Introduction
Advances in the molecular genetics of schizophrenia increasingly support polygenic risk models based on many genes of small effect (Gottesman & Shields, Reference Gottesman and Shields1967; Purcell et al. Reference Purcell, Wray, Stone, Visscher, O'Donovan, Sullivan and Sklar2009; Shi et al. Reference Shi, Levinson, Duan, Sanders, Zheng, Pe'er, Dudbridge, Holmans, Whittemore, Mowry, Olincy, Amin, Cloninger, Silverman, Buccola, Byerley, Black, Crowe, Oksenberg, Mirel, Kendler, Freedman and Gejman2009; Stefansson et al. Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietiläinen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Børglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Böttcher, Olesen, Breuer, Möller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Réthelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jönsson, Terenius, Agartz, Petursson, Nöthen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009). For example, in a recent large-scale genome-wide association study, Purcell and colleagues of the International Schizophrenia Consortium (2009) reported that at least one-third of the variance in schizophrenia liability could be explained by a polygenic model involving thousands of commonly occurring alleles. Polygenic models suggest that the genetic liability may manifest as individual differences in specific neural circuits, producing observable neurocognitive intermediate phenotypes (Gottesman & Gould, Reference Gottesman and Gould2003; Meyer-Lindenberg & Weinberger, Reference Meyer-Lindenberg and Weinberger2006; Braff et al. Reference Braff, Freedman, Schork and Gottesman2007; Ivleva et al. Reference Ivleva, Morris, Moates, Suppes, Thaker and Tamminga2010).
Based on the criteria proposed by Gottesman & Gould (Reference Gottesman and Gould2003), deficits in face emotion recognition (FER) provide a potential intermediate phenotype for schizophrenia and related disorders (Gur et al. 2007 a, b). FER deficits are consistently related to schizophrenia (Mueser et al. Reference Mueser, Penn, Blanchard and Bellack1997; Mandal et al. Reference Mandal, Pandey and Prasad1998; Hooker & Park, Reference Hooker and Park2002; Kohler & Brennan, Reference Kohler and Brennan2004), are observable in early (Edwards et al. Reference Edwards, Pattison, Jackson and Wales2001) and late psychosis (Mueser et al. Reference Mueser, Penn, Blanchard and Bellack1997), remain after treatment (Herbener et al. Reference Herbener, Hill, Marvin and Sweeney2005), and are related to familial risk (Kee et al. Reference Kee, Horan, Mintz and Green2004; Bediou et al. Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007). Evidence suggests that FER ability is also highly heritable (Gur et al. 2007 a, b). FER provides the advantage of implicating a well-studied neural network, including the amygdala, superior temporal sulcus and inferior parietal lobe (Adolphs, Reference Adolphs2002), whose function can be dissociated from the function of neural networks concerned with static face features (Haxby et al. Reference Haxby, Hoffman and Gobbini2000). Notably, people with schizophrenia spectrum disorders have structural and functional abnormalities in neural regions that support FER processing (Aleman & Kahn, Reference Aleman and Kahn2005; Brunet-Gouet & Decety, Reference Brunet-Gouet and Decety2006), but relatively normal function of neural regions such as the fusiform gyrus that support face identity processing (Foxe et al. Reference Foxe, Murray and Javitt2005; Yoon et al. Reference Yoon, D'Esposito and Carter2006).
Recent evidence suggests that FER deficits are not limited to individuals with schizophrenia, but are more broadly related to psychosis vulnerability (Phillips & Seidman, Reference Phillips and Seidman2008). FER deficits have been reported in the first-degree relatives of schizophrenia patients (Kee et al. Reference Kee, Horan, Mintz and Green2004; Bediou et al. Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007), even where other face processing abilities are unimpaired (Bediou et al. Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007). If FER deficits contribute to the development of psychosis by influencing the development of psychosis-like characteristics, they may also be observable in healthy, high-risk individuals with psychosis-like or subthreshold characteristics (schizotypy or psychosis-proneness). Individuals with high familial risk vary widely in how much they express schizotypal or psychosis-like traits (Kremen et al. Reference Kremen, Faraone, Toomey, Seidman and Tsuang1998; Tsuang et al. Reference Tsuang, Stone and Faraone1999; Vollema et al. Reference Vollema, Sitskoorn, Appels and Kahn2002), so studies of psychometric psychosis-proneness provide a crucial means of addressing the relationship between FER, phenotype and psychosis vulnerability.
