Our earliest relationship begins well before birth. Historical works, including the Hindu Mahabharata, the Tibetan Epic of King Gesar, the Judeo-Christian Bible, and numerous contemporary works of fiction and folklore are replete with examples of how this relationship is expressed. The circumstance of the neural and physiological development of an individual embedded in the psychological and physiological milieu of another is unique in human ontogeny. While it is clear that both members of the dyad express dynamic adaptation in response to the other and in concert with genomic, physiological, and experiential characteristics of each, particulars of how these processes unfold remain ill-defined. Research endeavors on the transition from pregnant woman to mother and from fetus to child exist as relatively distinct within different disciplines. The complexity of these intertwined systems in the proximal and distal arms of development of the infant, mother and dyadic relationship requires a multidimensional framework that bridges traditional academic and clinical boundaries. A Research Domain Criteria (RDoC) informed approach (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010), directed at integrating well-operationalized observable behaviors with underlying neurobiology, can help consolidate these disparate fields and methodologies into a more coherent whole.
Pregnancy generates profound and conspicuous physical and physiological alterations to women's bodies and brains. Normative processes that support pregnancy and prepare women for impending motherhood include both central and peripheral effects on the nervous system and complex endocrine adaptations (Almanza-Sepulveda, Fleming, & Jonas, Reference Almanza-Sepulveda, Fleming and Jonas2020; Dahan, Reference Dahan2021; Glynn, Howland, & Fox, Reference Glynn, Howland and Fox2018; Howland, Sandman, & Glynn, Reference Howland, Sandman and Glynn2017). At the same time, physical growth and differentiation of function of the fetus unfolds within this adapting intrauterine context. Fetal development, like infant and child development, progresses in relatively predictable ways but at different rates in individual fetuses. Maturation of the autonomic nervous system is marked by increasing parasympathetic tone. The rapid escalation in fetal heart rate variability commencing mid-way through gestation and continuing through term is regarded as a key indicator of this underlying process, as well as central shifts to higher cortical control (DiPietro, Costigan, & Voegtline, Reference DiPietro, Costigan and Voegtline2015a; Martin, Reference Martin1978; Mulkey & du Plessis, Reference Mulkey and du Plessis2019; Schneider et al., Reference Schneider, Bode, Schmidt, Nowack, Rudolph, Dolker and Hoyer2018).
The gestational foundation of the maternal–fetal relationship takes root within this dynamic context (DiPietro, Reference DiPietro2010; Van den Bergh, Reference Van den Bergh2010). After birth, the bidirectional nature of the mother–infant dyad, particularly with respect to social communication, has been established (Bell, Reference Bell1968; Feldman, Reference Feldman2007), although the contribution of infants has been less well appreciated. Extension of this perspective to the period before birth can be found in recognition of the fetal role in initiation of parturition (Challis et al., Reference Challis, Bloomfield, Bocking, Casciani, Chisaka, Connor and Premyslova2005; Reinl & England, Reference Reinl and England2015). Furthermore, it is well accepted that fetal behaviors contribute to the intrauterine milieu which, in turn, contributes to ontogeny (Hepper, Reference Hepper2015; Prechtl, Reference Prechtl and Prechtl1984; Smotherman & Robinson, Reference Smotherman and Robinson1987). Evidence of the bidirectional nature of the maternal–fetal relationship through an assortment of fetal signaling processes is steadily gaining traction (DiPietro et al., Reference DiPietro, Caulfield, Irizarry, Chen, Merialdi and Zavaleta2006, Reference DiPietro, Voegtline, Costigan, Aguirre, Kivlighan and Chen2013, Reference DiPietro, Raghunathan, Wu, Bai, Watson, Sgambati and Pien2021; Glynn et al., Reference Glynn, Howland and Fox2018; Sandman, Reference Sandman2018).
