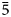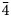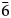Introduction
Arrojadite-group minerals are complex primary pegmatite phosphates with the general structural formula A 2B2CNa2+xM 13Al(PO4)11(PO3OH1–x)W 2 (after Cámara et al., Reference Cámara, Oberti, Chopin and Medenbach2006; with the Ca site labelled as C). Eleven members of the group are currently known on the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) list (Pasero, Reference Pasero2020). Following the IMA-accepted nomenclature (Chopin et al., Reference Chopin, Oberti and Cámara2006), they are named according to the combination of the predominant cations at the M sites [rootname; Fe2+– arrojadite, Mn2+ – dickinsonite (Chopin et al., Reference Chopin, Oberti and Cámara2006) and Mg – carmoite (Cámara et al., Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi and Bracco2019)] and the dominant cation of the dominant valence state at the A1 and B1 sites [two suffix-modifiers, e.g. arrojadite-(KNa)]. A third suffix is added in case the sum of non-(P,Al) cations exceeds 20.5 atoms per formula unit (apfu) [which implies that the Na3 site is more than half occupied, e.g. dickinsonite-(KMnNa)] and prefixes may be added to the rootname in the case of dominance of F over OH at the W site or of Fe3+ over Al at the Al site [e.g. fluorarrojadite-(BaFe)]. The nomenclature also proposed handling potential monovalent substitutions at the C site in the same way as for the Al site, i.e. by using an appropriate prefix like bario- or strontio-.
Manganoarrojadite-(KNa), ideally KNa5MnFe13Al(PO4)11(PO3OH)(OH)2, is a new member of the arrojadite group described in this paper. It was found while examining a specimen labelled ‘dickinsonite-(KMnNa)’ in the collection of the Canadian Museum of Nature, Ottawa, Canada (CMNMC 47194). Manganoarrojadite-(KNa) was named in accordance with the IMA nomenclature of the arrojadite group (Chopin et al., Reference Chopin, Oberti and Cámara2006) as a mineral with Fe2+ as the dominant cation at the M sites [rootname arrojadite], K at the A1 site, Na at the B1 site site [two suffix-modifiers (KNa)] and Mn2+ at the C site [prefix mangano-]. Both the new mineral and the name have been approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification, proposal IMA2020-003 (Lykova et al., Reference Lykova, Rowe, Poirier, Friis and Helwig2020). The specimen CMNMC 47194 became the holotype of manganoarrojadite-(KNa).
Occurrence and general appearance
Manganoarrojadite-(KNa) occurs at the Palermo No. 1 mine, Grafton Co., New Hampshire, USA. The mine exploited a large phosphate-bearing granite pegmatite and is famous worldwide as a source of numerous rare primary and secondary phosphates (Segeler et al., Reference Segeler, Ulrich, Kampf and Whitmore1981; Whitmore and Lawrence, Reference Whitmore and Lawrence2004). The new mineral forms anhedral slightly elongated grains up to 1 cm × 1.5 cm in size (Fig. 1) combined in aggregates. Manganoarrojadite-(KNa) was partially replaced along intergrain fractures by vivianite Fe2+3(PO4)2⋅8H2O (Fig. 2) during late stages of the pegmatite formation. The other associated minerals are goyazite SrAl3(PO4)(PO3OH)(OH)6, quartz and calcite.

Fig. 1. Olive-green manganoarrojadite-(KNa) partially replaced by vivianite, which causes the bluish-grey shade. White grains are goyazite. Field of view = 2.2 cm. Specimen CMNMC 47194. Photo: John Montgomery.