Results from studies looking at the relationship between psychometric psychosis-proneness and FER have thus far been mixed or unclear. Some studies have shown FER deficits in individuals high (versus low) in schizotypy or psychosis-proneness (Poreh et al. Reference Poreh, Whitman, Weber and Ross1994; Mikhailova et al. Reference Mikhailova, Vladimirova, Iznak and Tsusulkovskaya1996; Waldeck & Miller, Reference Waldeck and Miller2000; Williams et al. Reference Williams, Henry and Green2007; Aguirre et al. Reference Aguirre, Sergi and Levy2008) whereas other studies have not (Toomey & Schuldberg, Reference Toomey and Schuldberg1995; van ‘t Wout et al. Reference van ‘t Wout, Aleman, Kessels, Laroi and Kahn2004; Jahshan & Sergi, Reference Jahshan and Sergi2007). However, ceiling effects may have contributed to negative results (e.g. Toomey & Schuldberg, Reference Toomey and Schuldberg1995; Jahshan & Sergi, Reference Jahshan and Sergi2007) by reducing the ability to detect between-group differences. Sensitive FER tests are needed to detect individual differences in healthy populations.
Furthermore, general cognitive impairment is associated with schizophrenia patients in addition to those at risk; therefore, FER deficits could be part of more generalized deficits in face processing or in visual perception rather than emotion processing (Addington & Addington, Reference Addington and Addington1998). Of the studies that have used face processing-related control tasks, Poreh et al. (2004) found evidence of general face processing impairment in psychosis-prone individuals, whereas Williams et al. (Reference Williams, Henry and Green2007) reported that high psychosis-proneness was related to FER impairments but not face identity recognition impairments, based on the Benton Facial Recognition Test (BFRT; although the BFRT may be a suboptimal measure of face discrimination ability; see Duchaine & Nakayama, Reference Duchaine and Nakayama2004). Moreover, differences in procedure or face stimuli between tasks can contribute to misleading or artifactual results. Hence, it is not clear from current research whether the relationship between psychosis-proneness and FER, where observed, is related to more generic processes. Given the possible role of FER as an intermediate phenotype, good behavioral assays in schizophrenia and schizophrenia risk are an important tool, and more research is needed to determine how best to test, characterize and quantify the extent and specificity of ER deficits in individuals with schizophrenia or at risk for schizophrenia.
In addition, as evidence for polygenic models accumulates, it is increasingly important to characterize the relationship between psychosis liability and neurocognition across the continuum. FER differences may, for example, vary linearly with psychosis-proneness or only be observable in individuals with the highest levels of psychosis-proneness. Clarifying the nature of this relationship is needed for deciding whether a continuous individual differences model (Claridge, Reference Claridge and Claridge1997) or a discrete, discontinuous model (e.g. Meehl, Reference Meehl1962, Reference Meehl1990) is most appropriate for characterizing FER as an intermediate phenotype. Thus far, no study has examined the relationship between FER and psychosis liability at intermediate levels of psychosis-proneness.
In two experiments using very large, psychometrically defined samples, we tested the hypothesis that variations across the continuum of psychosis-proneness are related to FER ability but not to other face processing abilities. In Experiment 1, we administered tests of face emotion and face gender identification to extend Bediou et al.'s (Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007) finding of selective FER impairments in familial high-risk participants to a sample of participants with varying levels of psychometric risk. In Experiment 2, we replicated our results from Experiment 1 using a test of face emotion and face identity discrimination [the Queen Square Face Discrimination Test (QFDT); Garrido et al. Reference Garrido, Furl, Draganski, Weiskopf, Stevens, Tan, Driver, Dolan and Duchaine2009]. These discrimination tasks were designed to be sensitive to individual differences in face processing, closely matched to minimize difficulty or task-related artifacts, and have been shown to rely on specific and dissociable neural subsystems (Pitcher et al. Reference Pitcher, Garrido, Walsh and Duchaine2008; Garrido et al. Reference Garrido, Furl, Draganski, Weiskopf, Stevens, Tan, Driver, Dolan and Duchaine2009).
Experiment 1: Emotion identification versus gender identification
To determine whether individual differences in face emotion processing performance is related to psychosis-proneness, we administered a face emotion and a face gender identification task to individuals in the normal population with varying levels of psychosis-proneness based on scores from the brief version of the Schizotypal Personality Questionnaire (SPQ-B; Raine & Benishay, Reference Raine and Benishay1995).
