Introduction
Nitrogen is the most essential nutrient required by plants in large quantities. Its deficiency is the major problem of crop production in many tropical and subtropical areasReference Ali, Krishnamurthy, Saxena, Rupela, Kumar and Johansen1, Reference Beebe, Rojas-Pierce, Yan, Blair, Pedraza, Munóz, Tohme and Lynch2, particularly in East AfricaReference Sanchez3 including EthiopiaReference Mamo, Haque, Kamara, Jutzi, Haque, McIntire and Stares4, Reference Tsegaye5. Resource-poor farmers have striven to overcome the nitrogen deficiency problem since antiquity by integration of legumes in crop rotation systemsReference Hirsch6, and by manuring and fallowing. Different studies in many parts of the worldReference Adjei-Nsiah, Kuyper, Leeuwis, Abekoe, Cobbinah, Sakyi-Dawson and Giller7–Reference Yusuf, Iwuafor, Abaidoo, Olufajo and Sanginga11 including EthiopiaReference Gorfu12 have shown the importance of symbiotic nitrogen fixation not only to the legumes themselves but also to the cereals grown in association (as a mixed cropping) or rotation with them.
The amount of nitrogen fixed annually with symbiotic association is far more than the amount industrially producedReference Rengel13, Reference Jensen and Hauggaard-Nielsen14. Some estimates show that hundreds of millions of metric tons of nitrogen are symbiotically fixed each yearReference Jensen and Hauggaard-Nielsen14, Reference Hardarson and Atkins15. At the farm level, nitrogen fixation with annual legumes may contribute 20–120 kg of nitrogen ha−1 in a season in the temperate regionReference Pearson, Norman and Dixon16 and 15–120 kg ha−1 in tropical AfricaReference Dakora and Keya17.
Among the food legume crops, chickpea (Cicer arietinum L.) is a crop of manifold economic and ecological merit at the global level. Evidence indicates that chickpea fixes atmospheric nitrogen to the tune of 100 kg ha−1Reference Crouch, Buhariwalla, Blair, Mace, Jayashree, Serraj and Serraj18, Reference Shiferaw, Bantilan, Serraj and Serraj19 and contributes over 500,000 million tons of nitrogen every year to developing countriesReference Hardarson and Serraj20. Ethiopia, being the first producer of chickpea in Africa in terms of area coverage, gets a significant amount of nitrogen fixation from the cropReference Kassie, Shiferaw, Asfaw, Abate, Muricho, Ferede, Eshete and Assefa21. As in other legume crops, however, chickpea's effectiveness and efficiency to fully realize its potential depends on the type of genotype, compatibility of the micro-symbiont and several environmental factorsReference Shukla and Yadav22–Reference Lindemann and Glover24.
In most cases, farmers in Ethiopia do not use chemical fertilizers in chickpea productionReference Agegnehu, Fikre, Tadesse, Ali, Keneni, Ahmed, Malhotra, Beniwal, Makkouk and Halila25. With the increasing price of nitrogen fertilizer and with growing environmental concern, selection of nutrient-efficient host genotypes with effective endosymbionts is a good alternative to overcome the problem of nitrogen deficiency. Efforts made to enhance nitrogen fixation through selection of better strains of rhizobacteria in Ethiopia have resulted in identification of many effective and competitive strainsReference Hailemariam, Tsige, Ali, Keneni, Ahmed, Malhotra, Beniwal, Makkouk and Halila26. Nevertheless, development of better strains alone may not provide the required gains in productivity without compatible host genotypesReference Rengel13, Reference Crouch, Buhariwalla, Blair, Mace, Jayashree, Serraj and Serraj18, Reference Chen, Zhang, Terefework, Kaijalainen, Li and Lindström27, Reference Giongo, Luciane, João, Freire and de Sá28. Some reports indicate that host plants play rather a dominant role as compared to bacterial strain in enhancing symbiotic nitrogen fixationReference Hardarson and Serraj20, Reference Shantharam and Mattoo29. Even though genotypic variation for attributes of host symbiotic and agronomic significance have been reported in many legume crops including chickpeaReference Ali, Krishnamurthy, Saxena, Rupela, Kumar and Johansen1, Reference Krasilnikoff, Gahoonia and Nielsen30, Reference Walley, Kyei-Boahen, Hnatowich and Stevenson31, for a variety of reasons, the role of host plants attracted little attention in Ethiopia and elsewhereReference Hardarson and Serraj20, Reference Bejiga and Serraj32.
Ethiopia has a large collection of chickpea germplasm but, apart from some observations in specific trials by microbiologists, mostly with a few improved varieties, no systematic assessment was made to exploit these gene pools especially for improving host symbiotic nitrogen fixation combined with agronomic performance. This study was, therefore, conducted to characterize and evaluate Ethiopian chickpea germplasm accessions and some varieties, and to identify desirable genotypes for symbiotic and agronomic performance.
