shown as black; (c) flow plotted against time for the aorta and pulmonary arteries demonstrating pulmonary incompetence, with a calculated regurgitant fraction of 30%.

Figure 12. Short axis cine magnetic resonance image, in a steady state free precession sequence, showing dilation of the right ventricular outflow tract, with (a) diastolic and (b) systolic frames.
Postoperative outcomes for other pathologies requiring reconstruction of the right ventricular outflow tract as part of the initial corrective procedure have been extensively reported in the literature,19–22 and expanding on such results is beyond the scope of our review.
In recent years, the rates of reintervention rates for patients with congenital cardiac disease have increased, reflecting the ageing of the population of children undergoing cardiac surgical procedures. Most of the data regarding late reintervention on the right ventricular outflow tract originate from longitudinal follow-up of patients undergoing correction of tetralogy of Fallot and its variants since the late 1950s. This represents a large proportion of the group of patients undergoing surgical reintervention for congenital cardiac disease, this group also being the one with highest propensity to become symptomatic later in life, after years of well-being. In a large series of patients undergoing surgical correction of tetralogy of Fallot, and followed at a mean of 18 years postoperatively,23 over nine-tenths were in normal sinus rhythm and functional class I or II of the New York Heart Association. Almost four-fifths had normal right ventricular function on echocardiographic follow-up, and only one-tenth had severe pulmonary valvar regurgitation. These patients will typically tolerate chronic pulmonary insufficiency up to the fourth decade of life. Severe pulmonary insufficiency is strongly associated with transjunctional patching, right ventricular dysfunction and enlargement, decreased exercise tolerance and forced vital capacity, higher functional class, and with the need for treatment of cardiac failure.13, 23–26
When late complications occur in patients after childhood repair of tetralogy of Fallot, they are related in three-quarters of cases to the right ventricular outflow tract, involving pulmonary insufficiency, aneurysm at the site of the patch, or stenosis at the level of the pulmonary valves or within a conduit. They are followed in frequency by residual ventricular septal defects, and severe tricuspid regurgitation. Pulmonary regurgitation is by far the most common reason for late surgical reintervention in tetralogy of Fallot.27 The overall rate of reintervention for such patients, in a report with mean follow-up of almost 26 years, was nevertheless only 8%, with the vast majority of patients conducting relatively normal lives.28 Similarly, in the experience of the Toronto team responsible for adults with congenital cardiac lesions, only one-sixth of 330 adult patients with tetralogy of Fallot followed from operation into adulthood required late reintervention.27
Stenosis within the right ventricular outflow tract (Fig. 13) can be tolerated for years, especially in the setting of a competent pulmonary valve. An elevated ratio between the peak systolic pressures in the right and left ventricles immediately following repair, nevertheless, remains an early predictor of reintervention.29 The mean interval between operative correction to reoperation for complications involving the right ventricular outflow tract in patients with tetralogy of Fallot is around 20 years.27