Method
Participants
Subjects were individuals who navigated to the website www.testmybrain.org and clicked on a link labeled ‘Recognizing Emotion and Gender from Faces’. Data collected from face processing tests offered on testmybrain.org (different from the ones described here) have been included in a previously published study (Wilmer et al. Reference Wilmer, Germine, Chabris, Chatterjee, Nakayama, Williams, Loken and Duchaine2010). There was no specific advertising conducted for the study or the website. Most users arrived at the site through self-generated internet searches and by following links posted by other volunteers on social networking websites and blogs. Subjects were given feedback on their performance at the conclusion of the test as incentive for participating. There were no limitations on who could participate in the experiment, but subjects in the reported sample had to meet several criteria. After filling out an online consent form, participants completed a questionnaire assessing demographics, psychiatric, neurological and medical history. Participants were excluded if they endorsed any of the following: age <16 or >65 years, neurological problems, psychological problems, vision problems, a physical disability that might impact their performance, Asperger's disorder or other autistic spectrum disorder. At the end of the experiment, subjects who indicated that they had had technical problems were also excluded, as were those who may have participated in the experiment before (as indicated by self-report and/or checking the individual's web browser for a ‘cookie’ that indicated previous participation).
Our final group comprised 2332 subjects. Table 1 show age, gender and SPQ information for this sample.
Table 1. Mean performance and participant information

SPQ-B, Schizotypal Personality Questionnaire – Brief version; s.d., standard deviation.
a Mean SPQ-B score from this sample was approximately equal to the mean obtained from a sample of adults with a similar gender distribution (Irwin, Reference Irwin2001: mean=9.25, where 63% were female).
b Proportion correct out of 60.
c Proportion correct out of 15.
d Proportion correct out of 40.
Procedure
All subjects began by completing a test of face gender identification and then a test of face emotion identification, both using morphed face stimuli and adapted from tests previously administered to schizophrenia patients and their relatives (Bediou et al. Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007).
Example stimuli from face emotion and gender identification tests are shown in Fig. 1. In the face gender identification task, faces were created by morphing a gender neutral face with each of four male and four female faces. Each face stimulus contained 20, 30, 40, 50 or 60% of the target gender (male or female), yielding 40 face stimuli (eight identities×five percentage categories). In the face emotion identification task, stimuli were faces morphed between a neutral expression and an emotional expression. There were four different emotional expressions: happy, disgusted, angry, and fearful. Faces were created from one male and two female face identities. The faces contained 20, 30, 40, 50 and 60% of the emotional expression for each identity and each type of facial expression. This yielded 60 face trials (four emotion types×three identities×five percentage categories). The original tasks used by Bediou et al. (Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007) each contained 10 percentage categories, with trials containing 10–100% of the target gender or expression. Based on the control data reported by Bediou et al. (Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007), the range 20–60% was chosen for the current experiment to maximize the range of difficulty levels in a minimal number of trials. The different increments of emotion and gender intensities created varying levels of difficulty, and therefore increased the sensitivity of the task to reveal individual differences in performance.
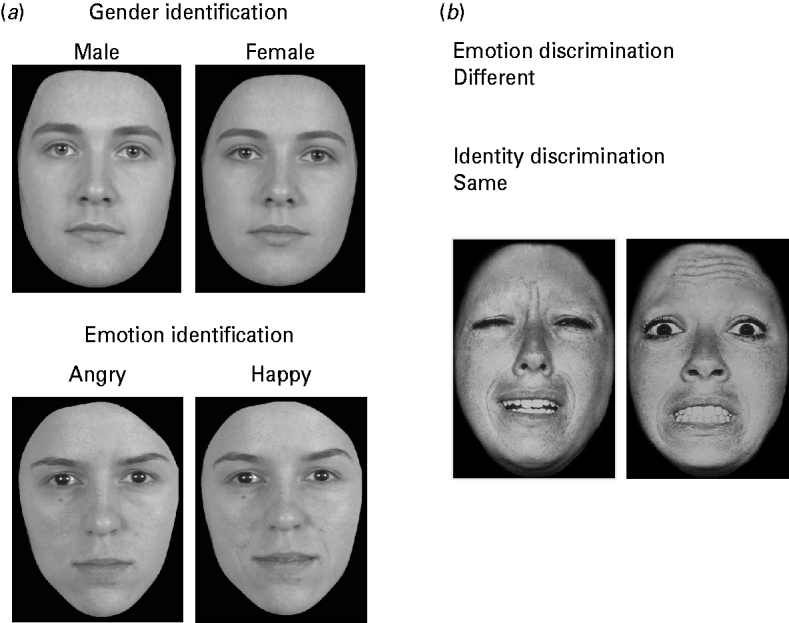
Fig. 1. Stimuli from Experiments 1 and 2. (a) Images from Experiment 1 are shown (Bediou et al. Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007). In the gender identification task, participants had to label each morphed face as male or female. In the emotion identification task, participants had to label each emotion as fearful, angry, disgusted, or happy. Correct responses for each image/trial are shown in italics. (b) Images from Experiment 2 are shown (Garrido et al. Reference Garrido, Furl, Draganski, Weiskopf, Stevens, Tan, Driver, Dolan and Duchaine2009). Participants had to judge whether two sequentially presented faces had the same or different emotion (emotion discrimination task) or the same or different identity (identity discrimination task). Correct responses for this image pair in each task are shown in italics.