Materials and Methods
Plant materials
In this study, a total of 155 chickpea genotypes were evaluated. They include 139 accessions collected from different regions of Ethiopia kindly provided by the Ethiopian Institute of Biodiversity Conservation (IBC), five improved genotypes from the International Crop Research Institute for the Semi-Arid Tropics (ICRISAT), eight originally introduced commercial cultivars released in Ethiopia and three genetically non-nodulating reference checks received from ICRISAT and the International Center for Agricultural Research in the Dry Areas (ICARDA). These chickpeas will be called ‘genotypes’ hereafter for experimental purpose. The passport description of the genotypes and the map of the areas of collection for the Ethiopian accessions are presented as online supplementary materials in Annexes A and B (at http://journals.cambridge.org/). All genotypes were rejuvenated during 2008/2009 under the same conditions at Ginchi to reasonably minimize initial variation due to differences in seed age and indigenous seed nitrogen contentsReference Liao, Hocking, Dong, Delhaize, Richardson and Ryan33.
The test environment
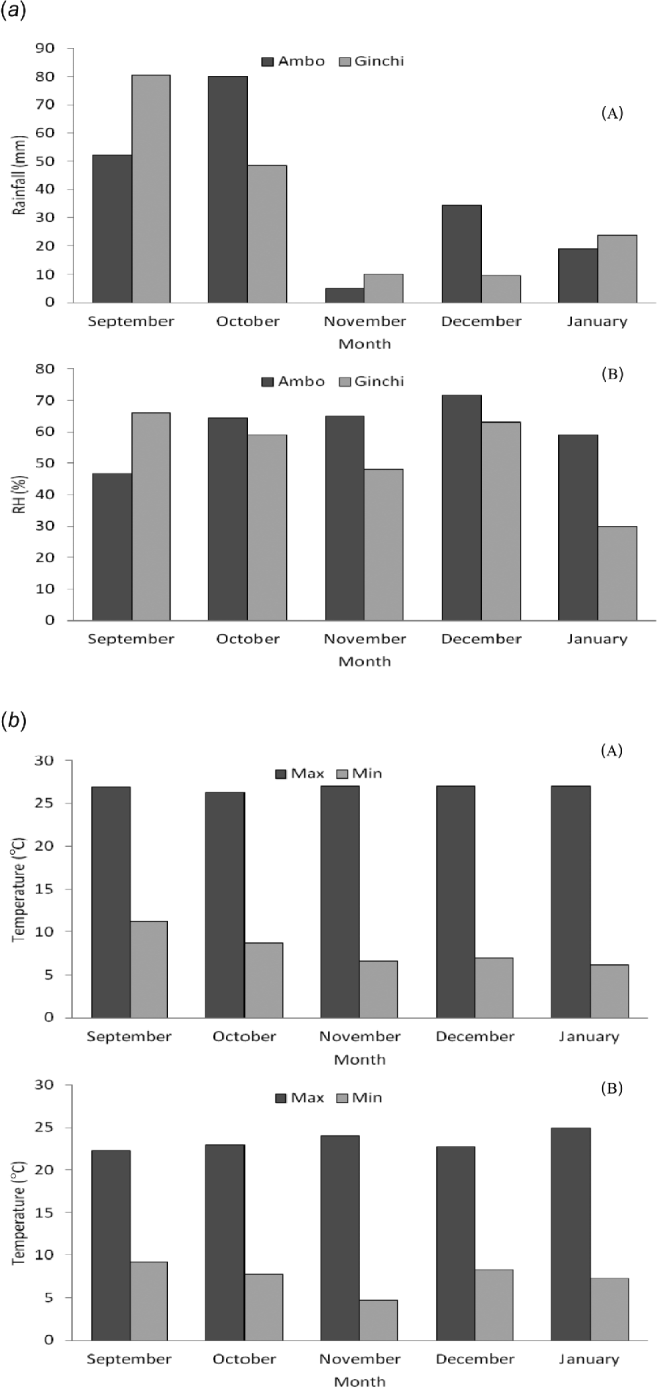
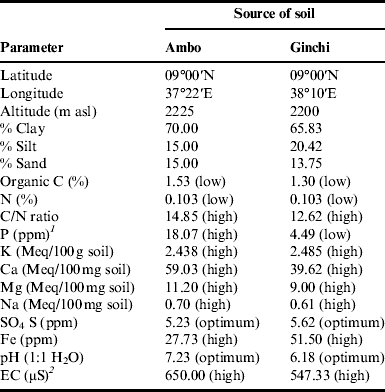
The experiment was conducted under field conditions at two locations (Ginchi and Ambo) in the central part of Ethiopia for 1 year during the main cropping season of 2009/2010 (September–January). The two locations are assumed to represent the major chickpea production areas of Ethiopia. Climatic data of the two locations during the growing period were taken from Ambo and Holetta Research Centers (Fig. 1a and b). Soil samples from both locations were collected from the rhizosphere (top 20 cm) for physico-chemical characterization (Table 1).

Figure 1. (a) Rainfall (mm) (A) and relative humidity (%) (B) at Ambo and Ginchi during the growing season. (b) Maximum and minimum temperatures (°C) at (A) Ambo and (B) Ginchi during the growing season.
Table 1. Description of the test locations for geographical position and physico-chemical properties of the soils.

1ppm, parts per million; 2μS, micro Siemens. EC, electrical conductivity.
Rhizobium inoculant and inoculation
An effective isolate of Rhizobium for chickpea, CP EAL 004, originally isolated by the National Soil Laboratory from a collection of Ada'a District of East Shewa Zone, Ethiopia, was used for the study. The isolate was found to be efficient in nodulation and symbiotic nitrogen fixation in previous studiesReference Hailemariam, Tsige, Ali, Keneni, Ahmed, Malhotra, Beniwal, Makkouk and Halila26. The inoculum was received at the concentration of approximately 109 cells g−1 of peat carrier. The concentration and purity of the inoculum was confirmed in the Soil Microbiology Laboratory at Holetta Research Center immediately before planting. Seeds of all genotypes were coated with the inoculant at the rate of approximately 2 g of inoculum for 80 seeds using 40% gum Arabic as an adhesive.