Fig. 2. Manganoarrojadite-(KNa) partially replaced by vivianite. Polished section. Back-scattered electron image.
Physical and optical properties
In separate grains manganoarrojadite-(KNa) is olive green with a pale green streak and vitreous to greasy lustre. In aggregates it is grey–green and bluish-grey due to the vivianite inclusions (Fig. 1). The cleavage is good in one direction [probably {001}]. The Mohs hardness is 4½. The fracture is uneven, stepped. The mineral is non-fluorescent under ultraviolet light. The density calculated using the empirical formula and unit-cell volume refined from the single-crystal X-ray diffraction (XRD) data is 3.53 g/cm3.
Manganoarrojadite-(KNa) is optically biaxial (–), α = 1.658(2), β = 1.666(2), γ = 1.670(2), 2Vmeas. = 67(1)° (from a spindle-stage extinction curve) and 2Vcalc. = 70° (589 nm). Pleochroism is very weak and the absorption scheme is as follows: X ≈ Y very pale green < Z pale green.
Experimental methods
Chemical data for manganoarrojadite-(KNa) were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five wavelength dispersive spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) with an acceleration voltage of 20 kV, a beam current of 10 nA and a beam diameter of 20 μm. The following reference materials were used: albite (Na), sanidine (K and Al), fluorapatite (Ca, P and F), diopside (Mg), tephroite (Mn) and hematite (Fe). The intensity data were corrected for Time Dependent Intensity (TDI) loss (or gain) using a self-calibrated correction for KKα, NaKα, FeKα, FKα and PKα.
The Fourier-transform infrared (FTIR) spectrum of manganoarrojadite-(KNa) was obtained at the Canadian Conservation Institute, Canada using a Bruker Hyperion 2000 microscope interfaced to a Tensor 27 spectrometer with a wide-band mercury cadmium telluride (MCT) detector. A small fragment of manganoarrojadite-(KNa) was mounted on a low-pressure diamond anvil microsample cell and analysed in transmission mode. The spectrum was collected between 400–4000 cm–1 with the co-addition of 150 scans at a 4 cm–1 resolution.
Powder XRD data were collected at the Canadian Museum of Nature, Canada using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and IμS microfocus X-ray source (λCuKα = 1.54184 Å). The instrument was calibrated using a statistical calibration method (Rowe, Reference Rowe2009). A powder ball 200 μm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10° rocking motion along the Psi axis of the Centric Eulerian Cradle stage.
Single-crystal X-ray studies were carried out at room temperature on a Rigaku XtaLAB Synergy-S diffractometer equipped with a HyPix 6000HE detector (λMoKα = 0.71073 Å) operating at 50 kV and 1 mA, housed at the Natural History Museum, University of Oslo, Norway. The data were processed, including face indexed absorption correction, using Rigaku's CrysAlis Pro software.
Results
Chemical data
Chemical data for manganoarrojadite-(KNa) are given in Table 1. The data based on 11 analyses of four crystal fragments from different parts of the specimen show that the mineral is homogeneous. One of the analysed fragments was later used for the crystal-structure study. The empirical formula calculated on the basis of 12 P and (O+OH+F) = 50 apfu is Na4.73K0.80Ca0.12Mg1.13Mn2+3.65Fe2+9.13Al1.00P12.00O46.59OH3.08F0.33. The O/OH ratio was calculated by the charge balance. All Fe was assumed to be 2+.
Table 1. Compositional results (in wt.%, average of 11 analyses) for manganoarrojadite-(KNa).

*calculated from stoichiometry; S.D. – standard deviation.
The simplified formula is KNa5Mn2+(Fe2+,Mn2+,Mg)13Al(PO4)11(PO3OH)(OH)2. The end-member formula is KNa5MnFe13Al(PO4)11(PO3OH)(OH)2, which requires K2O 2.20, Na2O 7.25, MnO 3.32, FeO 43.71, Al2O3 2.39, P2O5 39.86, H2O 1.27, total 100 wt.%.
Manganoarrojadite-(KNa) does not react with a diluted aqueous HCl solution at room temperature.
Infrared spectroscopy
The infrared (IR) spectrum of manganoarrojadite-(KNa) (Fig. 3) shows IR bands of O–H stretching (in the range from 3530 to 3560 cm–1) and P–O stretching (1000–1100 cm–1) vibrations. The bands in the ranges 560–650 and 425–460 cm–1 correspond to M–O stretching vibrations where M = Fe, Mn, Mg and Al. The weak band at 901 cm–1 can be explained by the presence of acid orthophosphate groups, confirming structural data that show protonation of O7 and, partially, of O30 and O31 (see below). The band assignment was made in accordance with Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016).