In both tasks, each trial began with a fixation cross for 250 ms, then the face was presented on screen for 1000 ms, followed by the list of answer choices. Participants made a choice between ‘male or female’ in the face gender test, and ‘angry, disgusted, fearful, or happy’ in the face emotion test. The answer choices remained on screen for 7 s or until the participant responded. Participants indicated their response by pressing a key (‘m’ or ‘f’; ‘a’, ‘d’, ‘f’, or ‘h’). For each task, participants who failed to respond within the time limit on more than 10% of trials were excluded from analysis.
After completing both tests, subjects responded to items from the SPQ-B, a measure of psychosis-proneness. The SPQ-B is a 22-item self-report questionnaire that indexes the degree to which an individual has schizophrenia-like cognitive-perceptual (e.g. ‘Have you ever noticed a common event or object that seemed to be a special sign for you?’), interpersonal (e.g. ‘I feel I have to be on my guard even with my friends’), and disorganized features (e.g. ‘I sometimes use words in unusual words’).
Results
A summary of mean performance for this sample is given in Table 1. Paired-sample t test results show that participants were more accurate on gender identification as compared with emotion identification [t(2331)=59.4, p<0.001].
Multiple regression was conducted (SPSS version 16.0; SPSS Inc., USA) to test the hypothesis that individual differences in psychosis-proneness were related to emotion identification but not to gender identification performance, by using age, participant sex, and SPQ-B scores as predictors of face emotion identification. Previous research has indicated that face processing ability is related to both participant sex and age (McClure, Reference McClure2000; Bowles et al. Reference Bowles, McKone, Dawel, Duchaine, Palermo, Schmalzl, Rivolta and Wilson2009), so we controlled for these effects in our analysis. As both SPQ-B scores and age (r=−0.21) and SPQ-B scores and sex (r=0.06) were significantly related in this sample, controlling for age and sex also allowed us to focus on variations in face processing with psychosis-proneness that were not due to variations in age and sex. As expected, the SPQ-B score significantly predicted emotion identification performance (β=−0.09, p<0.001), controlling for the effects of sex (β=−0.18, p<0.001) and age (β=−0.07, p<0.01). The relationship between psychosis-proneness and emotion identification did not change when gender identification performance was added as a predictor (β=−0.09, p<0.001).
Two subgroups were defined by total SPQ-B score such that they roughly represented the bottom and top 10% of the sample. The top 10% is traditionally defined as high risk in studies of psychometric schizotypy, and individuals with schizophrenia spectrum disorders such as schizotypal personality disorder are likely to be in the top 10% of scorers (Raine & Benishay, Reference Raine and Benishay1995) whereas the bottom 10% is unlikely to contain individual with schizophrenia spectrum diagnoses (Raine, Reference Raine1991). Individuals with the lowest SPQ-B scores (from 0 to 2, bottom 10%) were significantly more accurate than those with the highest SPQ-B scores (⩾17, top 9%) [mean (s.d.) for low SPQ-B scorers: 0.66 (0.1); mean for high SPQ-B scorers=0.69 (0.1); independent samples t test: t(430)=2.7, p<0.01] and corresponded to a Cohen's d effect size of 0.24. This relationship was not driven entirely by high SPQ-B scorers (those with possible schizophrenia spectrum disorders); SPQ-B scores predicted emotion identification performance even when individuals with high SPQ-B scores (scores of 16/22 or higher) were excluded (2023 participants remaining; β=−0.11, p<0.001).