An RDoC approach to the development of the dyad, from pregnancy to postpartum
In this study we seek to evaluate the separate contributions of each individual over time (i.e., pregnant woman and mother; fetus and infant) to the dyad. Our model encompasses two general levels of maternal function. The first involves the evolution of maternal psychological processes specific to the experience of pregnancy, including feelings of maternal attachment to the fetus and infant and the affective valence of the maternal experience of pregnancy and early motherhood. The second relies on characteristic patterns of maternal physiological arousal and regulation as both signal to the developing fetus and framework for response patterns to the infant. In turn, characteristics of fetal physiological arousal and regulation, influenced in part by maternal response characteristics, provide the foundation for infant temperament and reactions to maternal affect and behavior, as expressed during mother-infant interactions. The multiple sources of data necessary for integrating these elements are well suited to an RDoC-informed approach and center around key RDoC domains of positive and negative valence systems, arousal and regulatory systems, and social processes, which includes attachment and social communication (Cuthbert & Insel, Reference Cuthbert and Insel2013).
Maternal antenatal attachment and affective valence towards pregnancy
Frequently measured maternal psychological processes in pregnancy and early motherhood include nonpregnancy-related elements of psychological distress and strain as well as pregnancy and motherhood-specific indicators of distress (Abdi, Navidpour, & Dolatian, Reference Abdi, Navidpour and Dolatian2018; Blackmore, Gustafsson, Gilchrist, Wyman, & O'Connor, Reference Blackmore, Gustafsson, Gilchrist, Wyman and O'Connor2016; Huizink, Mulder, Robles de Medina, Visser, & Buitelaar, Reference Huizink, Mulder, Robles de Medina, Visser and Buitelaar2004). However, pregnancy is often also a time of joy and anticipation such that when women are queried about both its uplifting and distressing features, the ratio of these reveals an overall positive valence (DiPietro, Ghera, Costigan, & Hawkins, Reference DiPietro, Ghera, Costigan and Hawkins2004; van der Zwan, de Vente, Koot, & Huizink, Reference van der Zwan, de Vente, Koot and Huizink2017). Although long-term effects of positive affective valence during pregnancy are not widely studied, it has been linked to more optimal parenting behaviors that foster better child health outcomes, including breastfeeding (Baumgartner et al., Reference Baumgartner, Bhamidipalli, Guise, Daggy, Parker, Westermann and Haas2020; McManus, Khalessi, Lin, Ashraf, & Reich, Reference McManus, Khalessi, Lin, Ashraf and Reich2017).
Parents form working mental representations of the fetus well before delivery (Reading, Cox, Sledmere, & Campbell, Reference Reading, Cox, Sledmere and Campbell1984; Zeanah, Zeanah, & Stewart, Reference Zeanah, Zeanah and Stewart1990). These include features of temperament, such as activity level and rhythmicity, but also more nuanced feelings of empathy, acceptance, and affiliation (Vreeswijk, Maas, & van Bakel, Reference Vreeswijk, Maas and van Bakel2012). The construct of attachment in developmental sciences most often refers to the constellation of affective, cognitive and behavioral expressions towards a caregiver, most often the mother, by either a child or an adult, and measured using standard protocols. The manner in which mothers and infants communicate their affective and motivational states during social interaction, and implications of these interactions for subsequent emotional, regulatory, and cognitive development during childhood and into adulthood, has generated a large body of research and theory in developmental sciences (Cassidy & Shaver, Reference Cassidy and Shaver2018). Conversely, the term “attachment” is also applied to the construct of parental affiliation towards offspring, including feelings, attitudes and behaviors directed to the fetus, and subsequently to infants (Cannella, Reference Cannella2005; Cranley, Reference Cranley1981; Van den Bergh & Simons, Reference Van den Bergh and Simons2009).