Figure 13. Angiographic image of the right ventricular outflow tract.
As the timing of surgical correction shifts toward early infancy and the neonatal period, so does the need for early reintervention on the connection between the right ventricle and pulmonary arteries. This is an inexorable consequence of the deterioration of the conduit or the somatic growth of the child. In patients with tetralogy of Fallot and pulmonary atresia rather than stenosis, the rates of late reintervention are much higher than for those with stenosis. Actuarial freedom from reoperation for those with atresia has been reported as 55% and 29% at 10 and 20 years postoperatively.19 Such rates of reintervention are particularly high for children younger than 3 years at the time of repair.20 For children with common arterial trunk, reintervention for replacement of the conduit in the intermediate term is even higher, and is reported as needed in more than two-fifths of patients after no more than 3 years.21, 22 The crucial question that cardiologists and surgeons face regarding such late reintervention on the right ventricular outflow tract revolves around the timing of intervention and the choice of conduit or prosthesis used to restore both optimal flow to the pulmonary vascular bed as well as valvar competence.
The timing of surgical intervention in the typical setting of stenosis of the right ventricular outflow tract or pulmonary regurgitation is critical, as the pathology must be addressed surgically before the intervention of irreversible problems with contractility and pump dysfunction. Untimely intervention on patients with severe right ventricular impairment may jeopardise their chance for ventricular recovery, and often leads to poor outcome. Conversely, premature reintervention commits the patient to an early reoperative procedure on the right ventricular outflow tract.
Whereas, in adults with corrected tetralogy of Fallot with either stenosis or atresia, pulmonary regurgitation and stenosis of the right ventricular outflow tract represent the majority of surgical indications, these are slightly different when neonates and children face early reintervention. The highest rates for reintervention in the short and intermediate term are observed in patients undergoing surgery in the neonatal period.4 Concomitant lesions typically seen in smaller children are, for example, a residual ventricular septal defect, seen in up to 95% of children, and stenosis of the left pulmonary artery, found in up to 22%. Patients with pathology of the right ventricular outflow tract may present earlier to surgical attention if such complications are present, along with tricuspid regurgitation. All abnormalities should be treated upon reintervention, in order to provide the best opportunity for functional recovery and remodelling of the right ventricle. The latter process is often limited, most likely as a consequence of already established structural myocardial deterioration. Avoidance of this irreversible damage is the cornerstone of early reintervention.29 Accepted indications for surgical reintervention are, first, sustained arrhythmias, second, exercise intolerance and progression of symptoms, third, evidence of progressive ventricular dysfunction and development of tricuspid regurgitation, and fourth, presence of a severe but asymptomatic pressure gradient across the right ventricular outflow tract.30, 31
Late sudden death of presumed cardiac cause is mainly thought to be secondary to malignant ventricular arrhythmias in the setting of ventricular dysfunction, and can be as high as 6%.14–16, 27 There is a strong association between ventricular dysfunction, stress on the right ventricular wall, and ventricular irritability. In unselected patients with tetralogy of Fallot who have been surgically corrected, it is possible to induce monomorphic ventricular tachycardia in more than one-quarter. Risk factors include increased right ventricular end-systolic and diastolic volumes, elevated right ventricular systolic pressure, a longer period of follow-up, and older age at operation.15, 32 Supraventricular tachycardia is comparatively less common.
In order to identify patients at risk of sudden or sustained disturbance of rhythm, several electrocardiographic parameters have been investigated. In a large series of 793 patients followed after intra-cardiac repair for tetralogy of Fallot, the duration of the QRS complex increased at an average rate of 2 ms each year. This increase correlated well with the degree of pulmonary regurgitation and ventricular dysfunction. A duration of the QRS complex in excess of 180 ms was predictive of sustained ventricular tachycardia, tri-fascicular block, and late sudden death.33 If concomitant pathology of the right ventricular outflow tract is also present, reintervention should therefore be strongly considered in those patients in whom the duration of the QRS complex approaches this value, or in those with an increase in duration greater than 3.5 ms each year. Patients at risk of late arrhythmic events should be aggressively screened with electrocardiography, 24-h Holter monitoring, radionuclide angiography and exercise testing.15 Up to one-third of patients requiring reoperation will manifest sustained episodes of ventricular tachycardia on preoperative Holter monitoring, and confirmation of inducibility of sustained monomorphic ventricular tachycardia should be considered preoperatively. Most frequently, the triggering focus is located in the muscular outlet septum adjacent to the patch used to repair the ventricular septal defect. Intra-operative mapping and ablation has been strongly recommended by several authors.27, 30
The presence of symptoms in the setting of an abnormal right ventricular outflow tract fulfils the criterions for surgical reintervention. Sub-clinical deterioration in functional reserve can often be detected only with exercise testing.34 The most reliable indicators of exercise reserve reported in the literature are maximal uptake of oxygen uptake and the ventricular anaerobic threshold. In one study, correction after initial palliation with a shunt brought the maximal uptake of oxygen from a mean of 18.2–36.4 mm/kg/min, averaging 85% of the predicted value for age. Though not always clinically evident, patients with tetralogy of Fallot who have undergone intra-cardiac repair have normal resting but lower maximal and sub-maximal heart rates when compared to healthy controls. The observed reduction in performance may, therefore, be attributed to sub-optimal increases in heart rate with exercise. Worse postoperative results are observed in patients corrected late,25 as work performance by objective testing appears to be reduced in case of concomitant cardiac enlargement, increased right ventricular systolic pressures, pulmonary regurgitation, residual ventricular septal defects, and disturbances of rhythm.
Pulmonary regurgitation usually indicates the need for reintervention in the setting of associated progressive symptoms, right ventricular dilation, tricuspid regurgitation, and arrhythmias.24 The reduction of ejection fraction seen late in patients after correction involving a transjunctional patch is not truly related to the degree of pulmonary insufficiency, but rather to the magnitude of right ventricular systolic and diastolic dysfunction.35 The most frequently associated procedure performed at the time of reintervention on the right ventricular outflow tract, needed in over two-fifths of patients, is repair of the tricuspid valve.30
In the patient with few symptoms but progressive deterioration of right ventricular function, decision on the timing of reintervention can be supported by three-dimensional echocardiography, or reconstruction of magnetic resonance images. Such advanced imaging techniques are, for practical purposes, problematic in smaller children, but possible in older patients who are evaluated late after initial intra-cardiac repair.36, 37 In the future, magnetic resonance imaging may prove to be the diagnostic tool of choice, allowing for accurate quantification of ventricular performance, as well as for detailed three-dimensional reconstruction of the pulmonary trunk, its right and left branches, and the distal pulmonary arterial anatomy.
In a large meta-analysis of patients with tetralogy of Fallot subsequent to surgical correction,15 most postoperative gradients exceeded 40 millimetres of mercury. This finding, coupled with the natural history that follows intra-cardiac repair, suggests that residual obstruction is well tolerated for a prolonged period of time, impacting on exercise performance only at a sub-clinical level. Exercise tolerance appears severely compromised when the ratio of systolic pressures between the right and left ventricles exceeds two-thirds, once the systolic pressure gradient is greater than 50 millimetres of mercury, or if there is coexisting stenosis in the right ventricular outflow tract or right and left pulmonary arteries. In the absence of symptoms, reintervention may also be indicated in those patients with haemodynamically significant lesions who require an optimal physical state in order to tolerate the haemodynamic perturbations and fluid shifts associated with pregnancy.
Reintervention on the right ventricular outflow tract can be accomplished with almost no perioperative mortality, minimal morbidity, and excellent late survival.20, 30 In the experience reported from Toronto, actuarial survival at 10 years following reintervention was 92%. Over nine-tenths of the patients in this series were in the first or second functional classes of the New York Heart Association postoperatively, as opposed to two-thirds prior to reintervention.27 These functional findings are supported by several other reports.38, 39 There is scarce consensus in the literature about echocardiographic evidence of right ventricular remodelling and improvement. Pulmonary valvar replacement in adults may be followed by less ventricular regression than that typically observed within one year after correction of aortic lesions.40 In the experience reported from the Mayo Clinic, a marked reduction was observed in the proportion of patients with moderate-to-severe right ventricular depression at a mean of 9 years postoperatively, specifically from three-fifths to one-fifth.30 Others, however, observed no change in right ventricular ejection fraction, stroke volume and end-diastolic volume in 25 consecutive adults undergoing radionuclide ventricular angiography at a mean of 28 months after reintervention. Their postoperative exercise performance, nevertheless, was increased,41 although amelioration in maximal uptake of oxygen and ventricular anaerobic threshold values showed poor correlation with the observed postoperative ventricular function.
Post-operative sinus rhythm is expected in up to nine-tenths of patients undergoing late reconstruction of the right ventricular outflow tract with a competent valve. These patients appear to maintain the duration of their QRS complexes when compared to an appropriately matched group of patients with tetralogy of Fallot who have not undergone reintervention. Even though the duration of the QRS complex is not reduced by replacement of the pulmonary valve, the prolongation seen over time in the matched cohort appears to be stopped in the group undergoing surgery.42 Ablation strategies, including right-sided Maze procedure, cryoablation, division of accessory conduction pathways, and right atrioplasty do not have yet a clear role. Up to three-quarters of instances of preoperative supraventricular tachycardia, and four-fifths of ventricular arrhythmias, nevertheless, can be cured by the combination of reintervention and ablation.27, 30, 42 Actuarial freedom from re-replacement of the pulmonary valve is as high as 93 and 70% at 5 and 10 years, with the need for a second reintervention correlating with younger age at the time of initial replacement.30
No “ideal conduit” or “gold standard” currently exists. The “ideal conduit” should retain its function over time, not degenerate, have good handling characteristics, be freely available and inexpensive, and grow with the patient. Several options are available when the surgeon is facing the challenge of restoring the connection between right ventricle and pulmonary arteries. The choice depends on the size and age of the patient, on the anatomy in terms of whether the connection is present, or there is atresia with or without discontinuous pulmonary arteries, on the type of procedure in terms of primary or reoperative reconstruction, and on the personal preference and experience of the surgeon. These surgical options have now also to be compared with catheter-based treatments.
When there is already continuity between right ventricle and the pulmonary arteries, and the right ventricular outflow tract is simply stenotic at the sub-valvar, valvar, or supravalvar level, the effective diameter of the outflow tract can be augmented by a longitudinal incision augmented with a patch (Fig. 14). Several materials have been utilised for such patching, including autologous tissue such as pericardium, bovine pericardium, and expanded polytetrafluoroethylene (Gore-Tex®; Flagstaff, AZ, USA). The typical anatomic substrate for performance of a transjunctional patch is tetralogy of Fallot with pulmonary stenosis. The incision can be carried proximally or distally to the valve, depending on the anatomy of the valve, which is often bicuspid, and on the degree of stenosis and ventricular hypertrophy. Excessive length of the patch has been associated with higher rates of reintervention.31 Extensive patching is also a predictor of early restrictive diastolic physiology, defined as forward diastolic flow in the pulmonary arteries. Although such early restriction is also predictive of late restriction, patients with restrictive physiology may be somewhat protected from late ventricular dilation.43, 44 Most typically, patients will not have restriction, but rather manifest late pulmonary insufficiency with variable severity and clinical impact. At late presentation, at a mean of 18 years follow-up in one report, pulmonary regurgitation was absent or mild in up to three-fifths of patients after initial intra-cardiac repair for tetralogy of Fallot.23 To avoid the consequences of severe chronic pulmonary regurgitation, and the late problems associated with ventricular scarring, caution should be exercised when extending the incision across the ventriculo-arterial junction into the ventricle, keeping the extent of the ventriculotomy to the strictly necessary length.16

