Across species and methodologies, the amygdala has been implicated in fear expression and conditioning (for reviews see Etkin & Wager, Reference Etkin and Wager2007; Grupe & Nitschke, Reference Grupe and Nitschke2013; Kim et al., Reference Kim, Loucks, Palmer, Brown, Solomon, Marchante and Whalen2011; and Tovote, Fadok, & Lüthi, Reference Tovote, Fadok and Lüthi2015), suggesting that this structure may be core to the exaggerated and persistent fear that characterizes anxiety disorders (Shin & Liberzon, Reference Shin and Liberzon2010). Attesting to the role of the amygdala in anxiety, both clinically anxious populations and typical individuals with high levels of anxiety exhibit amygdala hyperactivity and altered patterns of amygdala functional connectivity (e.g., Bishop, Duncan, & Lawrence, Reference Bishop, Duncan and Lawrence2004; Etkin et al., Reference Etkin, Klemenhagen, Dudman, Rogan, Hen, Kandel and Hirsch2004; Hahn et al., Reference Hahn, Stein, Windischberger, Weissenbacher, Spindelegger, Moser and Lanzenberger2011; Hyde, Gorka, Manuck, & Hariri, Reference Hyde, Gorka, Manuck and Hariri2011; Kim, Gee, Loucks, Davis, & Whalen, Reference Kim, Gee, Loucks, Davis and Whalen2011; Sehlmeyer et al., Reference Sehlmeyer, Dannlowski, Schöning, Kugel, Pyka, Pfleiderer and Konrad2011; Sripada, Wang, Sripada, & Liberzon, Reference Sripada, Wang, Sripada and Liberzon2012). In contrast to robust findings from functional neuroimaging, the link between amygdala structure and anxiety remains inconclusive, with adult studies finding both positive (e.g., Baur, Hänggi, & Jäncke, Reference Baur, Hänggi and Jäncke2012; Lyons-Ruth, Pechtel, Yoon, Anderson, & Teicher, Reference Lyons-Ruth, Pechtel, Yoon, Anderson and Teicher2016; Schienle, Ebner, & Schafer, Reference Schienle, Ebner and Schäfer2011) and negative (e.g., Fisler et al., Reference Fisler, Federspiel, Horn, Dierks, Schmitt, Wiest and Soravia2013; Hayano et al., Reference Hayano, Nakamura, Asami, Uehara, Yoshida, Roppongi and Hirayasu2009; Rogers et al., Reference Rogers, Yamasue, Abe, Yamada, Ohtani, Iwanami and Kasai2009; Spampinato, Wood, De Simone, & Grafman, Reference Spampinato, Wood, De Simone and Grafman2009) relations between anxiety and amygdala volume. Given the mixed adult literature, studies examining the link between amygdala volume and anxiety at earlier developmental time points will help clarify the structural brain bases of anxiety.
Understanding the structural brain bases of anxiety is important because structural magnetic resonance imaging (MRI) data can inform our understanding of neural mechanisms while avoiding some practical confounds of functional neuroimaging work. Recent human and animal literature has found important links between amygdala structure and anxiety behaviors. Smaller amygdalae in mice have been linked to increased fear responsivity and glucocorticoid response (Yang et al., Reference Yang, Mozhui, Karlsson, Cameron, Williams and Holmes2008) and, in depression and bipolar disorder, individual differences in amygdala volume are related to functional hyperactivity (Kalmar et al., Reference Kalmar, Wang, Chepenik, Womer, Jones, Pittman and Blumberg2009; Siegle, Konecky, Thase, & Carter, Reference Siegle, Konecky, Thase and Carter2003). Further, interventions designed to impact stress and anxiety can alter amygdala volume (Hölzel et al., Reference Hölzel, Carmody, Evans, Hoge, Dusek, Morgan and Lazar2009), suggesting that volumetric data could provide a neurobiological measure of intervention efficacy. Practically, structural studies allow for data to be collected from a wide range of ages and functioning levels, including from those who cannot complete task-based designs, and structural data acquisition is less susceptible to motion artifacts and in-scanner anxiety (Dennis, Gotlib, Thompson, & Thomason, Reference Dennis, Gotlib, Thompson and Thomason2011). Recent evidence also suggests that structural neuroimaging can be used to differentiate anxiety subtypes (e.g., Hilbert, Lueken, Muehlhan, & Beesdo-Baum, Reference Hilbert, Lueken, Muehlhan and Beesdo-Baum2017; Hilbert et al., Reference Hilbert, Pine, Muehlhan, Lueken, Steudte-Schmiedgen and Beesdo-Baum2015), indicating the method's potential promise as a biomarker. Thus, structural neuroimaging studies of developmental anxiety offer important complementary insight to functional neuroimaging, helping to illuminate neurobiological mechanisms of anxious behaviors.
In particular, elucidating the relation between amygdala volume and anxiety in middle to late childhood (roughly ages 6–13) is important for two reasons. First, although anxiety disorders continue to develop into adolescence, middle to late childhood is a time when many anxiety disorders first begin to emerge (Beesdo, Knappe, & Pine, Reference Beesdo, Knappe and Pine2009; Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005, Reference Kessler, Angermeyer, Anthony, De Graaf, Demyttenaere, Gasquet and Kawakami2007). Thus, examining whether and how the relation between amygdala volume and anxiety changes in this age range, even in typical samples, may help illuminate neural mechanisms underlying the development of more severe anxiety later in life (Beesdo et al., Reference Beesdo, Knappe and Pine2009). Second, middle to late childhood represents an important time in amygdala development. Recent research suggests that amygdala volume peaks around 11 years, with slightly earlier maturation in females versus males and in the left versus right amygdala (Uematsu et al., Reference Uematsu, Matsui, Tanaka, Takahashi, Noguchi, Suzuki and Nishijo2012), although other studies have found peaks in early (Hu, Pruessner, Coupé, & Collins, Reference Hu, Pruessner, Coupé and Collins2013) and late adolescence (Wierenga, Langen, Oranje, & Durston, Reference Wierenga, Langen, Oranje and Durston2014), with inconsistent evidence for sex differences (either opposite trajectories Bramen et al., Reference Bramen, Hranilovich, Dahl, Forbes, Chen, Toga and Sowell2011; or no differences, Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014). Despite the mixed findings of structural maturation, functional data does suggest that approximately 10 years of age may represent an inflection point in connectivity between the prefrontal cortex and amygdala (Gabard-Durnam et al., Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014; Gee et al., Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013), a circuit that has been linked to fearful or anxious behaviors.