Antenatal maternal attachment measures have been validated with key developmental outcomes. Narrative features of maternal descriptions of their fetuses are predictive of security of infant attachment (Benoit, Parker, & Zeanah, Reference Benoit, Parker and Zeanah1997) and a meta-analysis supports associations with maternal-child interaction quality based on observation (Foley & Hughes, Reference Foley and Hughes2018). Antenatal attachment has also been associated with maternal perception of positive features of infant temperament (Priel & Besser, Reference Priel and Besser2000) and developmental outcomes (Alhusen, Hayat, & Gross, Reference Alhusen, Hayat and Gross2013), including socioemotional maturity (Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021). Suggested pathways include protective or detrimental maternal health behaviors during pregnancy, influences on postnatal attachment, and mediation through mother-child interactions (Branjerdporn, Meredith, Strong, & Garcia, Reference Branjerdporn, Meredith, Strong and Garcia2017). There is relatively little information on whether and how maternal feelings of attachment that originate in pregnancy are affected by the experience of caring for an infant but maternal personality traits (e.g., extraversion) and infant temperament (e.g., negative affect) have both been implicated as contributory (de Cock et al., Reference de Cock, Henrichs, Vreeswijk, Maas, Rijk and van Bakel2016).
Maternal and fetal arousal and regulation
Maternal affective states can be transduced to the fetus through physiologic and neuroendocrine processes that remain relatively underspecified (Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer and Schwab2020). Studies that experimentally elicit maternal arousal using emotional and/or cognitively taxing challenges are useful in identifying physiological adaptations to perturbations during pregnancy (de Weerth & Buitelaar, Reference de Weerth and Buitelaar2005; Tung et al., Reference Tung, Krafty, Delcourt, Melhem, Jennings, Keenan and Hipwell2021; Vlisides-Henry et al., Reference Vlisides-Henry, Deboeck, Grill-Velasquez, Mackey, Ramadurai, Urry and Crowell2021). Furthermore, eliciting maternal arousal has an ancillary benefit in that it provides an opportunity to ascertain the fetal response to induced changes in the intrauterine environment. Manipulation of maternal arousal through a variety of challenges can provoke measurable and near instantaneous responses in fetal heart rate parameters and/or motor activity (Araki et al., Reference Araki, Nishitani, Ushimaru, Masuzaki, Oishi and Shinohara2010; DiPietro, Costigan, & Gurewitsch, Reference DiPietro, Costigan and Gurewitsch2003; Monk, Myers, Sloan, Ellman, & Fifer, Reference Monk, Myers, Sloan, Ellman and Fifer2003).
The utility of this approach is predicated on the assumptions that maternal and/or fetal responses depict a facet of the antenatal period that is stable within individuals and also exerts or reveals persistent influences on the developing fetus. There is limited, but supportive, evidence for both. The maternal physiological response to a standard laboratory challenge shows stability during gestation (DiPietro et al., Reference DiPietro, Costigan and Gurewitsch2003). There is also stability in the degree to which fetuses react to and recover from manipulations that elicit maternal reactivity (DiPietro et al., Reference DiPietro, Costigan and Gurewitsch2003) and to more direct presentations of stimuli to fetuses that do not require physiologic transduction through maternal processes (i.e., a vibroacoustic device placed on the maternal abdomen) (DiPietro, Hodgson, Costigan, & Johnson, Reference DiPietro, Hodgson, Costigan and Johnson1996). Variations in developmental outcomes have been linked to fetal responsivity to induced maternal arousal in the neonate (DiPietro, Ghera, & Costigan, Reference DiPietro, Ghera and Costigan2008b; Ostlund et al., Reference Ostlund, Vlisides-Henry, Crowell, Raby, Terrell, Brown and Conradt2019), infant (Werner et al., Reference Werner, Myers, Fifer, Cheng, Fang, Allen and Monk2007), and child (DiPietro, Voegtline, Pater, & Costigan, Reference DiPietro, Voegtline, Pater and Costigan2018). These findings bolster support for the premise that eliciting maternal arousal may provide contextual information regarding features of the intrauterine environment to which the fetus is routinely exposed and may reveal core reactive and regulatory attributes of the developing fetus. These attributes are widely regarded as the foundational elements of early individual differences (Rothbart & Derryberry, Reference Rothbart, Derryberry, Lamb and Brown1981).
