INTRODUCTION
Mathematical models were first used to show that the regulation of wildlife populations by parasites is possible (Anderson and May, Reference Anderson and May1978), contrary to the commonly held belief that host-parasite coevolution would lead to a benign relationship (Lack, Reference Lack1954). Over the past 3 decades more and more empirical evidence has become available to support these models, and the significance of parasites and pathogens in the population dynamics of their host species is becoming increasingly apparent (Irvine, Reference Irvine2006; Taraschewski, Reference Taraschewski, Hiepe, Lucius and Gottstein2006). These theoretical models and a number of field studies suggest that macroparasites, which usually occur as endemic infections, tend to cause morbidity in their hosts, as opposed to the epidemic infections with peaks of high mortality often caused by microparasites such as viruses or bacteria (Tompkins et al. Reference Tompkins, Dobson, Arneberg, Begon, Cattadori, Greenman, Heesterbeek, Hudson, Newborn, Pugliese, Rizzoli, Rosà, Rosso, Wilson, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2006). Although not usually directly life threatening, such morbidity is likely to have a significant effect on host fitness by directing energy resources away from normal maintenance and reproductive activities towards immune defence and regenerative activities, thus potentially influencing basic population parameters (Dobson et al. Reference Dobson, Hudson, Lyles and Crawley1992). In spite of the significance of parasite induced morbidity for our understanding of population dynamics generally, quantitative estimates of morbidity and its effect on population dynamics of wild animals are rare, and those which are available are dominated by avian species (Irvine, Reference Irvine2006; Tompkins et al. Reference Tompkins, Dobson, Arneberg, Begon, Cattadori, Greenman, Heesterbeek, Hudson, Newborn, Pugliese, Rizzoli, Rosà, Rosso, Wilson, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2006).
European hedgehogs are a common component of rural, semi-rural and urban ecosystems throughout much of Western and southern Scandinavia (Reeve, Reference Reeve1994) where they are a popular component of the local fauna. After the Bern Convention on the Conservation of European Wildlife and Natural Habitats from 1979 (Appendix III), protection of the European hedgehog has to be ensured in the member States of the Council of Europe (Council of Europe, 1979). Although a number of studies indicate that humans may influence the hedgehog's chances of survival, due to both traffic related mortality and habitat change (Huijser and Bergers, Reference Huijser and Bergers2000; Orlowski and Nowak, Reference Orlowski and Nowak2004), the critical factors influencing their population density remain unclear (Reeve, Reference Reeve1994).
E. europaeus hosts a wide range of both ecto- and endoparasite species (Saupe, Reference Saupe1988). To what extent parasitic infestations contribute to morbidity and/or mortality in hedgehogs has not been systematically investigated. Lienhardt (Reference Lienhardt1979) suggested that 95% of all naturally occurring deaths could be related to parasite infections. A more conservative estimate of 39% was made by Timme (Reference Timme1980), although both of these studies lack an adequate quantitative basis.
Ectoparasites of the hedgehog include 2 species of ixodid tick, Ixodes hexagonus and I. ricinus. The former is a nest-dwelling species largely specific to this host, with other records coming predominantly from mustelid species such as the least weasel (Mustela nivalis), martens (e.g. Martes foina), the Eurasian badger (Meles meles) (Liebisch and Walter, Reference Liebisch and Walter1986), and also foxes (Vulpes vulpes) (Aubert, Reference Aubert1975). I. ricinus is undoubtedly the most common tick species in Europe. It is a generalist species, feeding on a large variety of different hosts as well as being highly adaptable to a wide distribution of different habitats (Liebisch and Liebisch, Reference Liebisch, Liebisch and Horst2003). Some evidence suggests that I. ricinus is also more prevalent on E. europaeus inhabiting more rural, wooded areas where it may reach higher densities than I. hexagonus (Liebisch and Walter, Reference Liebisch and Walter1986; Gern et al. Reference Gern, Rouvinez, Toutoungi and Godfroid1997).
Ticks can play a significant role in animal health. Infestation may result in anaemia due to blood loss, irritation and the development of primary or secondary infections through either direct transmission of pathogens or superinfection of the lesions they cause, as well as reduced weight gain and milk yield in mammals (Gemmel et al. Reference Gemmell, Cepon, Green and Stewart1991; Spencer and Canfield, Reference Spencer and Canfield1993; Jonsson et al. Reference Jonsson, Mayer, Matschoss, Green and Ansell1998). They are also known to display immunomodulatory activities by inhibiting the proliferation of lymphocytes (Wikel and Alarcon-Chaidez, Reference Wikel and Alarcon-Chaidez2001). Thus, tick infestation is likely to lead to changed patterns of energy distribution leading to reduced host fitness.