Despite the importance of understanding brain–behavior relations in developmental populations, few studies have examined the link between amygdala volume and anxiety in children and adolescents. Further, even this small set of studies has yielded inconsistent findings: anxiety has been linked to both increased (Albaugh et al., Reference Albaugh, Nguyen, Ducharme, Collins, Botteron, D'Alberto and Hudziak2017; De Bellis et al., Reference De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas and Ryan2000; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Thomas2010; van der Plas, Boes, Wemmie, Tranel, & Nopoulos, Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010; Weems, Klabunde, Russell, Reiss, & Carrión, Reference Weems, Klabunde, Russell, Reiss and Carrión2015) and decreased amygdala volume (McQueeny et al., Reference McQueeny, Padula, Price, Medina, Logan and Tapert2011; Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013; Strawn et al., Reference Strawn, Hamm, Fitzgerald, Fitzgerald, Monk and Phan2015). Understanding the reasons for these contradictory findings may clarify the mechanisms linking amygdala volume to anxiety. Specifically, there are several candidate explanations for why previous developmental studies have found divergent results.
First, given that the amygdala continues to develop through middle to late childhood, one possibility is that the relation between anxiety and amygdala volume changes with age. Most of the studies finding a positive relation examined participants into middle adolescence (e.g., around ages 16–17), with one study finding that a positive relation between amygdala volume and posttraumatic stress disorder diagnosis was only present in participants older than 15.5 years, with younger participants showing a trend toward negative relations (Weems et al., Reference Weems, Klabunde, Russell, Reiss and Carrión2015). However, one study examining children aged 7–9 years found a positive relation between anxiety traits and amygdala volume (Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014), and negative relations between amygdala volume and anxiety have also been found in adults (e.g., Fisler et al., Reference Fisler, Federspiel, Horn, Dierks, Schmitt, Wiest and Soravia2013; Hayano et al., Reference Hayano, Nakamura, Asami, Uehara, Yoshida, Roppongi and Hirayasu2009; Rogers et al., Reference Rogers, Yamasue, Abe, Yamada, Ohtani, Iwanami and Kasai2009; Spampinato et al., Reference Spampinato, Wood, De Simone and Grafman2009). Further complicating the study of age effects, amygdala development appears to follow different trajectories for males and females (Bramen et al., Reference Bramen, Hranilovich, Dahl, Forbes, Chen, Toga and Sowell2011; but see Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014), and there is some evidence that the developmental relations between volume and anxiety are impacted by sex (van der Plas et al., Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010). Anxiety also has different prevalence between males and females, although sex differences typically do not emerge until late childhood or early adolescence (Beesdo et al., Reference Beesdo, Knappe and Pine2009; Lewinsohn, Gotlib, Lewinsohn, Seeley, & Allen, Reference Lewinsohn, Gotlib, Lewinsohn, Seeley and Allen1998). Thus, it is unknown if age or sex may be responsible for contradictory findings in previous developmental samples.
Second, another potential explanation for the inconsistent literature is that different methods of anxiety assessment (e.g., differences in informants, measures, and sample characteristics) may yield different results. Although most studies examining amygdala volume and anxiety in typical adults have used self-reported anxiety (e.g., Baur et al., Reference Baur, Hänggi and Jäncke2012; Spampinato et al., Reference Spampinato, Wood, De Simone and Grafman2009), almost all studies of amygdala volume in typical children have relied on parent report (e.g., Albaugh et al., Reference Albaugh, Nguyen, Ducharme, Collins, Botteron, D'Alberto and Hudziak2017; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Thomas2010; van der Plas et al., Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010), with the one exception being in older adolescents (McQueeny et al., Reference McQueeny, Padula, Price, Medina, Logan and Tapert2011). This reliance on parent report may be problematic given that the discrepancy between parent-reported anxiety and child self-report begins early in childhood (e.g., Comer & Kendall, Reference Comer and Kendall2004; Engel, Rodrigue, & Geffken, Reference Engel, Rodrigue and Geffken1994). Previous research has indicated that, at least in typical samples, child report may better reflect real-world symptomatology (Cosi, Canals, Hernández-Martinez, & Vigil-Colet, Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010) and that parental relative underreporting of anxiety symptoms (Cosi et al., Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010; Muris, Merckelbach, Van Brakel, & Mayer, Reference Muris, Merckelbach, Van Brakel and Mayer1999) may lead to statistical floor effects. In addition to issues of informant, some previous studies have used scales that measure internalizing behaviors more broadly (e.g., a combined anxiety/depression scale; Albaugh et al., Reference Albaugh, Nguyen, Ducharme, Collins, Botteron, D'Alberto and Hudziak2017; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014). Further, it is possible that a more precise investigation of different types of anxiety (e.g., social anxiety vs. generalized anxiety) may reveal different patterns of correlation with amygdala volume, as functional neuroimaging literature has indicated dissociations between anxiety subtypes in adults (Blair et al., Reference Blair, Shaywitz, Smith, Rhodes, Geraci, Jones and Jacobs2008) and children (Kessel, Kujawa, Proudfit, & Klein, Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015). Finally, much developmental research has examined clinical populations. Research in a typical sample may offer insight into the brain-based precursors of later clinical anxiety (Westenberg, Gullone, Bokhorst, Heyne, & King, Reference Westenberg, Gullone, Bokhorst, Heyne and King2007) and give insight into the relation between amygdala volume and anxious traits, traits that may be disruptive even at subclinical levels (Bell-Dolan, Last, & Strauss, Reference Bell-Dolan, Last and Strauss1990). Ultimately, examining brain–behavior relations across multiple ages, different informants, and varied types of anxiety within typical samples is critical, given that the small set of existing studies, with their wide range of methods and samples, precludes a meta-analytic approach.
Thus, the goal of the current study is to disambiguate, in a single developmental sample, factors that may influence the relation between amygdala volume and anxiety traits, and in doing so, to clarify both past findings and the potential mechanisms driving these brain–behavior associations. To this end, we examined the relation between parent-reported and child-reported anxiety in a sample of typical children aged 6–13 years. We analyzed three specific moderators of the relation between amygdala volume and anxiety: (a) informant (i.e., parent vs. child report), (b) sex, and (c) age. We did not hypothesize about whether the link between amygdala volume and anxiety traits would be positive or negative across these moderators. Rather, the goal of the study was to attempt to disambiguate factors driving the mixed findings of prior developmental research.