Experimental design and layout
A randomized complete block design with four replications was used. A blanket basal application of phosphorus was made to all plots in the form of superphosphate (TSP) at the recommended rate (20 g for a single row of 4 m). Seed rate was 5 cm between plants and 40 cm between adjacent rows. The plot size was 1 row 4 m long. The genotypes were assigned to plots at random within each block. Nitrogenous fertilizer was totally omitted and all other crop management and protection practices were applied uniformly to all genotypes as required.
Shoot and grain nitrogen analysis
After 45 days of growth (shortly before flowering), five plants from each plot were carefully dug up and their roots washed free from soils with water running over a sieve for collection of nodules. Representative shoot and grain samples collected at 90% physiological maturity from each plot were oven-dried to constant moisture at 70 °C for 18 h. The dry samples were ground to pass through a 1 mm mesh sieve to determine nitrogen using the Kjeldahl technique34 at Holetta and Debre Zeit Soil Science Research Laboratories. The amount of total nitrogen accumulated from fixation in shoots and grains of the test genotypes was estimated by the difference method using a non-nodulating reference genotype PM 233. The N-difference method using a non-nodulating line as a reference plant was found to be both reliable and economicalReference Pimratch, Jogloy, Vorasoot, Toomsan, Patanothai and Holbrook35. Protein contents of shoot and grain were determined by multiplying nitrogen percentages by the standard conversion factor of 6.2534. Based on the nitrogen contents, the following parameters were calculated:
where Nsfg is the amount of nitrogen in the shoot of the fixing genotype and Nsnfg is the amount of nitrogen in the shoot of the non-fixing genotype.
where Ngfg is the amount of nitrogen in the grain of the fixing genotype and Ngnfg is the amount of nitrogen in the grain of the non-fixing genotype.
 $${\eqalign{&{\rm N}\,{\rm fixed}\, {\rm in}\,{\rm biomass =} \cr&\quad\displaystyle{{{\rm (N}\,{\rm fixed}\,{\rm in}\,{\rm shoot + N}\,{\rm fixed}\,{\rm in}\, {\rm grain) \times 100}} \over {{\rm Nsfg + Ngfg}}}}$$
$${\eqalign{&{\rm N}\,{\rm fixed}\, {\rm in}\,{\rm biomass =} \cr&\quad\displaystyle{{{\rm (N}\,{\rm fixed}\,{\rm in}\,{\rm shoot + N}\,{\rm fixed}\,{\rm in}\, {\rm grain) \times 100}} \over {{\rm Nsfg + Ngfg}}}}$$Grain N yield=Grain N content×grain yield
Shoot N yield=Shoot N content×shoot yield
Biomass N yield=Grain N yield+shoot N yield
Nitrogen harvest index (NHI) was estimated as
Data collection of symbiotic and agronomic characters
Data were collected either on a plot basis or from randomly selected five plants36.
Records of symbiotic characters were taken as follows: (1) number of nodules, (2) nodule dry weight, (3) nodulation index (nodule weight to shoot weight ratio), (4) shoot nitrogen and protein contents, (5) shoot nitrogen fixation, (6) grain nitrogen and protein contents, (7) grain nitrogen fixation, (8) above-ground biomass nitrogen content, (9) above-ground biomass nitrogen fixation, (10) fixed nitrogen assimilation efficiency, (11) shoot, grain and above-ground biomass nitrogen yields, and (12) NHI.
Agronomic characters recorded include: (1) early vigor (as shoot dry weight before flowering), (2) shoot dry weight at maturity, (3) shoot dry weight ratios (of nodulating to non-nodulating genotypes) before flowering and at maturity, (4) days to 50% flowering and 90% maturity, (5) grain filling period (the number of days from 50% flowering to 90% physiological maturity), (6) number of pods and seeds, (7) total above-ground biomass weight, (8) harvest index (proportion of grain to total above-ground biomass), (9) grain production efficiency (grain filling duration divided by duration of vegetative period and then multiplied by grain yield), (10) above-ground biomass production rate (above-ground biomass weight divided by days to 90% physiological maturity and then multiplied by 100), (11) economic growth rate (grain weight divided by grain fill duration and then multiplied by 100), (12) thousand seed weight and (13) grain yield.
Statistical analysis
Data based on nodules (number, weight and nodulation index) were log transformed to offset heterogeneityReference Little and Hills37, Reference Gomez and Gomez38 for statistical analysis as suggested by DoughtonReference Doughton, Saffigna, Vallis and Mayer39. Pooled analysis of variance was conducted to quantify the total variation among the genotypes using the following model of analysis of variance:
where P ijk is the phenotypic observation on genotype j in block i (at location k) (i=1, …, B, j = 1, …,G, and k= 1, …, L) and G, L and B are the number of genotypes, location and block, respectively, μ is the grand mean, (b/l)ik is the effect of block i (within location k), g j is the effect of genotype j, l k is the effect of location k, (gl)jk is the interaction effect between genotype j and location k and e ijk is the residual or effects of random error.