Here we examine the relationship between the intensity of tick infestation on European hedgehogs and a variety of blood parameters potentially associated with host morbidity. The results shown here are part of a larger study dealing with the influence of parasites on the population dynamics of European hedgehogs.
MATERIALS AND METHODS
Hedgehogs
We conducted our study from March to October 2007 on a captive hedgehog population (16 females, 20 males, 1–3 years old) living in a natural grass and bush garden habitat (1100 m2) with enough opportunities to hide and build nests, including the addition of 40 nest boxes, and where they were naturally infested with ticks. Cat food and fresh water were supplied each evening. The animals were weighed and examined for wounds and injuries daily. To minimize possible confounding effects of other parasites, the feces of the hedgehogs were examined once a month for endoparasite eggs and larvae, and highly infested animals treated with anthelminthics. In addition, fleas were removed at regular intervals with Bolfo (Bayer Vital GmbH, Leverkusen 81368, Germany). Once a month every hedgehog was moved into a tick collecting box (60×38·5×29 cm) for 5 days. The boxes had a grid of holes 1 cm in diameter and separated from one another by 1 cm in the base, through which ticks detaching from the hedgehogs fell into a shallow, water-filled box. During the 5 days we collected the ticks detaching from the hedgehogs daily and took a single blood sample. Sampling of ticks and blood was made at intervals of 4 weeks for each hedgehog.
To investigate possible regenerative effects in the haematological parameters of the experimental hedgehog population, we compared the blood parameters of 18 individual hedgehogs (13 males, 5 females) with high tick infestation rates measured in tick weight in grams (high count sample), with the same parameters 4 weeks later after ticks had been completely removed. The hedgehogs were released back into their enclosed garden habitat after the initial counts and the new natural infestation was at least 30% lower than previously (low count sample).
To clarify whether our observed patterns represent compensations due to a previously high tick burden and not some other effect over time, we compared both high and low count samples with blood parameters from 46 samples from hedgehogs from the captive population with a low infestation rate (not including the samples from the high count/low count sample group). We chose 0·305 g total tick weight as a threshold for the low infestation group, since a quarter of all animals had this or lower infestation rates.
Ticks
Engorged ticks (I. ricinus and I. hexagonus) naturally detaching were collected and the ticks which did not detach over the 5 days were removed from the hedgehogs with forceps. All collected ticks were dried using tissue paper, the life-history stage determined and identified to species according to Arthur (Reference Arthur1963). They were then weighed. Collections were made between the end of hibernation in March 2007 and the next hibernation period in October 2007. Tick weight was measured in milligrams with an analytical balance (AB204, Mettler-Toledo GmbH, Giessen, 35353, Germany). To obtain a measure correlated to the blood loss induced by ticks we pooled the weight of engorged and unengorged ticks of both species and all life stages.
Blood sampling and parameters
A sample of 1000 μl of blood was collected from the lateral saphenous vein of each hedgehog once every 4 weeks. This volume is small in relation to that taken by the ticks. Within 1 h of collection, the blood was taken to the Institute for Medical Laboratory Diagnostics of the Städt. Klinikum Karlsruhe and analysed using a Sysmex XE-2100 instrument that is usually used in human medicine. Among other features this instrument allows for the measurement of platelets with 2 different methods: (i) radio-frequency resistance (impedance method) and (ii) flow cytometry (optical fluorescent method). Since microcytic or fragmented erythrocytes may be mistaken for platelets if measured by the impedance method the hedgehog thrombocytes were determined flow cytometrically. The flow cytometry on the Sysmex instrument series has already been successfully evaluated and established for the measurement of thrombocytes in various vertebrate species (XT-2000iV – Fluoreszenz-Durchflusszytometrie in der Tierblutanalytik, Sysmex Xtra 2/2007 www.sysmex.de/files/articles/Xtra_XT-VET_FFC.pdf). Furthermore, the XE-instrument counts nucleated red blood cells (NRBC) and subtracts their number from the white cell count. On other automated analysers those NRBC may be misclassified as leukocytes, thus falsely elevating the white blood cell count.