The current study
Here we examine affective and physiological processes that contribute to development of dyadic relationships from the 24th week of gestation through 6 months postpartum. In the postnatal period, we use a standard paradigm, the Face-to-Face Still-Face (FFSF) procedure (Tronick, Als, Adamson, Wise, & Brazelton, Reference Tronick, Als, Adamson, Wise and Brazelton1978), to assess dyadic social processes. Infant affective and behavioral responses to the FFSF, particularly to unexpected maternal disengagement, have been well documented since its introduction (Adamson & Frick, Reference Adamson and Frick2003). This procedure is used as an objective measure of positive and negative affective behaviors that communicate motivation toward affiliation and proximity, and regulation under social duress. We include infant temperament, assessed through maternal report, to ascertain maternal perceptions of infant reactivity and regulation. Figure 1 provides a conceptual schematic.
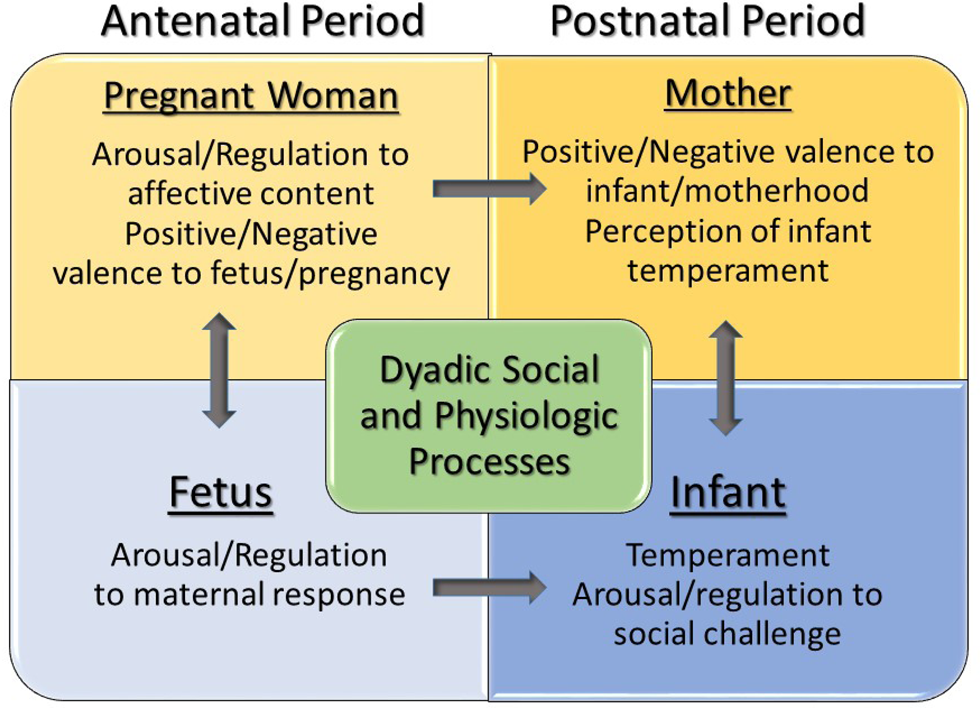
Figure 1. Schematic presentation of Research Domain Criteria (RDoC) informed contributions to the development of maternal attachment and dyadic social communication.
Our first objective centers on the affiliative and affective context of pregnancy and motherhood in relation to social processes in infancy and emerging infant temperament. Based on prior work (de Cock et al., Reference de Cock, Henrichs, Vreeswijk, Maas, Rijk and van Bakel2016; DiPietro, Goldshore, Kivlighan, Pater, & Costigan, Reference DiPietro, Goldshore, Kivlighan, Pater and Costigan2015b; Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021) we anticipate that individual differences in maternal attachment towards infants and the affective valence of early motherhood, using comparable measures used in the antepartum, will be predicated on maternal orientation to the fetus and pregnancy. Given that women form mental representations of the fetus during pregnancy, with relatively little information to guide those representations, we hypothesize that women expressing less maternal antenatal attachment and greater negative appraisal of pregnancy will be more likely to perceive their infants as having characteristics that are more challenging for infant care.