Method
Subjects
Eighty-four children were recruited via random sampling from a database of local families. All children were full-term, native English speakers, with normal or corrected to normal hearing and vision. As assessed via parent report, children had no history of psychiatric, psychological, or neurological conditions, no first-degree relatives with autism or schizophrenia, and no contraindications for MRI scanning. Racial and ethnic information were collected from a subset of n = 46 participants. Of this subset, 34 children (74%) were White and non-Hispanic/Latino, 6 were of more than one race, 2 were Black, 2 were White and Hispanic/Latino, 1 was Asian, and 1 was Native Hawaiian or Pacific Islander. Out of the subset of participants (n = 73) with household income and parental education data, the majority (81.0%) had a household income of over $75,000/year and the majority (63.0%) also had at least one parent with a graduate degree. Structural MRI and behavioral data were collected at two separate visits within 6 weeks of each other. Minor and parental consents were obtained from all participants, as approved by the Internal Review Board at the University of Maryland.
Behavioral data acquisition
The Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999) is a 41-item questionnaire that yields five anxiety factors: somatic/panic, generalized anxiety, separation anxiety, social phobia, and school phobia. We used two versions of the SCARED, one in which the child reported on his or her own anxiety (child SCARED) and one in which the parent reported on the anxiety levels of the child (parent SCARED). The same items are used on the child and parent SCARED, but pronouns are adjusted based on the informant, that is, “I feel …” versus “My child feels …” (Birmaher et al., Reference Birmaher, Khetarpal, Brent, Cully, Balach, Kaufman and Neer1997). Participants respond to prompts on a 3-point Likert scale (0 = not true or hardly ever true, 1 = sometimes true, and 2 = true or often true), with higher scores indicating greater anxiety. The SCARED has good internal validity, test–retest reliability, discriminant validity, and cross-cultural external validity (Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999; Su, Wang, Fan, Su, & Gao, Reference Su, Wang, Fan, Su and Gao2008; Weitkamp, Romer, Rosenthal, Wiegand-Grefe, & Daniels, Reference Weitkamp, Romer, Rosenthal, Wiegand-Grefe and Daniels2010). Parent-report and child-report have shown low to moderate levels of agreement (Cosi et al., Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010; Dirks et al., Reference Dirks, Weersing, Warnick, Gonzalez, Alton, Dauser and Woolston2014; Muris et al., Reference Muris, Merckelbach, Van Brakel and Mayer1999; Pereira et al., Reference Pereira, Muris, Barros, Goes, Marques and Russo2015), with children tending to report higher levels of anxiety overall and larger discrepancies emerging in somatic/panic and generalized anxiety (Cosi et al., Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010; Wren et al., Reference Wren, Berg, Heiden, Kinnamon, Ohlson, Bridge and Bernal2007).
In the current study, each child and each parent independently completed the SCARED, yielding both a child-report and a parent-report measure of anxiety. Children completed the SCARED by hand with the help of a researcher. The researcher read each item to the child and asked him or her to mark the box that corresponded with his or her feelings about that item. The researcher told the child that his or her answers would be kept private to ensure accurate responses on the self-report. One parent of each participant was instructed to complete the parent SCARED independently by hand. Consistent with previous SCARED research, the current study computed raw scores for each factor, which were then summed to obtain an overall parent SCARED score and child SCARED score for each participant.
MRI data acquisition
High-resolution T1-weighted images were acquired using a single Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions) using one three-dimensional T1 magnetization-prepared rapid gradient-echo sequence (176 contiguous sagittal slices, voxel size = 1.0 × 1.0 × 1.0 mm; repetition time/echo time/inversion time = 1900 ms/2.52 ms/900 ms; flip angle = 9°; pixel matrix = 256 × 256). For the final sample, data from 22 children were collected using a 12-channel head coil, and data for the other 50 children were collected on a 32-channel head coil. Age, amygdala volume, and SCARED scores did not significantly differ between the two groups of children (ps > .05), and inclusion of channel type as a covariate in analyses did not alter any patterns of results. Thus, we collapsed together both groups into a single sample for subsequent analyses.
The imaging data were analyzed using FreeSurfer software (Version 5.1.0). Freesurfer's automated segmentation of amygdala volume is highly correlated with expert hand tracing methods (Morey et al., Reference Morey, Petty, Xu, Hayes, Wagner, Lewis and McCarthy2009). FreeSurfer first automatically compared each participant's T1-weighted image to a probabilistic atlas, the use of which has been validated in pediatric populations (Burgund et al., Reference Burgund, Kang, Kelly, Buckner, Snyder, Petersen and Schlaggar2002; Ghosh et al., Reference Ghosh, Kakunoori, Augustinack, Nieto-Castanon, Kovelman, Gaab and Fischl2010), and then established boundaries for gray matter, white matter, and pial. Two trained, blind coders visually inspected the volumetric parcellation map of each participant, and edited the pial parcellation boundaries where necessary. After edits were made and agreement was reached between coders, automatic segmentation was rerun, yielding estimates of total gray matter volume and left and right amygdala volume. Total gray matter volume was calculated using the automatic algorithm in FreeSurfer. This algorithm sums together values from left cortex, right cortex, subcortical structures and cerebellum. For cortex, gray matter is calculated based on the volume inside the pial boundaries, subtracting out white matter and subcortical structures. Given concerns about unsupervised amygdala identification in pediatric populations (Schoemaker et al., Reference Schoemaker, Buss, Head, Sandman, Davis, Chakravarty and Pruessner2016), a trained coder compared the amygdala mask identified by FreeSurfer to the original T1 image to ensure accuracy (Figure 1).