To see whether the observed relationship between psychosis-proneness and face perception was specific to emotion processing, we conducted multiple regression of face gender performance on age, sex and SPQ-B score. The results indicated that although age significantly predicted gender identification performance (b=0.06, p<0.01), SPQ-B score and sex did not (SPQ-B: b=−0.02, p=0.43; sex: b=−0.002, p=0.99). Accordingly, high and low SPQ-B scorers did not differ significantly in gender identification performance [mean (s.d.) for low SPQ-B scorers=0.80 (0.08); mean for high SPQ-B scorers=0.81 (0.08); independent-samples t test: t(430)=1.0, p=0.3].
Scores on the SPQ-B can be divided into three subscales: an interpersonal factor, a cognitive-perceptual factor, and a disorganized factor. These three factors are analogous to the three symptom clusters observed in schizophrenia (Arndt et al. Reference Arndt, Alliger and Andreasen1991). After controlling for the effects of age and sex, multiple regression analysis revealed that each of the factors predicted emotion performance (interpersonal: β=−0.09, p<0.001; cognitive-perceptual: β=−0.06, p<0.01; disorganized: β=−0.04, p<0.05) but not gender performance (interpersonal: β=−0.03, p=0.23; cognitive-perceptual; β=0.01, p=0.66; disorganized: β=−0.02, p=0.27).
To identify whether the relationship between SPQ-B score and emotion identification was significantly greater than the relationship between SPQ-B score and gender identification, we used Steiger's Z1* statistic for comparing two correlation coefficients from the same sample (Steiger, Reference Steiger1980). This analysis showed that the partial correlation between SPQ-B score and emotion identification and SPQ-B score was significantly greater than the partial correlation between SPQ-B score and gender identification (Z=2.8, p<0.01).
Finally, to explore the relationship between SPQ-B scores and identification of specific emotions, we conducted multiple regression with SPQ-B score, age and participant sex as predictors of proportion correct for happy, angry, disgusted and fearful faces separately. Mean performance for individual emotions is shown in Table 1. SPQ-B scores significantly predicted identification of happy faces (β=−0.07, p<0.001), angry faces (β=−0.07, p<0.001), and fearful faces (β=−0.05, p<0.05), but predicted disgusted faces only at the trend level (β=−0.04, p=0.08). These results should be interpreted cautiously, however, as we did not have any a priori predictions about the relationship between psychosis-proneness and specific emotions, and the current task was not designed to reveal emotion-specific dissociations.
Fig. 2 shows performance on face emotion and gender identification across the range of SPQ-B scores, illustrating that differences in emotion identification begin to emerge at moderate levels of psychosis-proneness.

Fig. 2. Task performance and psychosis-proneness. Average proportion correct is shown for individuals at different levels of psychosis-proneness in (a) Experiment 1 and (b) Experiment 2. Although performance on both emotion tasks varied with psychosis-proneness, performance on identity and gender tasks did not. Psychosis-proneness was measured using the brief version of the Schizotypal Personality Questionnaire (SPQ-B; Raine & Benishay, Reference Raine and Benishay1995). For each experiment, proportion correct was binned by SPQ-B score. The median score for each bin is shown, with the exception of the highest bin, which reflects the high end of SPQ-B scorers (scores were positively skewed). Bars reflect ±1 standard error. Bins range in size from n=93 to n=495.
Experiment 2: Emotion discrimination versus identity discrimination
There was a significant difference in overall accuracy between the two tasks in Experiment 1, so it is possible that our findings were the result of differences in task difficulty or differences in task parameters (e.g. there were four response options for the emotion task and only two for the gender task). Differences in difficulty, in particular, pose a significant problem as more difficult tasks are often more sensitive to group differences. Thus, to replicate our findings from Experiment 1, exclude difficulty-related confounds, and investigate whether or not psychosis-proneness is related to another dimension of face perception (identity processing), we conducted a second experiment using a test of face emotion discrimination and a difficulty-matched test of face identity discrimination adapted from the QFDT (Garrido et al. Reference Garrido, Furl, Draganski, Weiskopf, Stevens, Tan, Driver, Dolan and Duchaine2009). These tests of identity and emotion discrimination have been used in two prior studies and were shown to tap into dissociable subsystems of face perception, behaviorally and neurally (Pitcher et al. Reference Pitcher, Garrido, Walsh and Duchaine2008; Garrido et al. Reference Garrido, Furl, Draganski, Weiskopf, Stevens, Tan, Driver, Dolan and Duchaine2009). Using a test of emotion discrimination would also allow us to generalize our results from Experiment 1 to face emotion processing more broadly. Whereas emotion discrimination is more purely perceptual, emotion identification relies on other cognitive abilities, such as verbal labeling, that make impairments difficult to interpret (Mandal et al. Reference Mandal, Pandey and Prasad1998).