Fig. 3. Infrared spectrum of manganoarrojadite-(KNa).
Characteristic bands of H–O–H bending vibrations of H2O molecules (in the range 1550–1750 cm–1) and CO32– anions (in the range 1350–1550 cm–1) are absent in the IR spectrum of manganoarrojadite-(KNa). The presence of NH4+ groups, previously reported in arrojadite-(KNa) from Rapid Creek, Yukon, Canada (Della Ventura et al., Reference Della Ventura, Bellatreccia, Radica, Chopin and Oberti2014), can also be excluded due to the absence of bands of H–O–H bending vibrations of NH4+ molecules in the range 1400–1440 cm–1.
X-ray diffraction data and description of the crystal structure
The indexed powder XRD data are given in Table 2. Parameters of the monoclinic unit cell refined from the powder data are as follows: a = 16.5732(2), b = 10.0535(1), c = 24.7002(2) Å, β = 106.025(1)° and V = 3955.60(7) Å3.
Table 2. Powder X-ray diffraction (d in Å) data for manganoarrojadite-(KNa).

1 Calculated from the crystal structure determination, only reflections with intensities ≥1 are given.
2 Calculated from PXRD Rietveld unit-cell refinement with a = 16.5732(2), b = 10.0535(1), c = 24.7002(2) Å, β = 106.0253(7)° and V = 3955.60(7) Å3.
The strongest lines are given in bold.
The single-crystal XRD data were indexed in the Cc space group with the following unit-cell parameters: a = 16.5345(3), b = 10.0406(2), c = 24.6261(5) Å, β = 105.891(2)° and V = 3932.09(14) Å3. The structure was refined to R 1 = 0.025 on the basis of 8515 independent reflections with I > 2σ(I) using the SHELXL-2018/3 program package (Sheldrick, Reference Sheldrick2015). The atomic coordinates of arrojadite-(KNa) (Cámara et al., Reference Cámara, Oberti, Chopin and Medenbach2006) were used as a starting model for the refinement. Crystal data, data collection and structure-refinement details are given in Table 3, atom coordinates, equivalent displacement parameters, site occupancy factors and bond valence sums (BVS) in Table 4 and selected interatomic distances in Table 5. The studied crystal of manganoarrojadite-(KNa) demonstrated twinning by merohedry Class I (Nespolo and Ferraris, Reference Nespolo and Ferraris2000), with a twin domain ratio of 96:4. The crystallographic information file for manganoarrojadite-(KNa) has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below). It was also deposited in the Inorganic Crystal Structure Database (ICSD; #CSD 2042959).
Table 3. Crystal data, data collection information and structure refinement details for manganoarrojadite-(KNa).

Table 4. Coordinates and equivalent displacement parameters (U eq, in Å2) of atoms, site occupancy factors (s.o.f.) and bond-valence sums for manganoarrojadite-(KNa).

1 According to the nomenclature by Chopin et al. (Reference Chopin, Oberti and Cámara2006), the Ca site was renamed as C to handle homovalent substitutions at the site.
2 Based on the e ref values (given in square brackets) and electron microprobe data, taking also into account coordination polyhedra character, BVS and interatomic distances.
3 BVS were calculated taking into account s.o.f. using bond-valence parameters of Brese and O'Keeffe (Reference Brese and O`Keeffe1991).
Table 5. Selected interatomic distances (Å) in the structure of manganoarrojadite-(KNa).