Due to the large variability in both white and red cell morphology we did not include the automated differential blood count in our analyses. Instead, conventional thin blood smears were prepared and dyed with May-Grünwald-Giemsa stain. Dyes and phosphate buffer tablets (pH 7·2) were purchased from Merck, Darmstadt, Germany. Blood films were microscopically investigated using an immersion-oil lens at a final 1000-fold magnification and a differential blood cell count was done on each 100 leukocytes per smear. The following parameters were measured: leukocytes (/nl) (monocytes (%), lymphocytes (%), neutrophils (%), eosinophils (%), basophils (%)), erythrocytes (/pl), haemoglobin (g/dl), haematocrit (%), MCV (mean corpuscular volume in fl), MCH (mean corpuscular haemoglobin in pg), MCHC (mean corpuscular haemoglobin concentration in g/dl), thrombocytes (/nl) and reticulocytes (0/00 and /nl). Correlations were sought between the blood parameters and the total weight of ticks (both species, all life stages) which infested the individual hedgehogs.
Statistics
Using the statistical program SPSS 11.5 for Windows, t-tests were used to investigate possible differences between female and male hedgehogs, an ANOVA was performed to determine differences over time, and bivariate correlations (Pearson-correlation) were used to relate tick burden (measured as cumulative tick weight) to the blood parameters.
RESULTS
Blood parameters and tick infestation
Of the 32 135 ticks counted, 82% were I. ricinus and 18% I. hexagonus. Although female ticks represented only 6·7% of all life stages (I. ricinus and I. hexagonus combined – larvae 65·22%, nymphs 28·11%, males 0·96%) they accounted for 80·66% of the total tick weight measured. Of these females, 66·7% were I. ricinus and 33·3% I. hexagonus. Thus the hedgehogs had relatively lower infestations with adult I. hexagonus than I. ricinus.
During the 8 month sampling period we obtained 200 blood samples from the 36 hedgehogs: 22 samples (11%) were not analysed due to technical problems (too low a volume or the specimen clotted). During our investigation 8 hedgehogs (22%) died, apparently unconnected to tick parasitization. The mortality rate of 22% from our captive animals is lower than the average annual mortality for subadult and adult individuals from wild populations of hedgehogs of 47% described by Reeve (Reference Reeve1994).
There were no significant differences in haematological parameters between male and female animals, and no seasonal influences on the blood parameters as measured monthly throughout the investigation (data not shown). Thus, for the statistical analysis we pooled the data from both sexes and all months.
Figures 1–3 show the correlations between hedgehog blood parameters and tick infestation (weight in grams for both tick species). There were significant negative correlations between tick weight and erythrocytes (r=−0·409, P=0·001, N=178), haemoglobin (r=−0·369, P=0·001, N=178), haematocrit (r=−0·247, P=0·001, N=178), MCHC (r=−0·453, P=0·001, N=178) and lymphocytes (r=−0·206, P=0·006, N=175), and significant positive correlations with reticulocytes (r=0·458, P=0·001, N=178), reticulocyte concentration (r=0·266, P=0·001, N=162), thrombocytes (r=0·198, P=0·008, N=178), neutrophils (r=0·191, P=0·012, N=174) and MCH (r=0·233, P=0·002, N=178). There were no correlations between tick weight and leukocytes (r=−0·128, P=0·088, N=178), basophils (r=0·062, P=0·470, N=137), eosinophils (r=−0·105, P=0·181, N=164) monocytes (r=0·001, P=0·991, N=170) and MCV (r=0·056, P=0·460, N=178).

Fig. 1. Relationship between different blood parameters in whole blood and the weight of the tick infrapopulation in grams for each blood sample during the period of investigation. (A) Erythrocyte concentration, (B) haemoglobin, (C) haematocrit. Results include data for both sexes and for all samples over the 8-month period of investigation (N=178). A Pearson correlation coefficient r was calculated, P indicates the significance. Note the negative significant correlation between tick infestation and erythrocyte concentration, haemoglobin and haematocrit.