Existence of significant differences among the genotypes, locations and genotype×location interaction effects were determined using the F-test. Mean separation was done using Duncan's multiple range test (DMRT) at 5% probability levelsReference Gomez and Gomez38.
Results and Discussion
Description of the test locations
The two locations received more or less similar amounts of rainfall with different patterns of distribution, but Ambo was more humid than Ginchi (Fig. 1a). It was witnessed that the weather variables recorded did not deviate much from the long-term trends at both locations40, indicating that the present findings will be reproducible over seasons.
The physico-chemical properties of the soils from the two test locations, Ambo and Ginchi, showed equally low levels of nitrogen contents (0.103%) but high levels of K, Ca, Mg, Na and FeReference Jones41 with variable amounts. The levels of exchangeable cations were also high with pH values close to neutral. The level of soil phosphorus was high at Ambo and low at Ginchi (Table 1). Similar results were reported from previous analysis of soils at the same locationsReference Dibabe, Bekele, Mamo, Dubale, Dibabe, Zeleke, Ayele and Kirub42.
Symbiotic performances of the genotypes
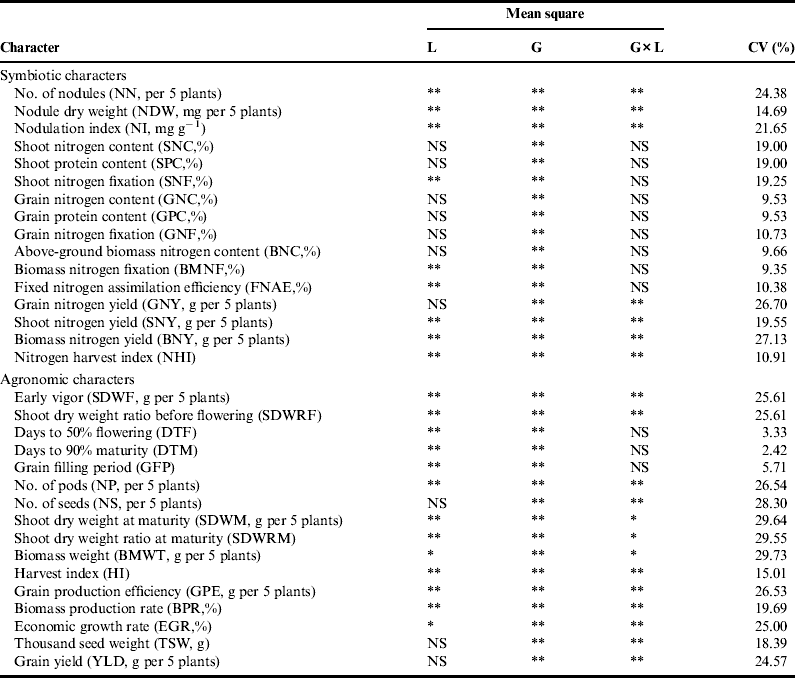
Differences among the genotypes were significant for symbiotic nitrogen fixation and a number of associated characters (Table 2 and see supplementary material Annex C). The larger coefficients of variation (CV) values (>20%) in many traits may be related to the production on residual moisture, where the crop was highly stressed, or a sample-based estimation of mean performances from only a few plants grown on small plots or both. Depending on host genotype, the amount of fixed nitrogen varied from 13 to 49% (![]() $\bar X$ = 30%) in foliage, 30 to 44% (
$\bar X$ = 30%) in foliage, 30 to 44% (![]() $\bar X$ = 36%) in grain and 28 to 40% (
$\bar X$ = 36%) in grain and 28 to 40% (![]() $\bar X$ = 34%) in total above ground biomass. The potential of fixation by the genotypes may be generally limited because of a shortage of soil moisture as the crop was grown with residual moisture. In SyriaReference Beck, Rupela, Saxena, Saxena, Johansen and Harris43, for instance, fixation in winter-sown chickpea reached over 80–81%, where as spring-sown chickpea, where moisture was a limiting factor, fixed only 8–27%. The difference method of determination of the amount of nitrogen fixation may also underestimate the magnitudeReference Dibabe, Bekele, Mamo, Dubale, Dibabe, Zeleke, Ayele and Kirub42. Protein contents of the shoot, grain and biomass, being derivative characters of plant tissue nitrogen, obviously followed the same pattern of variation as nitrogen contents. Other associated characters also showed wider ranges of variation.
$\bar X$ = 34%) in total above ground biomass. The potential of fixation by the genotypes may be generally limited because of a shortage of soil moisture as the crop was grown with residual moisture. In SyriaReference Beck, Rupela, Saxena, Saxena, Johansen and Harris43, for instance, fixation in winter-sown chickpea reached over 80–81%, where as spring-sown chickpea, where moisture was a limiting factor, fixed only 8–27%. The difference method of determination of the amount of nitrogen fixation may also underestimate the magnitudeReference Dibabe, Bekele, Mamo, Dubale, Dibabe, Zeleke, Ayele and Kirub42. Protein contents of the shoot, grain and biomass, being derivative characters of plant tissue nitrogen, obviously followed the same pattern of variation as nitrogen contents. Other associated characters also showed wider ranges of variation.
Table 2. Combined analysis of variance (over locations) for attributes of symbiotic and agronomic performance in 155 chickpea genotypes tested at two locations.