The crystal structure of manganoarrojadite-(KNa) is based on a framework of P-centred tetrahedra, five- and six coordinated M cations, and Al-centred octahedra linked together via common vertices (Fig. 4). There are 13 independent M sites in the structure. Fe, Mn and Mg atoms are distributed among these sites based on the microprobe data and refined site-scattering factors (e ref, in electrons per site) taking into account BVS and interatomic distances (Tables 4, 5). For example, the sites Fe4 and Fe5 with the lowest e ref values (20.8 and 20.6, respectively) are characterised by a significant admixture of Mg atoms (30% of the site occupancy), whereas the Fe10, Mn11 and Mn13 sites form the largest M-centred polyhedra with the average <M–O> distances of 2.220, 2.228 and 2.226 Å, respectively, and thus characterised by a significant presence of Mn atoms (40, 60 and 60% of the occupancy, respectively). According to arrojadite-group nomenclature (Chopin et al., Reference Chopin, Oberti and Cámara2006), only the dominant cation among all the M sites taken as a bulk is considered when choosing the root name. Therefore, manganoarrojadite-(KNa) belongs to the Fe-dominant arrojadite series. One Al and 13 P sites are fully occupied.

Fig. 4. General view of the crystal structure of manganoarrojadite-(KNa). Projection on (010).
There are five independent Na sites, four fully occupied and one partially occupied [Na5 with the occupancy of 75%], and one K site with an occupancy of 88%.
The Mn site [= the Ca site according to the nomenclature by Chopin et al. (Reference Chopin, Oberti and Cámara2006)] is occupied by 0.88Mn2+ + 0.12Ca [e ref = 24.3] with the average <Mn–O> distance of 2.286 Å. The bond-valence sum (BVS) at the Mn site is 1.74 valence units (vu, Table 4). The interatomic distances and the BVS unambiguously indicate that the dominant cation at the Mn site is Mn2+ and not Fe2+. If the Mn site were occupied by Fe atoms, the BVS would be unjustifiably low (1.62 vu).
The BVS at the O7 [1.16 vu], O49 [0.97 vu] and O50 [0.97 vu] sites show that they are occupied by hydroxyl groups, while the O30 and, possibly, O31 sites have mixed O/OH occupancies [1.71 and 1.75 vu, respectively] with a predominant O2– anion (Table 4). A similar partial protonation of an oxygen in one of the PO4 groups was observed in the recently described arrojadite-group mineral fluorcarmoite-(BaNa) BaNa4CaMg13Al(PO4)11(PO3OH)F2 (site O4a, 1.63 vu; Cámara et al., Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi and Bracco2019). The presence of hydroxyl groups at the O7, O30 and O31 sites means that PO3OH groups are present. The remaining 45 anion sites are occupied by O atoms. The bands at 3532 and 3557 cm–1 in the IR spectra of manganoarrojadite-(KNa) confirm the presence of hydroxyl groups (Fig. 3). Both BVS and IR data indicate the absence of H2O0.
Discussion
The resulting structural formula of manganoarrojadite-(KNa) K0.88Na4.75(Mn2+0.88Ca0.12)Σ1.00(Fe2+9.10Mn2+2.80Mg1.10)Σ13.00Al1.00(PO4)10.62(PO3OH)1.38(OH)2.00 is in good agreement with the empirical formula K0.80Na4.73(Mn2+0.91Ca0.12)Σ1.03(Fe2+9.13Mn2+2.74Mg1.13)Σ13.00Al1.00(PO4)10.59(PO3OH)1.41(OH1.67F0.33)Σ2.00. Combining our data with the nomenclature scheme established by Chopin et al. (Reference Chopin, Oberti and Cámara2006) leads to the ideal structural formula A 1KA 2NaB 1NaB 2NaNa1,2Na2Na3□CMnMFe13Al(PO4)11(PO3OH) W(OH)2 (the Ca site labelled as C; Tables 4, 6).
Table 6. Assignment of cation sites for minerals of the arrojadite group (compiled after Chopin et al., Reference Chopin, Oberti and Cámara2006, with additions)*.