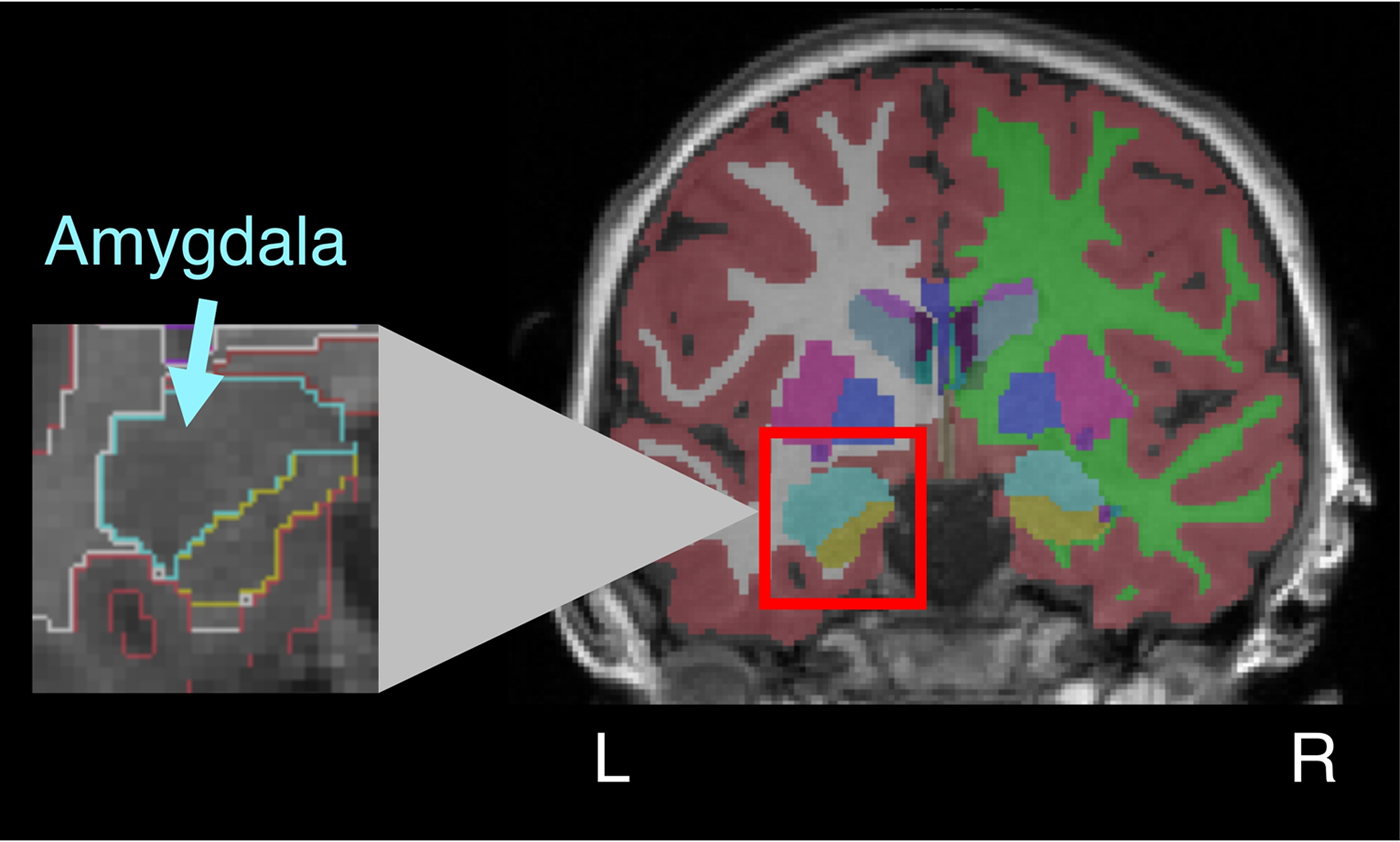
Figure 1. (Color online) Amygdala volume in childhood. The figure depicts a sample coronal slice from a participant (aged 7.58 years) after anatomical data were processed. After automatic parcellation was completed, coders inspected each map, made any needed edits to pial surface segmentation, and then reran the automatic parcellation. As a final step, the anatomical boundaries of the amygdala on the T1 image were manually compared to the computer-generated amygdala segmentation (depicted in teal online). Left and right amygdala volumes were computed separately.
From the original sample of 84 children, 5 participants (5 female, mean age = 9.82 years) did not have the parent SCARED measure, 1 participant (1 male, age = 8.49 years) did not complete the structural scan due to discomfort in the scanner, 4 participants (2 male, mean age = 9.27 years) did not have usable FreeSurfer data due to excessive motion in the scanner, as identified by overall poor image contrast, and 2 participants (2 male, mean age = 9.81 years) were excluded due to inaccurate amygdala/hippocampal segmentation revealed during manual inspection. The final sample (N = 72) did not differ from the excluded sample in age, sex, or anxiety (Table 1).
Table 1. Descriptive statistics

Note: All values are means (standard deviations). For sex, the groups were compared using a chi-squared test; for all other variables, a t test was used. Two children in the excluded sample were missing IQ data, two were missing child Screen for Child Anxiety Related Emotional Disorders (SCARED) scores, and five were missing parent SCARED scores. IQ was assessed using the Kaufman Brief Intelligence Test (Kaufman & Kaufman, Reference Kaufman and Kaufman2004). No brain-based averages are given for the excluded sample, because many in this sample were excluded due to poor data quality.
Statistical methods
Previous investigations of amygdala volume and anxiety have used a variety of methods to normalize across head size, including statistical corrections for total intracranial volume (e.g., Fisler et al., Reference Fisler, Federspiel, Horn, Dierks, Schmitt, Wiest and Soravia2013; van der Plas, Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010) and total gray matter (Lyons-Ruth et al., Reference Lyons-Ruth, Pechtel, Yoon, Anderson and Teicher2016), as well as dividing amygdala volume by total intracranial volume (e.g., Rogers et al., Reference Rogers, Yamasue, Abe, Yamada, Ohtani, Iwanami and Kasai2009). A meta-analysis has indicated that subcortical structures most closely scale with gray matter volume and that controlling via regression may best prevent bias (Van Petten, Reference Van Petten2004). We thus controlled for total gray matter volume in all of our brain-based analyses.