**Highly significant (P⩽0.01), *significant (P⩽0.05), and NS=non-significant (P>0.05).
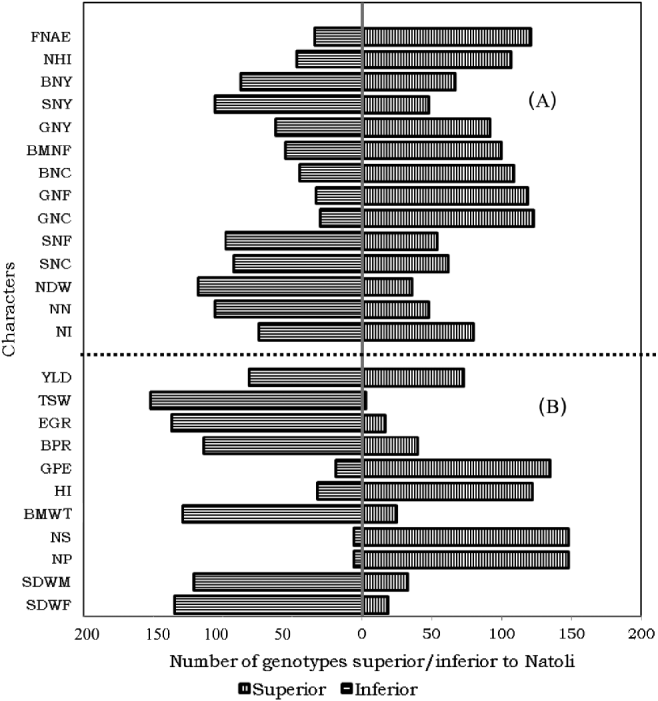
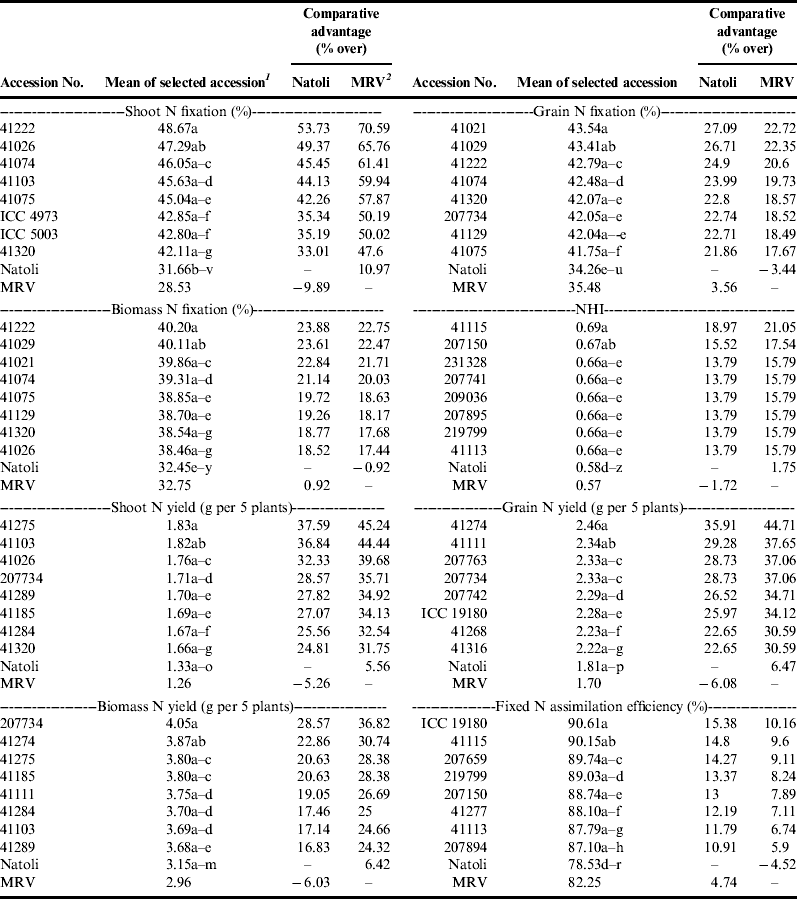
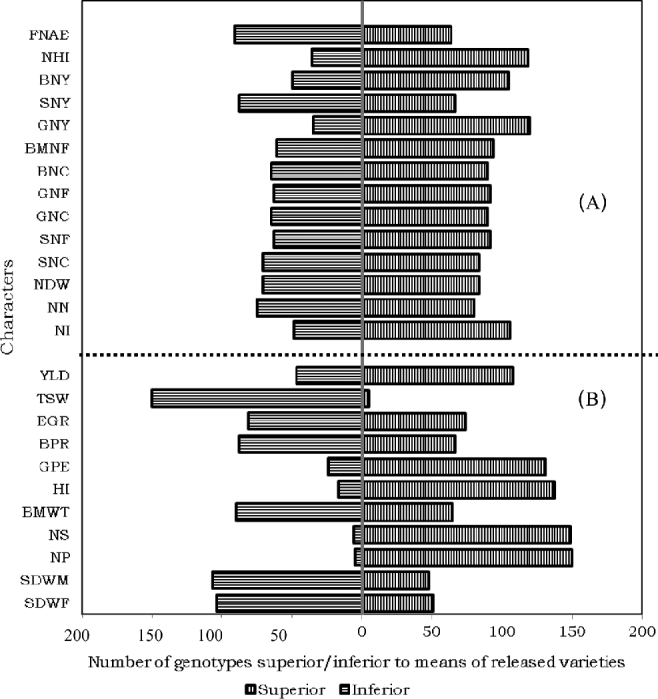
Comparison of the test genotypes with a recently released variety, Natoli, and with the overall mean performances of eight released varieties (Shasho, Arerti, Worku, Akaki, Ejere, Teji, Habru and Natoli) identified a number of accessions with superior symbiotic performance including the amount of nitrogen fixation in shoot, grain and total above-ground biomass (Figs 2 and 4). The best 5% of the accessions for total (shoot+grain) nitrogen fixation include accession nos. 41222, 41029, 41021, 41074, 41075, 41129, 41320 and 41026.