Methods
Participants
Subjects were individuals who navigated to the website www.testmybrain.org and clicked on a link labeled ‘Recognizing Emotion and Identity from Faces’. Experiments 1 and 2 were never available on our website at the same time, so participant overlap between the two experiments was unlikely to be significant. Exclusion criteria were the same as for Experiment 1, except that we included two additional question prompts to serve as validity checks. Participants were excluded if they responded ‘No’ to the statement ‘I am paying attention to my responses on this questionnaire’ or ‘Yes’ to the statement ‘I responded to most of the last 47 questions without reading them’. Our final group comprised 1514 participants. Details of this sample are given in Table 1. All subjects first completed a test of face identity discrimination followed by a test of face emotion discrimination.
Procedure
Stimuli were the same for both emotion and identity discrimination tests, and comprised six female models taken from the Ekman & Friesen (Reference Ekman and Friesen1976) facial affect series expressing either happiness, sadness, surprise, fear, anger or disgust. Pictures were grayscale and cropped, using the same contour to hide the hair and neck. For both tasks, face pairs were presented sequentially for 500 ms per face with 500 ms fixation between images. Participants then had up to 7 s to indicate whether the two faces had the same or different identity (identity discrimination test) or were expressing the same or different emotion (emotion discrimination test). Half the trials on each test showed pairs with the same identity/emotion and half the trials showed pairs with different identities/emotions. In the emotion test, identity always varied between the face pairs. In the identity test, emotion always varied between the face pairs. Each test contained 40 trials.
After finishing both tests, subjects again completed items from the SPQ-B, the same measure of psychosis-proneness used in Experiment 1.
Results
Mean performance for this sample is given in Table 1. Participants were more accurate on emotion discrimination as compared with identity discrimination [paired-samples t test: t(1513)=14.5, p<0.001].
To test the hypothesis that psychosis-proneness was significantly related to emotion discrimination performance, multiple regression was conducted in SPSS (version 16.0; 2007) with age, participant sex, and total SPQ-B score as predictors of face emotion discrimination performance. SPQ-B scores in this sample were significantly related to participant age (r=−0.21) but not to sex. Participant sex significantly predicted emotion discrimination performance (β=−0.10, p<0.001) whereas age did not (β=−0.014, p=0.6). Psychosis-proneness, as measured by the SPQ-B, significantly predicted emotion discrimination performance (β=−0.11, p<0.001), even when controlling for identity discrimination performance (β=−0.10, p<0.001). Performance was again significantly different between the participants lowest in psychosis-proneness (SPQ-B scores 0–2, bottom 8%) and those highest in psychosis-proneness (SPQ-B scores ⩾17, top 9%) [mean (s.d.) for low SPQ-B scorers: 0.83 (0.8); mean for high SPQ-B scorers=0.79 (0.1); independent samples t test: t(261)=3.3, p<0.001], corresponding to a Cohen's d effect size of 0.38. As in Experiment 1, the relationship between SPQ-B score and emotion recognition performance was not being driven entirely by individuals with the highest levels of psychosis-proneness and possible schizophrenia spectrum diagnoses. When individuals with scores of ⩾16 (out of 22) were excluded from analysis, multiple regression again showed that SPQ-B score significantly predicted emotion discrimination (1322 participants remaining; β=−0.07, p<0.05).
To see whether differences related to psychosis-proneness were limited to emotion discrimination, we conducted multiple regression of face identity discrimination on age, sex, and SPQ-B score. Age and sex predicted identity discrimination performance (age: β=−0.17, p<0.001; sex: β=−0.14, p<0.001) whereas psychosis-proneness did not (β=−0.03, p=0.22). This occurred even though overall performance on the identity discrimination task was significantly lower than on the emotion discrimination task, in contrast to Experiment 1 where the emotion task was more difficult. Hence, the observed relationship between psychosis-proneness and emotion processing cannot be explained by difficulty-related confounds.
Multiple regression of emotion discrimination performance on age, sex, and the three factors of the SPQ-B again demonstrated a significant relationship between emotion performance and all three factors (interpersonal: β=−0.07, p<0.05; cognitive-perceptual: β=−0.10, p<0.001; disorganized: β=−0.08, p<0.01). Only the interpersonal factor of psychosis-proneness predicted identity discrimination performance (interpersonal: β=−0.05, p<0.05; cognitive-perceptual: β=0.01, p=0.82; disorganized: β=−0.02, p=0.54).