Figure 14. The transjunctional patch inserted to relieve obstruction of the right ventricular outflow tract in tetralogy of Fallot, as viewed intra-operatively from the right side of the patient. Reproduced with permission from: Kouchoukos NT, Blackstone EH, Doty DB, Hanley FL, Karp RB (eds). Ventricular septal defect with pulmonary stenosis or atresia. Kirklin/Barratt-Boyes: Cardiac Surgery, 3rd edn. Elsevier Science, Philadelphia, USA, 2003, 976.
An alternative technique to transjunctional patching is direct anastomosis of the pulmonary arterial confluence to the right ventricle, with anterior augmentation of the anastomotic site using a patch. This solution, when anatomically feasible, has the advantage of utilising autologous tissue, the potential for growth, and possibly for a lower incidence of reintervention for obstruction than is seen following reconstruction with aortic or pulmonary homografts.45 Another reported variation in technique is the use of the left atrial appendage to augment the outflow tract posteriorly, followed by anterior augmentation with a transjunctional patch.46
Patch arterioplasty in neonates should be extended well beyond the pulmonary bifurcation into the left pulmonary artery, as stenosis of the left pulmonary artery at the site of insertion of the arterial duct is a frequent cause of late reintervention in neonates, in whom a transjunctional patch is also more likely to be utilised.12
Since relief of right ventricular outflow tract obstruction by transjunctional patching results in immediate pulmonary regurgitation, several authors have advocated the insertion of monocusp valves in neonates and infants (Fig. 15). The reaction of the pulmonary vasculature typically observed in this age group may be blunted in its detrimental effect on acute right ventricular pressure and volume overload by insertion of a competent valve. Though the comparative overall morbidity and mortality are similar between patients with monocusp valves and those with unvalved transjunctional patches, it appears that the postoperative course and stay in the intensive care unit might be smoother and shorter in duration for those having a valve. In the intermediate term, the development of pulmonary regurgitation, with no stenosis, compares favourably with the outcome of unvalved reconstructions.47–52 Polytetrafluoroethylene is preferred to autologous tissue by several authors because of the decreased incidence of earlier degeneration. A monocusp valve can also be fashioned from an aortic or pulmonary homograft. If an aortic homograft is used, the retained aortic leaflet of the mitral valve can then be used to reconstruct the proximal aspect of the take-off of the conduit from the right ventricle.