We conducted both planned and exploratory brain–behavior analyses. In our planned analysis, we had two core goals: (a) to establish the relation between amygdala volume and anxiety, and, if this relation was significant, (b) to determine if this brain–behavior relation was moderated by informant, sex, or age. To accomplish the first goal, we calculated the relation between amygdala volume, which was corrected for age, sex, and total gray matter volume, and total child SCARED score. We then repeated this analysis for total parent SCARED score. For these tests, we analyzed the right and left amygdala separately, as previous developmental literature has found relations between anxiety and amygdala volume specific to both the right (e.g., De Bellis et al., Reference De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas and Ryan2000; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013; Weems et al., Reference Weems, Klabunde, Russell, Reiss and Carrión2015) and the left (e.g., Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014) amygdala. Although there is substantial literature on amygdala lateralization (for reviews, see Fusar-Poli, Placentino, Carletti, Landi, & Abbamonte, Reference Fusar-Poli, Placentino, Carletti, Landi and Abbamonte2009; McMenamin & Marsolek, Reference McMenamin and Marsolek2013; and Sergerie, Chochol, & Armony, Reference Sergerie, Chochol and Armony2008), the lack of a clear laterality pattern in previous brain–behavior results meant that we did not have specific laterality hypotheses. Given the analysis of both the left and the right amygdala, and both parent and child SCARED scores, we examined our data using Bonferroni-corrected p values (α = 0.0125).
Our planned moderation analysis had two phases. In the first phase, we tested whether informant significantly affected brain–behavior relations. Specifically, for both the left and the right amygdala, we compared brain–behavior relations for the child SCARED to brain–behavior relations for the parent SCARED. The second phase of moderation analyses examined whether age or sex moderated the brain–behavior relations found to be significant after correcting for multiple comparisons. As we had no a priori reason to expect an interaction between age and sex, we planned to test two models: (a) a moderation model testing whether males showed significantly different relations between amygdala volume and anxiety as compared to females and (b) a moderation model testing whether children's age affected the strength of this brain–behavior relation. We did not have specific hypotheses about the direction of age or sex effects.
After conducting our planned analyses, we continued with exploratory analyses in order to determine whether brain–behavior relations differed across various SCARED subscales. Given the number of subscales (k = 5) and their interdependence, we did not correct for multiple comparisons in this analysis. Rather, given that much past literature has separately examined SCARED subscales (e.g., Becker, Jensen-Doss, Kendall, Birmaher, & Ginsburg, Reference Becker, Jensen-Doss, Kendall, Birmaher and Ginsburg2016; Kessel et al., Reference Kessel, Kujawa, Hajcak Proudfit and Klein2015; Scaini et al., Reference Scaini, Ogliari, De Carolis, Bellodi, Di Serio and Brombin2017), the goal of these analyses was to give descriptive statistics and suggest future directions for analysis.
Data were analyzed in SPSS 24.0. For moderation analyses, we used the SPSS add-on package PROCESS (Hayes, Reference Hayes2013) in order to determine the conditional effects of anxiety on amygdala volume. For dichotomous moderators (e.g., sex), PROCESS produces effect estimates at each value of the moderator. For continuous moderators (i.e., age), PROCESS produces effect estimates at the mean value of the moderator, as well as at ±1 SD. We further employed the Johnson–Neyman technique (Bauer & Curran, Reference Bauer and Curran2005; Preacher, Curran, & Bauer, Reference Preacher, Curran and Bauer2006) in PROCESS, in order to determine the moderator value(s) at which the relation between amygdala volume and anxiety transitioned in significance.
Results
Behavioral results
Consistent with prior literature, there was low agreement between the child SCARED total and parent SCARED total (Table 2), and scores were consistently higher on the child SCARED. For both child and parent report, child age was negatively correlated with total anxiety, although this correlation was not significant for parent report (child: r = –.24, p = .047; parent: r = –.15, p = .22). Total anxiety scores did not differ by sex for either child or parent report (ps > .1). Neither age nor sex moderated informant discrepancy (Fs < 1). That is, informant discrepancies were not significantly different for males versus females or for children of different ages.
Table 2. Distributions of child- and parent-reported anxiety and correlations between parent and child report

Note: SCARED, Screen for Child Anxiety Related Emotional Disorders.
*p < .05. ***p < .001.
Neuroanatomical results
Right amygdala volume was significantly larger than left amygdala volume, t (71) = 2.62, p = .01, although the volumes of the structures were significantly correlated (r = .636, p < .001). Left and right amygdala volumes were significantly correlated with total gray matter volume (left: r = .250, p < .05; right: r = .368, p < .01), which was controlled for in subsequent brain–behavior analyses. Controlling for total gray matter volume, left and right amygdala volume were significantly correlated with age (left: r = .344, p < .01; right: r = .393, p < .001). Males showed significantly larger left and right amygdala volumes than females: left, 1694.80 versus 1567.24 mm3, t (70) = 2.77, p = .007; right, 1751.91 versus 1612.41 mm3, t (70) = 3.57, p < .001, when not controlling for total gray matter volume. However, after controlling for total gray matter volume, the effects of sex on amygdala volume were no longer significant: right, F (1, 69) = 3.72, p = .058; left, F (1, 69) = 3.20, p = .078.
Given recent evidence that the rate of amygdala maturation may differ between males and females (e.g., Hu et al., Reference Hu, Pruessner, Coupé and Collins2013; Uematsu et al., Reference Uematsu, Matsui, Tanaka, Takahashi, Noguchi, Suzuki and Nishijo2012), we examined the moderating effect of sex on the relation between amygdala volume and age. Males and females had very similar age distributions in the current sample (male: 10.23 years, SD = 1.92, range = 6.89–13.99; female: 10.18 years, SD = 1.90, range = 6.64–13.99). For the left amygdala, sex had a marginal moderation effect, F (1, 67) = 3.04, p = .086, such that, controlling for total gray matter volume, males had a stronger relation between amygdala volume and age (male: r = .451, p = .007; female: r = .140, p = .41). The effect was in the same direction but not significant for the right amygdala, F (1, 67) = 2.73, p = .11; male r = .512, p = .002; female r = .248, p = .145.
Relation between amygdala volume and anxiety
Controlling for age, sex, and total gray matter volume, total child SCARED scores were negatively correlated with both left and right amygdala volume (Table 3, Figure 2), and the correlation with left amygdala remained significant after correcting for multiple comparisons. The strength of the relation between amygdala volume and anxiety did not significantly differ between the left and the right amygdala (z = –0.58, p = .56).

Figure 2. Partial relations between amygdala volume and child- and parent-reported anxiety. Children's (a) left and (b) right amygdala volume was correlated with their self-reported anxiety, although only the correlation with (a) the left amygdala volume survived correction for multiple comparisons. Children's (c) left and (d) right amygdala volume was not significantly correlated with parent-reported anxiety. Anxiety was measured by the total score on the Screen for Child Anxiety Related Emotional Disorders. All values are corrected for total gray matter volume, age, and sex. Gray lines around the line of best fit represent 95% confidence intervals. The correlation between child-reported anxiety and amygdala volume is not significantly different than the correlation between parent-reported anxiety and amygdala volume.