*Does not include arrojadite-(BaNa) as its structure was reported in the C2/c space group (Vignola et al., Reference Vignola, Hatert, Baijot, Dal Bo, Andò, Bersani, Pavese, Risplendente and Vanini2016) and it is difficult to compare its cation distribution with the nomenclature scheme established by Cámara et al. (Reference Cámara, Oberti, Chopin and Medenbach2006) and Chopin et al. (Reference Chopin, Oberti and Cámara2006).
References: [1] Chopin et al. (Reference Chopin, Oberti and Cámara2006); [2] Števko et al. (Reference Števko, Sejkora, Uher, Cámara, Škoda and Vaculovič2018); [3] Cámara et al. (Reference Cámara, Bittarello, Ciriotti, Nestola, Radica, Massimi and Bracco2019); [4] this work.
**The general structural formula for arrojadite-group minerals is A 2B2CNa2+xM 13Al(PO4)11(PO3OH1–x)W 2 (after Cámara et al., Reference Cámara, Oberti, Chopin and Medenbach2006; with the Ca site labelled as C to handle homovalent substitutions at the site).
□ = vacancy.
Manganoarrojadite-(KNa) is a Mn2+ dominant analogue [with Mn2+ > Ca at the C site] of arrojadite-(KNa), ideally KNa5CaFe13Al(PO4)11(PO3OH)(OH)2 (Cámara et al., Reference Cámara, Oberti, Chopin and Medenbach2006; Chopin et al., Reference Chopin, Oberti and Cámara2006; Tables 6, 7). It is the first arrojadite group with a dominant bivalent cation (Mn2+) other than Ca at the C site (Table 6), without Ca as the dominant cation at any cation site in the structure and with such a low Ca content (0.82 wt.% CaO, 0.12 apfu Ca) in general. An arrojadite-group mineral from Sidi-bou-Kricha, Morocco with a low Ca content (0.32 apfu) was mentioned by Huvelin et al. (Reference Huvelin, Orliac and Permingeat1972) and, as suggested by Chopin et al. (Reference Chopin, Oberti and Cámara2006), could be due to Ba atoms occupying the C site resulting in ‘fluorbarioarrojadite-(NaFe)’. However, a phase with such composition has never been studied in detail. Moreover, all analyses of arrojadite-group minerals from Sidi-bou-Kricha obtained by Chopin et al. (Reference Chopin, Oberti and Cámara2006) show CaO contents in the range 2.2–2.4 wt.%; thus, the low Ca value in the original analysis might have been an error. Arrojadite-(BaNa) is the only other arrojadite-group mineral with significant differences in the occupancy of the C site (Vignola et al., Reference Vignola, Hatert, Baijot, Dal Bo, Andò, Bersani, Pavese, Risplendente and Vanini2016; Tables 6, 7). Its structure was reported in the C2/c space group, and so it is difficult to compare it with other arrojadite-group minerals reported in the Cc space group; nevertheless, the site generally populated by Ca atoms in arrojadite-group minerals hosts Na atoms in arrojadite-(BaNa) and the M(1) site, dominated by Fe atoms in arrojadite-group minerals, is occupied by Ca atoms in arrojadite-(BaNa).
Table 7. Comparative data for manganoarrojadite-(KNa) and selected arrojadite-group minerals.

We propose revising the currently used general structural formula for the arrojadite group A 2B2CaNa2+xM 13Al(PO4)11(PO3OH1–x)W 2 (Cámara et al., Reference Cámara, Oberti, Chopin and Medenbach2006) as:
where C = Ca, Mn2+ to handle homovalent substitutions at the C site.
The existing criteria for arrojadite-group nomenclature are based on the assumption that the C site is always occupied by Ca and/or larger bivalent cations (Sr, Ba), and thus it is postulated that when calculating chemical analyses for classification purposes the C site is filled with Ca and A 2+ cations of increasing radius before bivalent cations are assigned to the A1 site (Chopin et al., Reference Chopin, Oberti and Cámara2006). The discovery of manganoarrojadite-(KNa) indicates that the C site can be occupied by smaller bivalent cations as well. The available data are not sufficient to analyse the implications of this fact, but it shows that a systematic structural study of arrojadite-group minerals is required to fully understand the distribution of Ca between various sites in the arrojadite type structure.
Acknowledgements
We thank Christian Chopin and an anonymous referee for their valuable comments. We are grateful to John Montgomery for his help in obtaining the colour photo.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.88