The second objective involves evaluating the significance of evoked maternal arousal in revealing maternal characteristic response patterns that serve as indicators of individual differences in maternal reactivity and regulation but also as providing variation in the intrauterine milieu experienced by the developing fetus. In turn, measurement of the effect of evoked maternal arousal on the fetus provides information on the characteristic response of the fetus with implications for subsequent temperament and capacity to communicate and regulate within the maternal–infant dyad. We hypothesize that the fetus will respond to the pregnancy-relevant stimulus used in this study (i.e., positive and negative videos depicting the uplifting and discomforting features of pregnancy, delivery and early motherhood) independent of affective valence, and that the size of the fetal response will correspond, in part, to the size of the maternal physiologic response.
A sizable developmental literature indicates that suppression of parasympathetic input (i.e., vagal tone) in response to experimental challenges is associated with better emotion regulation in infants and young children (Calkins, Reference Calkins1997; Moore & Calkins, Reference Moore and Calkins2004; Porges, Reference Porges2007; Provenzi et al., Reference Provenzi, Casini, de Simone, Reni, Borgatti and Montirosso2015). Thus, we hypothesize that fetuses showing suppression of heart rate variability, as opposed to activation, to maternal viewing of the videos will exhibit more optimal regulation strategies and social processes during the FFSF. Motor activity is a fairly weak proxy for fetal sympathetic activation, but since motor suppression has been previously associated with regulatory outcomes in boys (DiPietro et al., Reference DiPietro, Voegtline, Pater and Costigan2018), it will be analyzed in parallel fashion.
In contrast, there are scant data on which to base hypotheses regarding the potential contributory role of maternal physiologic responsivity on outcomes in infancy. However, given the orthogonal nature of the branches of the autonomic nervous system (Bernston, Cacioppo, & Quigley, Reference Bernston, Cacioppo and Quigley1991), we expect that women exhibiting greater sympathetic activation (i.e., electrodermal activity) and/or less parasympathetic activation (i.e., respiratory sinus arrhythmia [RSA]) will provide a more activating and variable intrauterine environment. This exerts greater pressure on the fetus to maintain equilibrium and may either impede or accelerate the development of regulatory resources.
Lastly, although we expect some degree of stability in maternal reports of attachment from the antenatal to postnatal periods, modest levels of stability reported by others (de Cock et al., Reference de Cock, Henrichs, Vreeswijk, Maas, Rijk and van Bakel2016; Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021) suggest that attachment is not conserved in all individuals. Given the limited available information on the trajectory of maternal attachment from before to after birth, we engage in an exploration of antenatal and postnatal characteristics of the mother, fetus and dyad to consider sources of change and continuity. We hypothesize that stability in maternal feelings of attachment to the fetus and infant, whether high or low, will be predicated on maternal positive or negative affective valence to pregnancy and motherhood while infant characteristics will contribute to shifts in attachment.
Method
Participants
A total of 158 maternal–fetal pairs met inclusion criteria of singleton pregnancies absent of significant pregnancy conditions with healthy offspring without neonatal conditions requiring treatment. All women were nonsmokers and most (87%) reported that the pregnancy was planned. Women were relatively mature, M (SD) age = 32.0 (4.3) and well educated, M (SD) years of completed education = 17.1 (1.8). Most (75%) identified as non-Hispanic White, 8% identified as African-American, 10% as Asian, 3% as Hispanic, and 3% as more than one race. Over half (n = 89, 56%) was primiparous. All infants, with one exception delivered at 36 weeks, were born at term (i.e., ≥37 weeks’ gestation), M (SD) = 39.5 (1.1) weeks, at appropriate for gestational age weight, M (SD) birth weight = 3,404 g, (505), with normal 5-min Apgar scores, M (SD) = 8.9, (.5). Slightly less than half (n = 69, 44%) of the infants was female.