Fig. 2. Relationship between different blood parameters in whole blood and the weight of the tick infrapopulation in grams for each blood sample during the period of investigation. (A) Relative reticulocyte concentration (in 0/00 of red blood cells) (N=178), (B) absolute reticulocyte concentration (/nl) (N=162), (C) MCHC (mean corpuscular haemoglobin concentration) (N=178), (D) MCH (mean corpuscular haemoglobin) (N=178). Results include data for both sexes and for all samples over the 8-month period of investigation. A Pearson correlation coefficient r was calculated, P indicates the significance. Note the positive correlation between tick infestation and reticulocytes and the MCH and the negative correlation of the tick infestation and the MCHC.

Fig. 3. Relationship between different blood parameters in whole blood and the weight of the tick infrapopulation in grams for each blood sample during the period of investigation. (A) Thrombocyte concentration (N=178), (B) relative concentration of lymphocytes (in respect to all leukocytes) (N=175), (C) relative concentration of neutrophils (in respect to all leukocytes) (N=174). Results include data for both sexes and for all samples over the 8-month period of investigation. A Pearson correlation coefficient r was calculated, P indicates the significance. Note the positive correlation between tick infestation and thrombocyte and neutrophil concentration and the negative correlation between tick infestation and lymphocyte concentration.
Longitudinal blood counts in individual hedgehogs
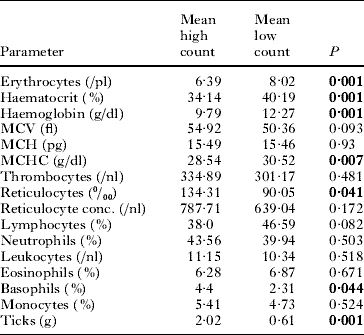
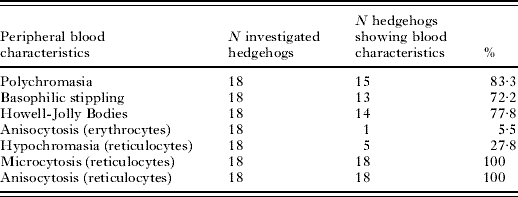
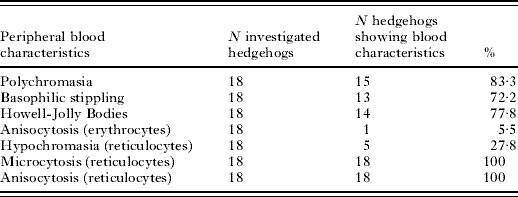
Regenerative effects in the haematological parameters for high count and low count hedgehogs are given in Table 1. Erythrocytes, haemoglobin, haematocrit and MCHC were significantly higher 4 weeks after the tick removal. The reticulocyte counts and the basophils decreased. In total, 83·3% of the hedgehogs showed polychromasia (low count) implying a high percentage of immature red blood cells (Table 2). A total of 72·2% of the hedgehogs showed basophilic stippling, 77·8% showed Howel-Jolly bodies and 100% microcytotic reticulocytes. All of the hedgehogs showed anisocytosis of the reticulocytes and 5·5% showed anisocytosis of the erythrocytes, while 27·8% had hypochromasia.
Table 1. Comparison of mean results of the blood parameters measured both for high (high count) and low (low count) tick infestation rates (both tick species, all life stages) in 18 individual hedgehogs from the experimental population (t-test)
(Note that the low counts were measured 4 weeks after the high count was determined, and ticks were removed, and the hedgehogs were re-infested with a at least 30% lower tick rate than before. Data for male and female hedgehogs are pooled. P indicates the significance. Significant values are in bold.)
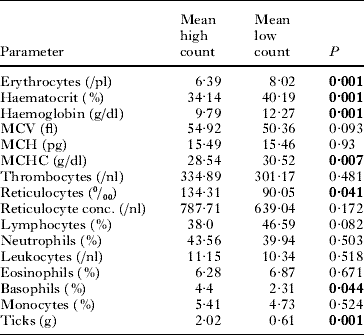
Table 2. Morphology of red blood cells in the regeneration phase of the low count hedgehogs (N=18) shown in Table 1
(Note that the blood samples for this group were drawn 1 month after the high counts were determined, the ticks were removed and the hedgehogs were re-infested with a lower tick rate than before.)