Table 3. Correlations between amygdala volume and child-reported anxiety

Note: Full sample correlations are controlling for age, sex, and total grey matter volume. Sex-stratified correlations control for age and total grey matter volume.
†p < .1. *p < .05. **p < .01. ***p < .001.
Compared to the correlations between total child SCARED scores and amygdala volume, total parent SCARED scores showed weaker brain–behavior relations for both the left and the right amygdala. Direct comparison of parent and child brain–behavior correlations, however, revealed no significant differences (left: z = 0.99, p = .32; right: z = 1.19, p = .23).
Effects of age and sex
Brain–behavior relations are stronger in boys
We next examined whether brain–behavior relations differed for males and for females. Given that only the correlation between left amygdala volume and total child SCARED survived correction for multiple comparisons, we examined whether sex significantly moderated this relation. Controlling for total gray matter volume and age, sex significantly moderated the relation between child-reported anxiety and left amygdala volume, F (1, 66) = 4.97, p = .029. Post hoc analyses found that correlations between left amygdala volume and child-reported anxiety were greater for males than females (Table 3; Figure 3).

Figure 3. Moderating effect of sex on brain–behavior relations. (a) Pyramid histogram of the distribution of age by sex. (b) Relations between left amygdala volume and total child-reported anxiety, stratified by sex. All values are corrected for total gray matter volume and age. Gray lines around the line of best fit represent 95% confidence intervals. Sex significantly moderated the relation between anxiety and amygdala volume, such that males showed stronger correlations than females (p < .05).
Brain–behavior relations weaken with age
Our next moderation analysis examined the effect of age on the relation between amygdala volume and anxiety. We again examined the relation between the left amygdala and child SCARED. Controlling for sex and total gray matter volume, age significantly moderated the link between left amygdala volume and total child SCARED scores, F (1, 66) = 4.66, p = .035; Figure 4. Follow-up Johnson–Neyman analysis revealed a significant inflection point at 11.18 years, such that children younger than this age (n = 47) showed a significant negative relation between amygdala volume and total anxiety, and children older than this cutpoint (n = 25) showed no relation.

Figure 4. (Color online) The relation between left amygdala volume and child-reported anxiety, as measured by the Screen for Child Anxiety Related Emotional Disorders, is significantly moderated by age, such that older children show weaker brain–behavior relations than younger children. Moderation analyses controlled for sex and total gray matter volume. The lines represent fit at the mean age and ±1 SD. Johnson–Neyman analysis revealed an inflection point at 11.18 years of age, where the relation between anxiety and amygdala volume changed from significant to nonsignificant.
Given that we found effects of both age and sex on brain–behavior relations, we next conducted a post hoc analysis examining potential interactions between age and sex on the relation between amygdala volume and anxiety, specifically in the model examining left amygdala volume and total child SCARED. The interaction between age and sex was not significant; that is, the effect of weakening brain–behavior relations with age was not significantly greater for males than for females, F (1, 64) = 0.05, p = .83.
Effects of SCARED subscale
For the child SCARED, each subscale was significantly correlated behaviorally with all other subscales (rs = .29–.65). For the parent SCARED, all pairwise subscale correlations were significant except for the relation between school and somatic/panic anxiety (r = .12, p = .33; all other rs = .26–.61). Levels of informant discrepancy significantly differed between subscales. Specifically, the social subscale showed significantly more concordance than the school, separation, and social phobia subscales (ps < .05) and marginally more concordance than the generalized subscale (p = .054). An important caveat to these analyses, however, is that for several of the subscales, the parent report produced a very limited range of responses. For example, for each of the somatic, separation, and school subscales, over 70% of parents reported total subscale scores of 0, 1, or 2. Thus, it is possible that more nuanced measures would reveal different patterns of informant discrepancy.
In additional, exploratory analyses, we repeated our brain–behavior analyses for the five subscales of the child SCARED by correlating each subscale with left and right amygdala volume. The child reports for the somatic/panic subscale showed the strongest brain–behavior relation and generalized anxiety the weakest, but the differences between subscales were not significant (Table 3).
Discussion
The current study examined, in a single typical, developmental sample, the moderating effects of informant, sex, and age on the relation between amygdala volume and individual differences in anxiety traits. Overall, we found a significant negative relation between children's self-reported anxiety and amygdala volume. Although we did not find significant effects of informant, the strongest correlations emerged between children's self-reported anxiety and amygdala volume. We further found significant moderating effects for sex and age; boys showed stronger brain–behavior relations than girls, and brain–behavior relations significantly weakened with age. These findings help to clarify previously contradictory literature and help suggest neurobiological mechanisms underlying anxiety in middle to late childhood.
Using brain–behavior relations to understand informant discrepancy
Previous research on informant discrepancy has been hampered by difficulty in determining true symptomatology or impairment (see De Los Reyes & Kazdin, Reference De Los Reyes and Kazdin2005; Dirks, De Los Reyes, Briggs-Gowan, Cella, & Wakschlag, Reference Dirks, De Los Reyes, Briggs-Gowan, Cella and Wakschlag2012; Martel, Markon, & Smith, Reference Martel, Markon and Smith2017, for theoretical and methodological reviews of informant discrepancy). Examining correlations with physiological (e.g., Bitsika, Sharpley, Andronicos, & Agnew, Reference Bitsika, Sharpley, Andronicos and Agnew2015) or brain-based measures provides a novel method to assess the validity of various informants.
In this sample, consistent with previous studies of nonclinical samples (Cosi et al., Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010; Pereira et al., Reference Pereira, Muris, Barros, Goes, Marques and Russo2015; Wren, Bridge, & Birmaher Reference Wren, Bridge and Birmaher2004; although see Kircanski et al., Reference Kircanski, Zhang, Stringaris, Wiggins, Towbin, Pine and Brotman2017), correlations between child and parent SCARED were fairly weak, with self-reported anxiety significantly higher than parent-reported anxiety. That is, children reported themselves to be more anxious than their parents reported them to be. Two possibilities thus arise: that children overreport their anxiety or that parents underreport their child's anxiety. The brain–behavior correlations provide a way to address these two possibilities; assuming a biological model for anxiety disorders, the fact that children's reports of their own anxiety show a significant correlation with amygdala volume supports the validity of child report. The brain-based evidence from the current sample converges with existing findings that child SCARED scores better correlate with clinical interviews than parent SCARED scores (Cosi et al., Reference Cosi, Canals, Hernández-Martinez and Vigil-Colet2010), that self-reported anxiety is more strongly correlated with cortisol levels (Bitsika et al., Reference Bitsika, Sharpley, Andronicos and Agnew2015), and that parents overestimate children's well-being more generally (Lagattuta, Sayfan, & Bamford, Reference Lagattuta, Sayfan and Bamford2012).