Design overview
The longitudinal design included data collection at 24 (n = 151), 30 (n = 153) and 36 (n = 149) weeks’ gestation and a follow-up visit at 6 months postpartum. During each antental visit, women completed questionnaires concerning their feelings regarding pregnancy and the fetus; these were further probed during an interview at the 36-week visit. At 30 weeks, maternal–fetal physiological monitoring was conducted while women viewed videos that portrayed positive and negative aspects of pregnancy, delivery, and the transition to motherhood. Baseline, undisturbed data were collected as part of a larger study; longitudinal results are provided elsewhere and are not part of this report (Cohorts VII and VIII) (DiPietro, Costigan, et al., Reference DiPietro, Costigan and Voegtline2015a). The maternal interview and video protocols were administered in Cohort VIII but not Cohort VII so include only a subset of the full sample (n = 98). At the 6-month follow-up (n = 140), women completed a similar set of questionnaires pertaining to early motherhood, an additional questionnaire regarding infant temperament, and mothers and infants participated in a standard procedure to evaluate dyadic social processes.
Measures that align with constructs identified within the RDoC framework, as presented in Figure 1, are indicated at the end of each measure. These include those that access arousal and regulatory systems (AR), positive and/or negative valence (PV/NV), and social processes (SP).
Antenatal procedure and measures
Maternal Antenatal Attachment Scale (MAAS)
This scale assesses maternal feelings of closeness, preoccupation, and emotionality towards the fetus within the time frame of the previous two weeks of pregnancy (Condon, Reference Condon1993). It, and the postnatal version, were used in two recent, population-based studies of maternal attachment totaling nearly 3,000 participants (Ertmann et al., Reference Ertmann, Bang, Kriegbaum, Væver, Kragstrup, Siersma and Smith-Nielsen2021; Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021). Unlike some other antenatal attachment scales which include feelings about being pregnant, all MAAS items are directed specifically towards the fetus. Nineteen items, scored on 5-point scales, include “I have been trying to picture in my mind what my developing baby looks like [almost all the time to not at all],” “When I think about the baby my thoughts are [always tender and loving to contain a lot of irritation],” and “I have found myself talking to my baby when I am alone “almost all of the time to not at all.” After reverse scoring of relevant items, the total summed score reflects the global quality of maternal–fetus attachment with higher values suggesting stronger feelings of attachment. (SP)
Pregnancy Experience Scale (PES)
The PES is a pregnancy-specific, validated measure that assesses the degree to which women perceive their pregnancies as consisting of both uplifting and positive experiences and hassling and stressful ones (DiPietro et al., Reference DiPietro, Ghera, Costigan and Hawkins2004). It is based on the premise that the same experience can elicit both positive and negative affect and quantified in terms of frequency and intensity of this subjective appraisal. The PES consists of 41 neutrally worded items, scored on 4-point scales ranging from 0 (not at all) to 3 (a great deal). Respondents are instructed to score each item as to “How happy, positive or uplifted” and “How unhappy, negative, or upset” it makes them feel. Items include ordinary circumstances such as body changes due to pregnancy, fetal motor activity, and conversations about potential baby names. Scores are calculated as the total number of endorsed items not equal to 0 (frequency) and intensity (i.e., sum of scores divided by number of endorsed items) for both hassles and uplifts. (PV/NV)
Maternal pregnancy interview
An open-ended interview was conducted to allow women to identify and express their own positive and negative views on pregnancy and the fetus. Women were asked “What has been the best (or worst) part of your pregnancy so far?” with the order of best/worst counterbalanced at random. After approximately 5 min, the question was switched to the opposite valence, and continued for another 5 min. A short list of questions was on hand as prompts, if necessary, although women tended to speak quite freely. Responses were recorded and subsequently scored by two coders on the following dimensions generated from common themes expressed by women: happy thoughts about the fetus; planning for baby; and special attention because of pregnancy (positive); and physical discomforts, constraints on activities, and worries about the fetus/pregnancy (negative). The interview also generated qualitative information not otherwise measured, including how animated and forthcoming women were during each segment. Coding discrepancies were resolved by consensus. (PV/NV/SP)
Experimental manipulation of maternal arousal and fetal response
Maternal-fetal monitoring was conducted while women viewed two videos, each 5 min long, of women sharing their uplifting and heart-warming recollections of pregnancy, labor and delivery as well as potentially distressing and harrowing recollections, including sections containing images of childbirth. The positive and negative segments were edited from a longer video (Birth Stories, Cinema Guild, Brooklyn, NY); women viewed the videos wearing over-ear headphones. The order of the positive and negative segments was randomly assigned and counterbalanced, with a brief intermission between them.