For low infestation animals, we found higher erythrocyte concentrations (P=0·001), haemoglobin (P=0·001), haematocrit (P=0·001), MCHC (P=0·001) and lymphocyte concentrations (P=0·003) compared to the high count animals. MCV (P=0·003), thrombocyte concentration (P=0·019), neutrophils (P=0·016), reticulocyte counts (P=0·001) and reticulocyte concentration (P=0·006) were lower in low infestation animals than in the high-count animals. We found no differences in low count animals and low infestation animals except for higher erythrocyte concentrations (P=0·033) and lower reticulocyte counts (P=0·04) in low infestation animals. The tick infestation of the low infestation animals was lower compared to the high count (P=0·001) and the low count (P=0·001) individuals.
DISCUSSION
The data presented here indicate that tick infestation can have a significant and haematologically definable effect on hedgehogs. In cases of high infestation levels the effect is pathological and leads to the need for the hedgehog to divert resources away from normal maintenance activities and potential reproduction to produce new blood cells. If these ticks are allowed to detach and the hedgehog recover with a significantly lower tick burden, then this pathological condition is normalized. All of the qualitative changes in red blood cell morphology observed indicate that high densities of ticks cause a macrocytic, hypochromic, regenerative anaemia.
A female I. ricinus ingests about 0·45 ml of blood during a bloodmeal on its host (Heath, Reference Heath1951), gaining weight from about 2 mg to about 234·6 to 286 mg when fully engorged (Lees, Reference Lees1952; Bowessidjaou et al. Reference Bowessidjaou, Brossard and Aeschlimann1977; Brossard, Reference Brossard1982). Unengorged female I. hexagonus weigh between 2·3 and 3·8 mg, and the mean weight of an engorged female is 114·84 mg (Toutoungi et al. Reference Toutoungi, Gern and Aeschlimann1995). To our knowldege there are no data available on the volume of blood that a female I. hexagonus ingests.
Weight gain does not reflect the total amount of ingested blood because large quantities of blood are digested, partly assimilated and partly excreted with the feces during the feeding process by ixodid ticks (Balashov, Reference Balashov1968; Koch and Sauer, Reference Koch and Sauer1984), and excess liquid is regurgitated back into the host (Bowman et al. Reference Bowman, Coons, Needham and Sauer1997). Koch et al. (Reference Koch, Sauer and Hair1974) suggested concentration factors of 1·59 for larvae, 2·39 for nymphs and 2·78 for females based on data from 4 American species of ixodid tick (Amblyomma americanum, Rhipicephalus sanguineus, Dermacentor variabilis, Ixodes scapularis) with the blood volume imbibed by I. scapularis, a species closely related to I. ricinus, being 0·51 ml (Koch and Sauer, Reference Koch and Sauer1984).
The blood volume of a hedgehog is about 8% of its body weight (Eliassen, Reference Eliassen1961); thus a hedgehog of 1 kg has about 80 ml of blood. The hedgehogs in our research population had a mean weight of 1006 g and a mean tick load of 0·909 g. The maximum weight of ticks on a hedgehog was 5 g (415 I. ricinus – 16 females; 246 I. hexagonus – 14 females). Female ticks preponderate in the weight of ticks infesting a hedgehog, and if female I. hexagonus imbibe the same amount of blood as female I. ricinus, we would calculate a maximum blood loss of 9–10 ml (or 11–12% of total volume) per week. This estimate is likely to be on the low side since a higher potential blood loss due to already digested and excreted blood components, or the assumption that ticks continuously feed on the hosts, has not been accounted for. Potential additional blood loss caused by the feeding of hedgehog fleas is likely to be insignificant as fleas were removed from the hedgehogs if infestations were observed.
The decrease in erythrocytic values (erythrocytes, haemoglobin, haematocrit) with increasing tick load indicates tick-induced anaemia. This has also been demonstrated in other animals infested with ticks, such as cattle (Rechav, Reference Rechav1987), rabbits (Jellison and Kohls, Reference Jellison and Kohls1938) and lizards (Dunlap and Mathies, Reference Dunlap and Mathies1993). In the 18 individual hedgehogs we demonstrate significantly lower haemoglobin concentrations in animals with high tick infestation rates than in the same animals when the ticks had been removed and the new natural infestation rates were substantially lower. Since there were no apparent seasonal patterns in the data, this is most likely a recovery response from high tick infestation. Blood loss leads to an increased erythropoietic response. An increased production of reticulocytes, which are young erythrocytes, is usually directly correlated with the regenerative process. This so-called reticulocytosis is one of the most reliable indications of regenerative anaemia, because it occurs consistently during such an anaemia and is not caused by conditions resulting from a non-regenerative one (Tyler and Cowell, Reference Tyler and Cowell1996). In most healthy animals, reticulocytes circulate in peripheral blood due to continuous erythropoesis, but usually their concentration is very low (Canfield, Reference Canfield1998).