In addition, the correlations between SPQ-B score and emotion discrimination and SPQ-B score and identity discrimination were significantly different, based on Steiger's Z1* statistic (1980) for comparing two correlation coefficients from the same sample (Z=2.3, p<0.01).
We did not conduct analyses looking at the relationship between psychosis-proneness and specific emotions for this experiment, as the design (same/different; six emotion categories) was not conducive to this type of analysis.
Fig. 2 illustrates the relationship between psychosis-proneness based on SPQ-B scores and discrimination performance. Consistent with our previous result in Experiment 1, differences in emotion discrimination related to psychosis-proneness are visible at moderate SPQ-B scores.
Discussion
We have demonstrated in two large samples that increasing psychosis-proneness, as indicated by scores on the SPQ-B (Raine & Benishay, Reference Raine and Benishay1995), is related to reductions in the ability to identify and discriminate facial expressions of emotion. Furthermore, this relationship cannot be accounted for by differences in face processing, visual perception, or a general performance-related factor, as performance on a face gender test (Experiment 1) and a face identity discrimination task (Experiment 2) did not show reductions related to increasing psychosis-proneness. Finally, the relationship between FER and psychosis-proneness was significantly predicted by all three factors of our psychosis-proneness measure (interpersonal, cognitive-perceptual, and disorganized). This suggests that FER ability is broadly related to psychosis-like characteristics and not restricted to a single dimension of psychosis-proneness, such as positive or negative symptoms.
Our data indicate that the phenotypic expression of subthreshold or psychosis-like features is associated with small, but consistent, differences in the ability to decode facial expressions of emotion in the normal population. These differences are not likely to be clinically significant, but indicate that FER ability varies with individual differences in psychosis-proneness in the normal population. Schizotypal or psychosis-like features are related to genetic vulnerability to schizophrenia (Kendler & Walsh, Reference Kendler and Walsh1995; Vollema et al. Reference Vollema, Sitskoorn, Appels and Kahn2002) and elevated schizophrenia risk (Claridge, Reference Claridge and Claridge1997; Kwapil et al. Reference Kwapil, Miller, Zinser, Chapman and Chapman1997; Kwapil, Reference Kwapil1998; Vollema et al. Reference Vollema, Sitskoorn, Appels and Kahn2002). Our results suggest that FER deficits observed in schizophrenia and related disorders do not emerge solely as a result of disease-related confounds or secondary characteristics but instead may be a pre-existing or even predisposing neurocognitive feature that varies broadly in the normal population.
We have also shown that FER differences associated with psychosis vulnerability are not associated with more general differences in visual or face processing. Our results are consistent with those of Bediou et al. (Reference Bediou, Asri, Brunelin, Krolak-Salmon, D'Amato, Saoud and Tazi2007), who showed that schizophrenia patients and their relatives have FER impairments that are not related to deficits in another type of face processing. This specificity suggests that differences in the neural systems responsible for FER may be related to psychosis vulnerability and the expression of psychosis-like characteristics.
A polygenic model of vulnerability to schizophrenia (Gottesman & Shields, Reference Gottesman and Shields1967) suggests that vulnerability-related features may emerge in a continuous fashion across the spectrum of psychosis-proneness (Eysenck, Reference Eysenck and Eysenck1960; Chapman & Chapman, Reference Chapman and Chapman1980; Raine, Reference Raine2006). Differences in FER may, for example, reflect the expression of differing numbers of risk-conferring genes and hence were present even at moderate levels of psychosis-proneness in our samples (see Fig. 2). Differences in performance at moderate levels of psychosis-proneness also imply that reductions in FER ability are not attributable solely to early or subthreshold pathology in at-risk participants.