Maternal-fetal data collection and analysis used by our laboratory has been detailed elsewhere (DiPietro, Costigan, et al., Reference DiPietro, Costigan and Voegtline2015a). Briefly, maternal data included: a three-lead electrocardiogram (ECG; electrodermal activity, monitored from silver–silver chloride electrodes affixed to two fingers; and respiration, derived from a bellows apparatus stretched across the ribcage. Maternal electrocardiogram data underwent R-wave detection, manual editing for artifact, and conversion to heart rate (bpm) to retain consistency with fetal measures. Skin conductance level was measured by administering a constant 0.5 volt root-mean-square 30 Hz AC excitation signal and detecting the current flow; final values were scaled from 0 to 25 μS. Respirations were quantified via inspiration to inspiration and expiration to expiration periods based on the detected peaks and troughs of the respiratory waveforms, and used in computation of peak to valley changes in inter-beat intervals (IBI) from inspiration to expiration (ms) (Grossman, van Beek, & Wientjes, Reference Grossman, van Beek and Wientjes1990) as indicator of RSA. Variable quantification was derived by PHY General Physiology System and IBI Analysis Systems (James Long Company, Caroga Lake, NY).
Fetal heart rate and motor activity were collected and digitized from a fetal actocardiograph (MT320, Toitu, Toyko Japan), a Doppler-based device that detects and times the fetal heart beats and isolates fetal somatic activity. Maternal physiological signals were amplified using a multichannel, electrically isolated bioamplifier (Model JAD-04; James Long Company) Fetal data were analyzed using software developed to our specifications (GESTATE; James Long Company). Processing included use of an algorithm to detect and eliminate artifact in the fetal heart rate signal and yielded the following variables: (a) fetal heart rate (bpm); (b) fetal heart rate variability, quantified as the standard deviation per epoch; and (c) fetal motor activity, computed from mean values of all actograph data points, which are generated in arbitrary units. Maternal and fetal data channels were multiplexed into a single file for subsequent data analysis. Maternal and fetal responses to maternal viewing of the videos were quantified within the following segments: 5 min baseline, comprising the final 5 min of the 50 min recording; positive video/negative video (order varied); 5 min recovery period. (AR/PV/NV)
Postnatal procedure and measures
The 140 women and infants attending the 6-month [M (SD) infant age = 191 days (9.1)] laboratory-based visit reflect 88.6% of the original sample. Of the 18 nonattendees, 7 had moved out of the area and 11 either declined or had scheduling issues. No differences on maternal sociodemographic measures were detected between women who did not attend the visit and those that did. Most visits took place in the morning at approximately 10:00.
Maternal Postnatal Attachment Scale (MPAS)
This the postnatal companion to the antenatal scale and focuses on how women regard the experience of caring for a young infant (Condon & Corkindale, Reference Condon and Corkindale1998). It is also a 19-item instrument that assesses perception of the maternal–infant relationship around three constructs: acceptance/tolerance; competence as a parent; and pleasure in proximity. Items include “When I am caring for the baby, I get feelings of annoyance or irritation [Very frequently to never],” and “When I have to leave the baby, I feel [sadness to relief].” The total (summed) score reflects the global quality of maternal–infant satisfaction with and attachment to the infant. (SP)
Maternal Experience Scale (MES)
This postnatal companion scale to the PES is designed to capture the pleasures and challenges of caring for a young infant (DiPietro, Goldshore, et al., Reference DiPietro, Goldshore, Kivlighan, Pater and Costigan2015b). Like the PES, it provides information about maternal positive and negative affective valence towards motherhood. Items include those derived from the antenatal scale that are also relevant to the postpartum (e.g., body changes; sleep) with additional items specific to infants (e.g., baby's appearance). Respondents are instructed to score each item as to “How happy, positive or uplifted” and “How unhappy, negative, or upset” it makes them feel. The MES scale also includes 41 items; scoring is as described above for the antenatal version. Participants in this study were also participants in the MES validation study so there is overlap in description of MES values (DiPietro, Goldshore, et al., Reference DiPietro, Goldshore, Kivlighan, Pater and Costigan2015b). (PV/NV)