Figure 15. Insertion of a monocusp at the time of reconstruction of the right ventricular outflow tract. The heart is schematically drawn in an echocardiographic para-sternal long-axis plane. The monocusp valve is seen in the closed (left) and open (right) position against the interventricular septum. Reproduced with permission from: Turrentine et al. Polytetrafluoroethylene monocusp valve technique for right ventricular outflow tract reconstruction. Elsevier Inc., New York, USA. Ann Thorac Surg 2002; 74: 2203.
Limited reports have described the use of pedicled or non-pedicled autologous pericardium to re-establish competent continuity between the right ventricle and the pulmonary arteries.52 At a mean follow-up of 50 months, nevertheless, enlargement of the conduit was observed in over half the patients in one series.53
When continuity needs to be reestablished in such setting as common arterial trunk or teralogy with pulmonary atresia, and similarly in the Ross procedure, many surgeons currently regard pulmonary homografts as the “gold standard”, albeit one that is sub-standard. Homografts (Fig. 16) represent a very versatile solution to correction of pathology within the right ventricular outflow tract, and can be implanted as a free-standing pulmonary root or, alternatively, in the junctional or sub-junctional positions. Limitations of allograft use include availability, smaller sizes in particular, and expense. Late structural deterioration, typically presenting as late calcific stenosis of the valve or the conduit, still remains a major concern. Overall freedom from reintervention because of structural deterioration of aortic and pulmonary homografts in children can be projected at 84, 58 and 34% at 5, 10 and 15 years after implantation.54