Our study was conducted using a sample recruited entirely on the internet. An increasingly large body of research demonstrates that results from populations tested over the internet are reliable and empirically valid (McGraw et al. Reference McGraw, Tew and Williams2000; Birnbaum, Reference Birnbaum2004; Gosling et al. Reference Gosling, Vazire, Srivastava and John2004; Kraut et al. Reference Kraut, Olson, Banaji, Bruckman, Cohen and Couper2004; Haworth et al. Reference Haworth, Harlaar, Kovas, Davis, Oliver, Hayiou-Thomas, Frances, Busfield, McMillan, Dale and Plomin2007; Wilmer et al. Reference Wilmer, Germine, Chabris, Chatterjee, Nakayama, Williams, Loken and Duchaine2010) and of broad theoretical interest (Owen et al. Reference Owen, Hampshire, Grahn, Stenton, Sajan, Burns, Howard and Ballard2010; Wilmer et al. Reference Wilmer, Germine, Chabris, Chatterjee, Nakayama, Williams, Loken and Duchaine2010). A recent analysis of data collected from our website (www.testmybrain.org) on a test of face recognition memory found that performance and reliability from the internet-based sample was the same as from a traditional laboratory-based sample (Wilmer et al. Reference Wilmer, Germine, Chabris, Chatterjee, Nakayama, Williams, Loken and Duchaine2010). Our average psychosis-proneness scores were also almost identical to those reported in a community sample with a similar gender distribution (Irwin, Reference Irwin2001). However, despite many precautions taken here to ensure valid data, it was not possible to monitor the performance of each participant in real time, control for biases in self-selection, and verify the accuracy of information provided by participants. These factors most probably added noise to the data and may have interacted with our results in ways that cannot be ascertained based on available data. Ultimately, testing over the internet allowed us to sample a large and diverse population that would not have been practically feasible if this study were conducted in a traditional laboratory setting. This large sample increased our ability to detect small but potentially meaningful effects on both our FER and face processing control tasks.
Variations in face emotion processing have been documented for several psychiatric disorders, including mood disorders (see Leppanen, 2006 for a review) and anxiety disorders (e.g. McClure et al. Reference McClure, Pope, Hoberman, Pine and Leibenluft2003). Thus, it is possible that our results were partially driven by the overlap between psychosis-like characteristics indexed by the interpersonal factor of the SPQ-B and social anxiety. FER ability was related to multiple subscales of the SPQ-B, however, including scores on the cognitive-perceptual factor, indicating that our results cannot be fully explained by overlap between mood/anxiety symptoms and psychosis-proneness.
Our results recommend an individual differences approach to psychosis-proneness. An individual differences approach has the advantage of complementing the increasing appreciation that schizophrenia and other psychotic disorders are likely to arise from the influence of many common genes of very small effect (Gottesman & Shields, Reference Gottesman and Shields1967; Purcell et al. Reference Purcell, Wray, Stone, Visscher, O'Donovan, Sullivan and Sklar2009; Shi et al. Reference Shi, Levinson, Duan, Sanders, Zheng, Pe'er, Dudbridge, Holmans, Whittemore, Mowry, Olincy, Amin, Cloninger, Silverman, Buccola, Byerley, Black, Crowe, Oksenberg, Mirel, Kendler, Freedman and Gejman2009; Stefansson et al. Reference Stefansson, Ophoff, Steinberg, Andreassen, Cichon, Rujescu, Werge, Pietiläinen, Mors, Mortensen, Sigurdsson, Gustafsson, Nyegaard, Tuulio-Henriksson, Ingason, Hansen, Suvisaari, Lonnqvist, Paunio, Børglum, Hartmann, Fink-Jensen, Nordentoft, Hougaard, Norgaard-Pedersen, Böttcher, Olesen, Breuer, Möller, Giegling, Rasmussen, Timm, Mattheisen, Bitter, Réthelyi, Magnusdottir, Sigmundsson, Olason, Masson, Gulcher, Haraldsson, Fossdal, Thorgeirsson, Thorsteinsdottir, Ruggeri, Tosato, Franke, Strengman, Kiemeney, Melle, Djurovic, Abramova, Kaleda, Sanjuan, de Frutos, Bramon, Vassos, Fraser, Ettinger, Picchioni, Walker, Toulopoulou, Need, Ge, Yoon, Shianna, Freimer, Cantor, Murray, Kong, Golimbet, Carracedo, Arango, Costas, Jönsson, Terenius, Agartz, Petursson, Nöthen, Rietschel, Matthews, Muglia, Peltonen, St Clair, Goldstein, Stefansson and Collier2009). The potential relationship between increasing vulnerability to developing psychosis and FER ability suggests that differences in social-emotional processing might contribute to the expression of psychosis-like traits and, ultimately, to psychosis development.
Acknowledgments
We thank B. Bediou and L. Garrido for providing us with the tasks and stimuli used in Experiments 1 and 2 respectively, and also K. Nakayama for his financial support of www.testmybrain.org, the website used to collect the current dataset. This research was supported by a National Science Foundation (NSF) Graduate Research Fellowship to L. Germine. The NSF had no further role in the research.
Declaration of Interest
None.