Figure 2. Proportion by number of the 155 chickpea genotypes superior and inferior to the recently released check, Natoli, for (A) symbiotic and (B) agronomic characters showing superiority of a number of landraces to the standard check (see Table 2 above for abbreviations of the characters).
These landraces could register additional fixation ranging from 18.52 to 23.88% over the standard check, Natoli, and 17.44 to 22.75% over the mean performance of the released varieties. These accessions also had the highest above-ground biomass nitrogen contents, which varied from 5.24 to 5.61%, the content of Natoli being 4.56%. As protein content follows the same trend as nitrogen contents, these accessions also contained the highest amount of protein in their biomasses (see supplementary table Annex C). There were some other genotypes that had better fixation of nitrogen, either in their shoots (e.g. 41103) or grains (e.g. 207734). Two introductions from ICRISAT, namely ICC 5003 and ICC 4973, were also among the best 5% for shoot fixation. The best genotypes for NHI, with superiorities of 13.79–18.97% over Natoli and 15.79–21.05% over the mean performances of the released varieties, were also landraces. This contradicts the tendency that modern cultivars usually show better NHI than landracesReference Sinclair and Vadez44.
The best assimilators of fixed nitrogen were accession nos. 41115, 207659, 219799, 207150, 41277, 41113 and 207894. A genotype introduced from ICRISAT as a non-nodulating check, ICC 19180, was found to be nodulating with best performance for grain nitrogen yield and assimilation efficiency of fixed nitrogen (Table 3). Whether a change in environment alone can induce nodulation of a genotype that is naturally non-nodulating in another environment needs to be sorted out in the future.
Table 3. Comparison of mean performances of 5% of the accessions selected for best symbiotic performance with Natoli, a recently released variety, and with mean performances of released varieties.

1Values sharing the same letter(s) or ranges of letters within the same column are non-significantly different; 2MRV=mean of released varieties. NHI, nitrogen harvest index.
These are accessions with desirable attributes of both symbiotic and agronomic significance. For example, accession no. 41274 possessed both better symbiotic (grain and biomass nitrogen yields) and agronomic (pod and seed setting, biomass weight, production efficiency, biomass production rate, economic growth rate and grain yield) attributes. Among the introductions, ICC 19180 demonstrated relatively better grain nitrogen yield and assimilation of fixed nitrogen with better biomass production and economic growth rates.
Comparison of nitrogen fixation patterns between modern cultivars and landraces does not appear to follow a simple trend as there are conflicting reports. Some found better nitrogen-fixing genotypes from among the old cultivars, landraces or wild relatives than commercial varietiesReference Sinclair and Vadez44,Reference Provorov and Tikhonovich45. In another study, field pea landraces introduced from Ethiopian to Germany were found to fix more nitrogen than a commercial cultivarReference Adgo and Schulze46. A similar result was also reported in Phaseolus vulgaris in AustriaReference de Oliveira, Meinhardt, Sessitsch and Tsai47. Our experience with primitive forms of Pisum sativum contrarily showed superiority of improved cultivars over the landracesReference Keneni, Assefa, Imtiaz, Bekele and Regassa48. In chickpea, both nodulation efficiency and grain yield were improved as the result of selection from commercial cultivarsReference Ali, Krishnamurthy, Saxena, Rupela, Kumar and Johansen1. Therefore, specific tests for specific breeding materials, strain and environments may be needed to improve the selection process.
Agronomic performance of the genotypes
Differences among the genotypes were significant for a number of agronomic characters (Table 2). The comparison of the different genotypes with the recently released variety Natoli showed the superiority of a number of landraces for a number of agronomic traits (see supplementary table Annex D). Accordingly, the best 5% of the accessions for yield include accession nos. 41274, 207763, 41111, 207742, 231328, 207563, 41053 and 212589. These accessions recorded yield advantages of 16.62–30.76% over Natoli and 26.84–42.22% over the mean performance of the released varieties. There were many other landraces that were superior for many other traits. The improved cultivars that were originally from exotic sources might be more affected by moisture stress than the primitive landraces that have co-existed with the stress, but the detail will be discussed later. Experience with soybean (Glycine max) also showed that exotic of genotypes are usually lower yielding than domestic cultivars when released directlyReference Sneller, Nelson, Carter, Cui and Kang49. Nevertheless, no landrace was comparable to the improved genotypes for seed size (Fig. 2), the top 5% of genotypes for this trait being ICC 4918, ICC 5003, ICC 19180, Natoli, Teji, Ejere, Arerti and Habru, which are introductions either from ICRISAT or ICARDA.