Figure 16. A pulmonary arterial homograft is shown revealing (a) bifurcation of the pulmonary arteries and (b) en face.
The incidence of calcific stenosis is typically reduced for pulmonary allografts, which seem to outperform aortic homografts when followed over the longer term.55–61 Nevertheless, in the experience reported from Toronto, involving 905 allografts, the use of aortic rather than pulmonary homografts, and size, were found to be the strongest predictors of late deterioration only for those patients undergoing complete repair between birth and 18 months of age. Following this period, size remained as the main predictor of early deterioration over the type of conduit used.62 In particular, smaller homografts, and neonatal reconstruction, are associated with early structural failure. Several reports have described accelerated calcification of aortic homografts, in addition to somatic outgrowth, as causes of structural failure in patients corrected as neonates.11 In smaller children, it may therefore be advisable to oversize the pulmonary homograft.63 When a small but oversized homograft is not available, “bicuspidalisation” of a larger graft can be carried out, augmenting the available homograft pool available to small infants and children.64, 65 Freedom from events related to the conduit is higher in older patients, with freedom from reintervention of up to 91 and 87% at 5 and 8 years, respectively.60 Even longer freedom from structural deterioration of the conduit can be expected in older patients undergoing the Ross procedure.66
Other risk factors for late stenosis of the homograft include younger donor age and shorter duration of cryopreservation.53, 66 These are, in turn, likely to be related to increased retention of cellular viability and the subsequent induced immune response in the recipient.67 In spite of these considerations, mismatch of blood type, and mode of preservation, have not consistently shown to have any bearing on late homograft failure.68 A recent innovation in the process of cryopreservation known as SynerGraft® (Cryolife Inc., Kennesaw, GA, USA) technology, in essence de-cellularisation, has the potential to minimise rejection and calcification, improving long-term durability.69
Xenografts have been used in the right-sided circulation either as bioprosthetic valves incorporated within a conduit (TissueMed®; TissueMed Ltd, Leeds, UK and Hancock®; Medtronic Inc., Minneapolis, MN, USA), or inserted directly into the right ventricular outflow tract, typically at the time of reintervention.
The relatively high failure rate for homografts inserted in infants70 has indeed prompted evaluation of xenograft-valved conduits in infants and neonates as a viable alternative. Several centres favour, when possible, the utilisation of a Dacron-xenograft-valved conduit (Fig. 17) because of the observed long-term durability in comparison to homografts.20, 30, 71–73 Nevertheless, no consensus emerges from a review of the literature comparing insertion of xenografts as oppsed to allografts in children. A recent retrospective review of 1,095 patients undergoing placement of conduits from the right ventricle to the pulmonary arteries at the Mayo Clinic reported superior long-term freedom from reintervention for xenograft valved conduits.74 In centres using xenografts as their conduit of choice, the use of homografts is mostly confined to the Ross procedure, and to the restoration of continuity in the right ventricular outflow tract in neonates and small children. For those falling within the latter ages, the group in Paris has recently proposed the use of a composite stentless porcine-valved conduit of bovine pericardium, with very encouraging results in the intermediate term.75 Xenograft-valved conduits will typically degenerate because of insufficiency or stenosis from calcific valvar degeneration, and/or neo-intimal deposit of peel within the conduit. In comparison to xenografts, several investigators have observed better freedom from reintervention with homografts, albeit without obvious advantages in children with allograft having diameters of <15 mm.46, 76–78