Although the findings from the current study support the validity of child self-report, they do not explain why informant discrepancy arises. One explanation for parental underreport, at least in typical populations, is that some anxiety symptoms are not behaviorally observable and that agreement tends to be higher for observable symptoms (Comer & Kendall, Reference Comer and Kendall2004). In the current study, and consistent with prior research (e.g., Wren et al., Reference Wren, Berg, Heiden, Kinnamon, Ohlson, Bridge and Bernal2007), informant discrepancy was lowest for the social phobia subscale and largest for the somatic/panic subscale, on which children self-reported over four times the level of symptomatology than their parents reported. The somatic/panic subscale contains items about internal physical states (e.g., heart racing), whereas social phobia items may be more observable, especially at younger ages when parents are more involved in their children's social schedules. Complicating the study of informant discrepancy, however, is that parent-reported SCARED values also suffered from restriction of range due to their low estimates of child anxiety (e.g., the mean parental score for somatic/panic was 1.04). The restriction of range on the parent SCARED is an important consideration in future studies of anxiety, as it is possible that more nuanced measures of parental report may reveal brain–behavior relations.
Future studies of amygdala volume and anxiety should also measure parents’ own anxiety and amygdala volume, in both clinical and typical samples. There is some evidence that parental overreporting may be linked to parental anxiety and psychopathology (Becker et al., Reference Becker, Jensen-Doss, Kendall, Birmaher and Ginsburg2016; Frick, Silverhorn, & Evans, Reference Frick, Silverthorn and Evans1994), and, although nonclinical samples generally show parental underreporting versus child report, some clinical samples show the opposite pattern (Blakeley-Smith, Reaven, Ridge, & Hepburn, Reference Blakeley-Smith, Reaven, Ridge and Hepburn2012; Phares & Danforth, Reference Phares and Danforth1994; Salbach-Andrae, Klinkowski, Lenz, & Lehmkuhl, Reference Salbach-Andrae, Klinkowski, Lenz and Lehmkuhl2009, but see Weitkamp et al., Reference Weitkamp, Romer, Rosenthal, Wiegand-Grefe and Daniels2010). Further, there may be genetic factors underlying both susceptibility for internalizing disorders and amygdala volume (Montag, Weber, Fliessbach, Elger, & Reuter, Reference Montag, Weber, Fliessbach, Elger and Reuter2009). Ultimately, although examining links with neurobiology may provide new perspectives on informant discrepancy, the critical question of interest to children, parents, and clinicians is how these convergent anxiety measures relate to real-world functioning. The current study does not link amygdala volume to long-term scholastic or social outcomes or to response to an anxiety intervention. Going forward, combining neurobiological studies with these behavioral and clinical approaches will offer the best insight into informant discrepancy.
Using moderators of brain–behavior relations to understand neural mechanisms of anxiety
The finding of a negative relation between children's self-report of anxiety and amygdala volume is consistent with some existing adult (e.g., Fisler et al., Reference Fisler, Federspiel, Horn, Dierks, Schmitt, Wiest and Soravia2013; Hayano et al., Reference Hayano, Nakamura, Asami, Uehara, Yoshida, Roppongi and Hirayasu2009; Rogers et al., Reference Rogers, Yamasue, Abe, Yamada, Ohtani, Iwanami and Kasai2009; Spampinato et al., Reference Spampinato, Wood, De Simone and Grafman2009) and developmental (e.g., McQueeny et al., Reference McQueeny, Padula, Price, Medina, Logan and Tapert2011; Milham et al., Reference Milham, Nugent, Drevets, Dickstein, Leibenluft, Ernst and Pine2005; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013; Strawn et al., Reference Strawn, Hamm, Fitzgerald, Fitzgerald, Monk and Phan2015) literature and is contrary to previous findings of a nonexistent or positive relation between anxiety and amygdala volume (e.g., De Bellis et al., Reference De Bellis, Casey, Dahl, Birmaher, Williamson, Thomas and Ryan2000; Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Thomas2010; van der Plas et al., Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010). Whereas positive associations may suggest that increased dendritic branching or spine density underlies anxiety (e.g., Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Thomas2010), negative relations suggest alternative mechanisms. Although these precise mechanisms are yet unknown, there are a few candidate explanations. Persistent amygdala hyperactivation, which may characterize anxiety, could lead to glutamatergic excitotoxicity and subsequent reduction of amygdala volume (Siegle et al., Reference Siegle, Konecky, Thase and Carter2003). Although this exact causal mechanism has not been proven in humans, decreased amygdala volume has been linked to individual differences in amygdala hyperactivity in depression and bipolar disorder (Kalmar et al., Reference Kalmar, Wang, Chepenik, Womer, Jones, Pittman and Blumberg2009; Siegle et al., Reference Siegle, Konecky, Thase and Carter2003). An alternative explanation is that rather than a history of hyperactivity causing a reduction in amygdala size, a smaller amygdala may predispose one to anxiety. Supporting this possibility, mice with smaller amygdalae, specifically basolateral amygdala, show stronger fear responses and increased glucocorticoid response (Yang et al., Reference Yang, Mozhui, Karlsson, Cameron, Williams and Holmes2008). A third possibility is that some other factor is driving both the reduction in amygdala volume and the increase in anxiety. This factor remains unknown, but certain genotypes have been associated with both traits (Montag et al., Reference Montag, Weber, Fliessbach, Elger and Reuter2009; Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). Further complicating any discussion of mechanisms is that the direction of the relation between amygdala volume and anxiety may change throughout development.
In the current sample, the relation between amygdala volume and anxiety was strongest at younger ages, with an inflection point emerging around age 11. After this age, the link between amygdala volume and anxiety was no longer significant. The mechanisms driving these age effects are unknown. One simple explanation is that decreased accuracy in self-report around age 11 could lead to the null finding observed in the older age group. Although future research should include more clinical and behavioral anxiety measures to address this possibility, there is no evidence to suggest that older children are worse at self-report than younger children.