The erythrocyte or corpuscular indices (MCV, MCHC, and to a lesser extent MCH) are commonly used to classify an anaemia (Tyler and Cowell, Reference Tyler and Cowell1996). During the peak response of the reticulocytosis, indicating regeneration, the corpuscular indices tend to an increased MCV (macrocytic) and a decreased MCHC (hypochromic) (Fernandez and Grindem, Reference Fernandez, Grindem, Feldman, Zinkl and Jain2000), as in our results for the 18 individual hedgehogs.
Thrombocyte counts in the experimental population were directly correlated with the tick burden. This is in agreement with the anaemic state of the animals, since anaemic and inflammatory stimuli influence the release of thrombocytes from the spleen pool or the bone marrow, a state commonly referred to as secondary thrombocytosis (Santhosh-Kumar et al. Reference Santhosh-Kumar, Yohannan, Higgy and al-Mashhadani1991).
Decreased lymphocytes with an increase in neutrophils, as in our results, can be an indication of an inflammatory or an immune response due to pathogen infection (Thomas, Reference Thomas2007). Hedgehogs can harbour a variety of different pathogens such as Borrelia burgdorferi sensu lato or the TBE-virus (Riley and Chomel, Reference Riley and Chomel2005; Skuballa et al. Reference Skuballa, Oehme, Hartelt, Petney, Bücher, Kimmig and Taraschewski2007), so transmission of one of these by ticks could lead to such an immunological response. A decrease in lymphocytes could also be the result of handling and the stress of captivity (Hadjuk et al. Reference Hadjuk, Copland and Schultz1992). This is likely not the case here, because the animals used were not taken directly from the wild and were used to being handled every day.
In 18 heavily infested hedgehogs, almost all values of the peripheral red blood count recovered significantly within a month, indicating that the anaemic response to the blood loss is regenerative. MCV did not recover significantly although there was a trend for improvement. It is likely that 1 month was too short a time for complete compensation to occur, given that some blood loss due to lower tick burdens was still ongoing. Data on either the lifespan of hedgehog erythrocytes or their regeneration rate are, to the best of our knowledge, not available. In humans the lifespan of a single mature erythrocyte is estimated to be about 120 days. Approximately 0·8% of the red blood cells are thus replaced daily under normal conditions. Assuming an unimpaired ‘normal’ regeneration rate it can be calculated that about only 24% of the erythrocytes in humans are replaced within 1 month.
The regenerative effect is strenghtend by the comparison with the low infestation animals. We found significant differences for almost all parameters when we compared the high count animals with the low infestation group. These differences disappeared when we compared the low count animals with the low infestation group. Only erythrocyte concentrations were higher and reticulocyte counts were lower in the low infestation group. This can be explained by the fact that the low count group still had a higher tick infestation rate than the low infestation group, which cause a higher blood loss and an erythropoetic response.
Morphological characteristics of red blood cells are very important for the classification of anaemia. As indicated, reticulocytosis is one of the most important characteristics of regenerative anaemia. The other is polychromasia (variation in coloration of red blood cells after staining, relating to different maturation of the cells). Both attributes are hallmarks of a regenerative anaemia (Tyler and Cowell, Reference Tyler and Cowell1996; Thomas, Reference Thomas2007). All 18 hedgehogs assessed 4 weeks after heavy tick infestation showed reticulocytosis and 15 animals showed polychromasia. The other morphological characteristics found in our study, basophilic stippling, Howell-Jolly bodies, anisocytosis, microcytosis and hypochromasia, are also often seen in regenerative anaemia (Tyler and Cowell, Reference Tyler and Cowell1996). As far as we know ticks do not inject haemolytic agents into the body, which destroy the blood cells (the tick Rhipicephalus (Boophilus) microplus even excretes a protein in its saliva which protects red blood cells from lysis; Sauer et al. Reference Sauer, McSwain, Bowman and Essenberg1995). We thus conclude that the anaemia observed here is haemorrhagic, with this type being common in infestations with ectoparasites such as ticks or fleas (Tyler and Cowell, Reference Tyler and Cowell1996).