Figure 17. A composite xenograft-valved conduit (Hancock®, Medtronic). The stented porcine bioprosthesis is visible within the short Dacron conduit.
While experimental replacement of the pulmonary root with Freestyle® (Medtronic Inc., Minneapolis, MN, USA) stentless aortic xenografts has been met with disappointment due to early calcification,79 early clinical experience with the Toronto stentless porcine valve (SPV®; St. Jude Medical, St. Paul, MN, USA) has shown promising results.80
Bovine valved jugular venous xenografts fixed with glutaraldehyde or genipin (Contegra®; Medtronic Inc., Minneapolis, MN, USA) have been recently introduced as an alternative in small children to the use of homografts and xenografts. These are available in sizes between 8 and 22 mm, and have disclosed minimal rates of stenosis and need for reintervention because of problems involving the conduit in preliminary short-term follow-up studies.81–84
In spite of all arguments, most conduits, whether xenografts or homografts, used to reestablish the connection between the right ventricle and the pulmonary arteries in neonates and small children are likely to require replacement in the short or intermediate term.85
Given the inherent thrombogenicity of mechanical prostheses in the low pressure right-sided circulation, mechanical valves are regarded by many as a sub-optimal solution when reintervention is required to restore pulmonary valvar competence. Although anticoagulation is for obvious reasons problematic in children and young adults, there have been several recent reports regarding use of mechanical valves for late reconstruction of the right ventricular outflow tract in patients with severe pulmonary regurgitation or high pulmonary resistance. In contrast to bi-leaflet valves, these reports indicated the virtual absence of thrombosis in prostheses with tilting discs, as well as the advantage of avoiding further reintervention because of late structural valvar deterioration.86, 87
Earlier reports of percutaneous dilation and stenting for recurrent pathology involving the right ventricular outflow tract have yielded discouraging results, providing reasons to avoid such therapeutic approaches to correct late stenosis.88 Catheter-based technology, nevertheless, is now finding fertile ground for clinical application in late regurgitation or stenosis of the pulmonary valve. Bovine jugular venous valves have recently been used, mounted within a Nitinol stent, and deployed percutaneously over an 18 French delivery system, to dilate and stent conduits and simultaneously to restore pulmonary valvar competence (Fig. 18). This approach, for now limited to small series of patients,89–91 awaits future validation by randomised trials and longer term follow-up.

Figure 18. Percutaneous insertion of a stentless bovine jugular venous valve mounted within a Nickel–Titanium (Nitinol) expandable stent. Severe pulmonary regurgitation (left) is absent following deployment of the valve (right). Photograph courtesy of Prof. P. Bonhoeffer, Great Ormond Street Hospital for Children NHS Trust, London, UK.
The timing of primary intervention for pathologies involving the right ventricular outflow tract has shifted. Early operative intervention on tetralogy of Fallot, and on congenital heart disease in general, is pushing clinicians to consider complete reconstruction of the right ventricular outflow tract at the time of the initial operation. This, in turn, results in higher rates of reintervention over the intermediate term in neonates and infants, but possibly in improved long-term ventricular function.
Late reintervention after transjunctional patching, and extensive right ventriculotomies for pulmonary insufficiency, are increasing. A more sensible transatrial approach, with limited patching onto the right ventricle, may in the future result in a lower incidence of significant pulmonary regurgitation, as well as descreased right ventricular dysfunction, dilation, and arrhythmias.
Though several new conduits, and techniques for their insertion, have been introduced in recent years, no perfect substitute currently exists to restore the connection between right ventricle and pulmonary arteries. At present, timely intervention and preservation of ventricular function must always be weighed against the need for future reoperation caused by failure of the conduit.
Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from Research and Development funding received from the NHS Executive.
Andrew Cook and Mazyar Kanani are supported by the British Heart Foundation.