Several other explanations of the age effects are possible, and future longitudinal studies would be needed to disambiguate between them. One explanation of the age-related findings is a delayed growth model. In this model, smaller amygdala volume may predispose children to develop anxiety, but as children age, their amygdala volume “catches up,” although the anxiety remains, and the statistical relation between amygdala size and anxiety disappears. In an alternative, but not orthogonal, model, early versus later onset anxiety may show different brain bases. Speculatively, early onset anxiety may be caused by neurobiological vulnerabilities rather than environmental experiences, whereas anxiety symptoms that emerge after puberty may be more varied in their etiology, producing, at the group level, a null correlation with amygdala volume. Yet another possibility is that the mechanisms linking amygdala volume and anxiety are consistent throughout development, but the onset of puberty complicates the discovery of that relation at a group level, given that puberty has been linked to differential rates of amygdala growth (Bramen et al., Reference Bramen, Hranilovich, Dahl, Forbes, Chen, Toga and Sowell2011). Finally, if the main mechanism linking anxiety and amygdala volume is that anxiety causes amygdala reduction, one might expect an increase in brain–behavior relations with age. The fact that we did not find this pattern indicates that these effects may have a sensitive period, such that late onset anxiety does not have the same effect as early onset anxiety. The current cross-sectional study, however, can only speculate about the causal relation between variables.
In addition to the effects of age, the current study found stronger brain–behavior relations in boys than in girls. One potential explanation is that boys’ amygdalae mature more slowly, potentially due to differences in pubertal timing (Bramen et al., Reference Bramen, Hranilovich, Dahl, Forbes, Chen, Toga and Sowell2011), and thus, the age range of the current study captures a key period of amygdala development for boys, but not for girls. This possibility is supported by the fact that boys, but not girls, show a significant relation between amygdala volume and age in the current sample. If this explanation is true, then studying girls in a younger age range (such as 4–10 years) might reveal similar negative correlations with amygdala volume. Alternatively, the brain bases of anxiety may be different across sexes (Bangasser & Valentino, Reference Bangasser and Valentino2014; Hamann, Reference Hamann2005; Maeng & Milad, Reference Maeng and Milad2015). For example, in typical adults, sex moderates the relation between amygdala hyperactivity and trait anxiety (Dickie & Armony, Reference Dickie and Armony2008), and in adolescents, differences in amygdala blood flow mediate the relation between sex and trait anxiety (Kaczkurkin et al., Reference Kaczkurkin, Moore, Ruparel, Ciric, Calkins, Shinohara and Gennatas2016). Further, testosterone has been linked to increased amygdala activity (Derntl et al., Reference Derntl, Windischberger, Robinson, Kryspin-Exner, Gur, Moser and Habel2009; van Wingen et al., Reference van Wingen, Zylicz, Pieters, Mattern, Verkes, Buitelaar and Fernández2009), suggesting that pubertal onset may have different effects for males versus females. Future studies should also include pubertal measures, both hormonal and self-report, to disentangle age, sex, and puberty effects (cf. Blakemore, Burnett, & Dahl, Reference Blakemore, Burnett and Dahl2010; Sisk & Zehr, Reference Sisk and Zehr2005).
Although we did not find significant differences for the right versus left amygdala, effects were numerically stronger in the left amygdala, a finding that should be explored further in future work. How these findings fit with existing literature is unclear, particularly because the existing volumetric literature on laterality of anxiety–amygdala relations is inconclusive. Functional literature suggests that the left and the right amygdala may play different roles in processing threat-relevant stimuli, such that the left amygdala is involved in more sustained responses (Sergerie et al., Reference Sergerie, Chochol and Armony2008), which may lead to a greater involvement in the ongoing fear that underlies anxiety. Future studies involving both structural and functional neuroimaging, using tasks designed to tap into both left and right amygdala processes, may provide more insight into the role of laterality. Such research should also continue to explore whether there is an effect of specific anxiety type (e.g., social anxiety and generalized anxiety) on brain–behavior relations.
Ultimately, although the current study offers insight into potential moderators of brain–behavior relations in anxiety, more research needs to be done before making firm conclusions about the relationship between amygdala volume and anxiety traits, and before applying findings from structural neuroimaging to clinical assays or pharmacological interventions. Although the current study had a large sample compared to many other developmental studies of amygdala volume and anxiety, future research should include even larger clinical and nonclinical samples that will allow for more nuanced investigation of the effects of various moderators. For example, although we did not find an interaction between age and sex, that may have been due to limited power. One of the advantages of structural neuroimaging is the ability to combine data across a wide variety of sites (e.g., BRAINS project; Job et al., Reference Job, Dickie, Rodriguez, Robson, Danso, Pernet and Waiter2017), and utilizing such approaches may resolve ongoing debates in this literature. Furthermore, multimodal approaches, such as examining functional and structural data in single samples, and including genotyping, will also help clarify the role of the amygdala in anxiety (Mueller et al., Reference Mueller, Aouidad, Gorodetsky, Goldman, Pine and Ernst2013). Finally, the amygdala is a complex structure made up of multiple nuclei, and these nuclei have different functions and show different patterns of functional connectivity with the rest of the brain (Saygin, Osher, Augustinack, Fischl, & Gabrieli, Reference Saygin, Osher, Augustinack, Fischl and Gabrieli2011). For example, there is some human (Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014) and animal evidence (Yang et al., Reference Yang, Mozhui, Karlsson, Cameron, Williams and Holmes2008) that basolateral amygdala volume may be especially predictive of individual differences in anxiety. Future research should continue to address this possibility. In the end, inconsistencies in the current literature will likely need multimethod, large-scale studies in order to be resolved.
Conclusions
The current study adds to the limited developmental literature on amygdala volume and anxiety by examining brain–behavior relations in a large, typical sample aged 6–13 years. We find that self-reported anxiety is negatively related to amygdala volume. Further, our results indicate that the relation between amygdala volume and anxiety weakens with age, which suggests that anxiety may have different neurobiological antecedents or consequences at different points in development. Such developmental trajectories may also interact with sex, as we find stronger brain–behavior relations in males. Although these findings do not fully explain previous inconsistencies in the literature, they provide important variables to consider for future large-scale, longitudinal, and multimodal studies that chart the brain bases of anxiety.