The landraces make the majority of the top 5% performers to the improved introductions for many attributes. Some of them, such as accession no. 41274, have displayed superiority for grain yield, pod and seed setting, biomass dry weight and production rate, grain production efficiency and economic growth rate. Another accession, accession no. 207563, was also among the top performers for grain yield, economic growth rate and pod setting. A number of other superior accessions for multiple traits of agronomic significance, including grain yield, were also identified (Table 4).
Table 4. Comparison of mean performances of 5% of the accessions selected for best agronomic performance with Natoli, a recently released variety, and with mean performances of released varieties.

1Values sharing the same letter(s) or ranges of letters within the same column are non-significantly different; 2MRV, mean of released varieties.
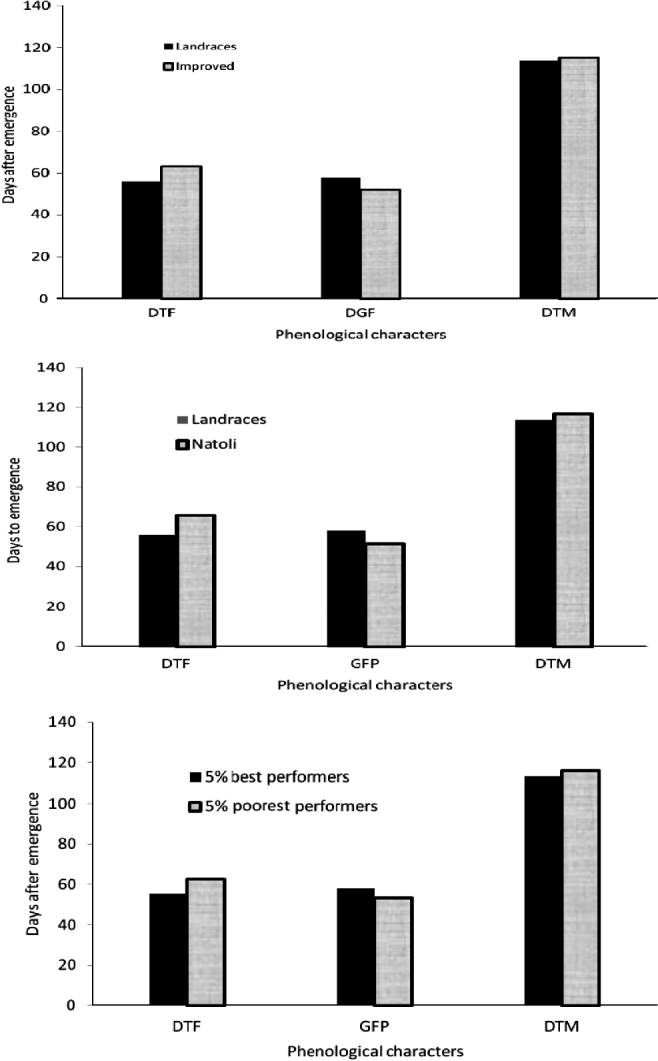
Detailed physiological reasons for the superiority of the landraces over the improved genotypes may need future study. From our observations, the landraces had relatively shorter period of vegetative growth and longer grain-filling periods. The comparison of the 5% best yielders for phenological characters with the 5% poorest yielders may also show, at least in part, that the genotypes tested here differed in grain yield for a similar reason (Fig. 3). Ideotypes with the faster developmental switch to reproductive growth earlier in the growing season when the soil moisture level is still adequate might have a better comparative advantage to mobilize assimilates and use them more efficiently for reproductive growth. The same mechanism might have provided the landraces with adequately longer period of time for grain filling. A similar observation was made in Ethiopian fenugreek (Trigonella foenum-graecum) landraces as compared to a released varietyReference Fikresilassie50. On the contrary, improved genotypes showed delays in flowering; the consequence may be higher investment in vegetative growth and longer exposure of their reproductive growth to an end-of-season moisture stress. It may be implied, therefore, that landraces may have better structural and functional fitness to survive and reproduce under moisture deficit condition than the improved genotypes.

Figure 3. Comparison of phenological characters in 155 genotypes for days to flowering (DTF), grain filling period (GFP) and days to maturity (DTM), showing shorter relative periods of vegetative and longer grain-filling periods in landraces than in improved genotype.

Figure 4. Proportion by number of the 155 chickpea genotypes superior and inferior to mean performances of all the released varieties for (A) symbiotic and (B) agronomic characters, showing superiority of a number of landraces to the standard check (see Table 2 above for abbreviations of the characters).
Effect of location
The two locations displayed significant differences for a number of symbiotic and agronomic characters. However, number and size of seeds, shoot and grain nitrogen and protein contents, grain and total nitrogen fixation, grain nitrogen yield and grain yield did not show marked differences between locations (Table 5).
Table 5. Mean performances of 155 chickpea genotypes for attributes of symbiotic nitrogen fixation and agronomic performance at two locations in Ethiopia.

* Values within a row sharing the same letter are statistically non-significant.