A schematic illustration of the arrangement and principal features of the right side of the normal heart. It also demonstrates the alternative terms for the supraventricular crest, infundibulum, septomarginal trabeculation, and the moderator band.

This illustrates the arrangement of the right heart in a case of tetralogy of Fallot, including the narrowed right ventricular outflow tract and the muscular outlet septum, which is deviated in the anterocephalad direction. It is this combination, together with hypertrophy of the septoparietal trabeculations, that accounts for narrowed outflow tract.

This close view of the right ventricular outflow tract, with the epicardium stripped away, demonstrates the circumferential nature of the myocardial fibres at the sub-pulmonary infundibulum.

This is a photograph of the normal heart, analogous to Figure 1, demonstrating the main features of the right ventricular outflow. Note the connection of the base of the anterior papillary muscle to the septomarginal trabeculation by way of the so-called moderator band.

This view from above demonstrates the tissue plane between the two arterial valves. Note their orientation relative to one another. LCS: left coronary sinus; RCS: right coronary sinus.

This shows the arterial supply to the sub-pulmonary infundibulum via branches of the right coronary artery that cross the atrio-ventricular groove.

This view of the normal heart emphasises the clear fibro-fatty tissue plane between the sub-pulmonary infundibulum and the root of the aorta. It also shows the muscular sleeve that is the freestanding sub-pulmonary infundibulum. Note the close proximity of the right coronary artery and its branches to the outflow tract.

This view of the normal heart shows the close relationship of the left coronary artery to the right ventricular outflow tract, and in particular the position of the first septal perforating artery which takes an oblique course deep to the sub-pulmonary infundibulum.

The morphologist’s view of the pulmonary valve, which has been opened out, and laid flat. The anatomic myocardial–arterial junction is marked by the red stars. As a consequence of the coronal attachment of the pulmonary leaflets, they straddle the myocardial–arterial junction, so that the tips of the three interleaflet triangles (green triangles) are arterial, and not muscular as might be expected.

This is a close-up view of the internal features of the right ventricular outflow tract, emphasising the relationships of the septomarginal trabeculation. The body of this structure has two distinct limbs. The superior points to the facing leaflets of the pulmonary valve, and the inferior arm directed towards the inner curvature of the heart and central fibrous body. It is from this latter arm that the medial papillary muscle takes origin.

Magnetic resonance phase contrast flow images through the pulmonary artery: (a) systole – forward flow shown as white; (b) disastole – reverse flow shown as black; (c) flow plotted against time for the aorta and pulmonary arteries demonstrating pulmonary incompetence, with a calculated regurgitant fraction of 30%.

Short axis cine magnetic resonance image, in a steady state free precession sequence, showing dilation of the right ventricular outflow tract, with (a) diastolic and (b) systolic frames.

Angiographic image of the right ventricular outflow tract.

The transjunctional patch inserted to relieve obstruction of the right ventricular outflow tract in tetralogy of Fallot, as viewed intra-operatively from the right side of the patient. Reproduced with permission from: Kouchoukos NT, Blackstone EH, Doty DB, Hanley FL, Karp RB (eds). Ventricular septal defect with pulmonary stenosis or atresia. Kirklin/Barratt-Boyes: Cardiac Surgery, 3rd edn. Elsevier Science, Philadelphia, USA, 2003, 976.

Insertion of a monocusp at the time of reconstruction of the right ventricular outflow tract. The heart is schematically drawn in an echocardiographic para-sternal long-axis plane. The monocusp valve is seen in the closed (left) and open (right) position against the interventricular septum. Reproduced with permission from: Turrentine et al. Polytetrafluoroethylene monocusp valve technique for right ventricular outflow tract reconstruction. Elsevier Inc., New York, USA. Ann Thorac Surg 2002; 74: 2203.

A pulmonary arterial homograft is shown revealing (a) bifurcation of the pulmonary arteries and (b) en face.

A composite xenograft-valved conduit (Hancock®, Medtronic). The stented porcine bioprosthesis is visible within the short Dacron conduit.

Percutaneous insertion of a stentless bovine jugular venous valve mounted within a Nickel–Titanium (Nitinol) expandable stent. Severe pulmonary regurgitation (left) is absent following deployment of the valve (right). Photograph courtesy of Prof. P. Bonhoeffer, Great Ormond Street Hospital for Children NHS Trust, London, UK.