A comparison of the means and the ranges of our haematologic parameters with values for ‘normal’ hedgehogs presented by Wenzel et al. (Reference Wenzel, Sachse and Arnold1977), Lewis et al. (Reference Lewis, Norcott, Frost and Cusdin2002) and von Wick (Reference Von Wick2006) proved to be of little value. The reported ranges in these references differ substantially from each other and assuming a broad reference range, covering all of those previously reported, we found very few outlying results.
It is important to remember that out data come from an experimental population of hedgehogs in a semi-natural environment in which tick densities are likely to be artificially high. It is therefore necessary to compare the tick burdens in our population with those found on non-captive animals. Preliminary data (Petney et al. unpublished) from wild animals brought into hedgehog care centres indicates tick prevalences of over 80% with the mean intensity of infestation being 74·3 (range of 0 to 1622), with 8/65 (12·3%) individuals having over 100 ticks. Maximum infestation by females was 94 females (86 I. ricinus and 8 I. hexagonus) on a single host. Thus, at least a small proportion of the natural hedgehog population in Europe is likely to suffer morbidity due to tick infestation.
There are no studies on the mortality rate in hedgehogs caused by tick infestation. We suggest that the chronic tick-induced blood loss observed here is not directly life-threatening for healthy animals, but that sick, young or old animals with lowered immunity could be severely affected. This may be particularly important for animals entering or just leaving hibernation, as this is a period of severe physical stress requiring substantial energy resources (Heldmaier et al. Reference Heldmaier, Ortmann and Elvert2004). This is supported by the fact that Ixodes ticks have immunosuppressive effects on their host by inhibiting B-cells, altering the the ratio of Th1 and Th2 cells or blocking complement activation (Leboulle et al. Reference Leboulle, Crippa, Decrem, Mejri, Brossard, Bollen and Godfroid2002; Hannier et al. Reference Hannier, Liversidge, Sternberg and Bowman2004). This effect might facilitate the transmission of tick-borne pathogens (Mejri et al. Reference Mejri, Rutti and Brossard2002). It is known that hedgehogs can be infected with tick-borne pathogens, but we do not know how they affect the health of the animals. It is possible that the immunosuppressive effect of ticks not only supports the transmission of pathogens, but also facilitates infection by other endoparasites such as Capillaria spp. or Crenosoma striatum. For weak animals, such co-infection would likely be life threatening. It is also likely that weight gain prior to hibernation may be reduced if the hedgehogs need to shift resources towards immunological defence and blood regeneration. As hibernation is a critical period in terms of hedgehog survival (Hoeck, Reference Hoeck1987), any reduction in resources is likely to be important and reduction below a survival threshold will lead to death.
Although we observed more I. ricinus than I. hexagonus feeding on hedgehogs, in most habitats the opposite is true (Martyn, Reference Martyn1988; Fischer, Reference Fischer2007). It was also shown by Lawrie et al. (Reference Lawrie, Randolph and Nuttall1999) that the complement activity of hedgehog serum is twice as strongly inhibited by I. hexagonus salivary gland extracts as those from I. ricinus. Thus, a natural infestation with I. hexagonus might have stronger effects on fitness than those shown in our study, since we suppressed other co-stressors such as infection with other parasites like Capillaria spp. or Crenosoma srtiatum or lack of food, etc. Although we cannot cleanly compare our results with natural populations of hedgehogs, we conclude that tick infestation primarily causes morbidity not mortality, due to the loss of energy invested in the immune response, to blood loss and regeneration, and to poor condition potentially leading to secondary infections (Beldomenico et al. Reference Beldomenico, Telfer, Gebert, Lukomski, Bennett and Begon2008).
Above all, most individual hedgehogs host a wide variety of both endo- and ectoparasites, the interactions between which currently remain unexplored. It is possible that synergistic effects between parasite species (Petney and Andrews, 2000), such as immunosuppression by the ticks studied here, may increase the pathogenic effect of the tick species under natural conditions.
The authors thank Dr Thomas Bücher and Miriam Maurer for their support in collecting and the team of the Institute for Medical Laboratory Diagnostics, Städt. Klinikum Karlsruhe, for analysing the blood samples. Financial funding was provided by grants of the Krieger Foundation and the Landesbank Baden-Württemberg Foundation. Dr Dan Tompkins kindly read and commented on an earlier version of the manuscript.