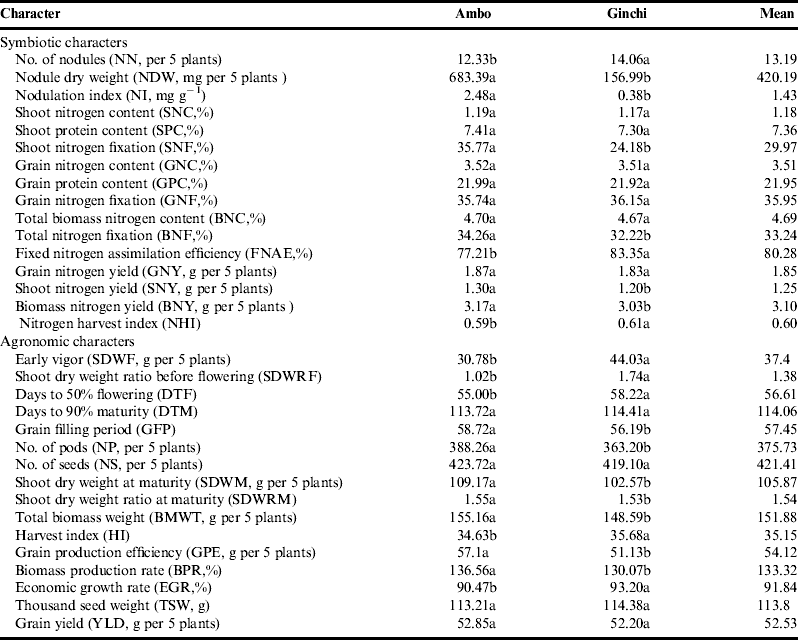
The number of nodules per five plants was higher at Ginchi and lower at Ambo, but nodule weight was heavier at Ambo. As nitrogen fixation was also better at Ambo than it was at Ginchi, this may imply that weight of nodules may be more important (within a limit) for fixation than their mere number. Better fixation at Ambo may also be attributed particularly to the higher level of phosphorus in the soil since phosphorus is of paramount importance in fixationReference Shukla and Yadav22.
Genotype by location interaction effects
Genotype by location interaction effects were significant for a number of symbiotic and agronomic characters (Table 2). Significant genotype by location interaction effects were mostly a ‘cross-over’ type; i.e., interactions were associated with rank order changes among the genotypes (data not shown). This indicated that the two locations were distinctly different for some of the characters and that better genotypes at one location may not also be better performing at another. Even though the inheritance of the process of nodulation was considered to be relatively simpleReference Rengel13, the genetic control of the whole process of symbiotic nitrogen fixation is complicated due to its polygenic natureReference Austin, Hayward, Bosemark and Romagosa51, Reference Tate52. Plant breeding commonly involves dealing with only a single organism at a time and, while this itself is not simple, breeding for symbiotic nitrogen fixation demands dealing simultaneously with both the host plant and the strain, which interact differently between themselves and with the environmentReference Crouch, Buhariwalla, Blair, Mace, Jayashree, Serraj and Serraj18. Most of the traits related to yield and agronomic yield components, nodulation and nitrogen yields showed significant genotype by location interaction. Fortunately, genotype by location interaction effects were non-significant for components related to plant tissue nitrogen contents, including the amount of fixation. This may indicate lesser sensitivity of traits for symbiotic nitrogen fixation to changes in the environment. In our previous study with primitive forms of P. sativum native to Ethiopia, we also found similar resultsReference Keneni, Assefa, Imtiaz, Bekele and Regassa48. Therefore, genotypes selected at one location for nitrogen (and hence protein) content and fixation levels may perform similarly in other locations.
Conclusions
The present study revealed that the Ethiopian chickpea landraces had considerable significance as sources of genotypes with desirable symbiotic and agronomic characters. Despite the important roles they could have played in chickpea–breeding programs, unfortunately, the potential of the Ethiopian chickpea landraces has not been properly exploited hitherto by the breeding efforts. Almost all of the released varieties currently under production in Ethiopia trace their genetic base to the introductions either from ICARDA or ICRISAT. The accessions selected here could serve as the basis for formulating an efficient scheme of multiple trait selection for both desirable symbiotic and agronomic characters. It can be expected that individual lines developed through selection from among the accessions identified here may result in even better performances than the ‘mother’ accessions themselves.
The past breeding strategy based on direct release of introduced genotypes has produced special cultivars that are substantially different from commonly grown landraces, particularly in terms of seed size and color. As far as a number of symbiotic and other agronomic merits are concerned, the Ethiopian chickpea landraces should constitute the predominant part in the genetic background of the breeding program. When the desired character does not exist in the available gene pool, as we indicated for seed size, screening of exotic germplasm becomes essential to identify good donor parents. Except for a few accessions, the majority of the landraces did not show a better combination of desirable symbiotic and agronomic characters, which may be of rare natural occurrence. In order to achieve varieties that combine many desirable symbiotic and agronomic attributes, therefore, there is a need to concomitantly incorporate many desirable traits from different sources into a single genotype through hybridization.
Acknowledgements
The authors wish to acknowledge the Ethiopian Institute of Agricultural Research, the International Center for Agricultural Research in the Dry Areas (ICARDA), and Addis Ababa University for material, technical and financial support to this study as a component of the PhD thesis of the first author. The test genotypes were received from the Ethiopian Institute of Biodiversity Conservation, the International Crop Research Institute for the Semi-Arid Tropics, ICARDA and the National Chickpea Research Project in Ethiopia. The inoculum used in this study was kindly provided by the National Soil Analysis Laboratory under Ministry of Agriculture and Rural Development. The authors also sincerely thank staff members of Holetta, Debre Zeit and Ambo Agricultural Research Centers who assisted in trial management and data collection.