Infant temperament
The Infant Characteristics Questionnaire (ICQ) (Bates, Freeland, & Lounsbury, Reference Bates, Freeland and Lounsbury1979) is an established and widely used instrument that ascertains maternal perception of infant temperament focused on difficultness. The 6-month version includes 32 items rated on 7-point scales that were aggregated into the following factors: fussy/difficultness, unadaptability, dullness, and unpredictability. (AR)
Mother–infant social communication
Infants were placed in an inclined infant seat and women were seated directly in front of them. Maternal and infant behavior was recorded using dual cameras in a split-screen format and coded offline. The FFSF (Tronick et al., Reference Tronick, Als, Adamson, Wise and Brazelton1978) procedure includes three standard episodes of 2 min each: (a) normal play engagement of infant in seat, without toys; (b) brief maternal turn away and turn back followed by maintenance of a neutral expression, without speaking or interacting, for the still-face episode; and (c) resumption of normal play in a reunion episode following another brief turn away/turn back.
Infant and maternal affective behaviors in each episode were coded by trained research assistants naïve to study hypotheses. Facial affect and gaze were coded in 1-s intervals using a modified Monadic phase coding system (Tronick, Reference Tronick1980). Facial affect was coded as positive, negative, or neutral; gaze was coded as away or toward partner. Coders were trained to reliability using a large pool of videotaped FFSF interactions from a separate study and a subset of interactions from the current study (15% of full sample) was randomly selected for double coding. Inter-coder reliability between coders, κ = 0.89 for infant and mother affect, and κ = 0.80 and 0.73 for infant and mother gaze, respectively. A prior report based on this sample of maternal–infant dyads (Busuito, Quigley, Moore, Voegtline, & DiPietro, Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) noted that infant behavioral variables during the FFSF were normally distributed but maternal behavioral variables showed the expected, limited distribution of negativity during normal play and the reunion episode, since nearly all women approached the task with positivity and engagement. As a result, maternal-only variables were excluded from this analysis.
Following prior work (Moore & Calkins, Reference Moore and Calkins2004), variable quantification included: (a) infant positive affect, (b) infant negative affect, (c) and infant gaze away, each computed as the proportion of valid time (i.e., not missing due to limited view) that the emotion was displayed during each FFSF episode; (d) positive affect matching, the proportion of time during normal play and reunion episodes in which mother and infant showed matched positive affect (i.e., both had positive facial expressions at the same time); and (e) infant smiling (yes/no) used as a social bid during the still-face episode only. (SP/PV/NV/AR)
Statistical analysis
Change during pregnancy for maternal psychological measures was established through repeated measures analysis of variance (rMANOVA). Pearson correlation coefficients were computed to ascertain intra-individual stability of these measures across the three gestational periods and 6-month visit. To reduce the number of analyses, mean values were computed across pregnancy and weighted values for uplifts (i.e., uplift intensity multiplied by uplift frequency) and hassles (i.e., hassle intensity multiplied by hassle frequency) were used in analyses with other variables. Maternal (i.e., heart rate, RSA, and skin conductance) and fetal (i.e., heart rate, heart rate variability, and motor activity) responses to maternal viewing of the videos were evaluated using rMANOVA. Individual differences in maternal and fetal responsivity, from baseline through recovery, were characterized through computation of area under the curve with respect to increase (AUCi) and used in linear analyses.
Associations between prenatal and postnatal measures were evaluated through correlational methods for continuous variables. Because the FFSF was not used as a method for ascertaining infant reaction to the still-face segment of the paradigm per se, mean values were computed for infant positive affect, infant negative affect, and infant gaze away across all three episodes; the mean for matched positive affect excluded the still-face episode. Associations with antenatal responsivity were evaluated by distribution of AUCi values into response suppression (AUCi ≤ 0) and activation (AUCi > 0) and evaluated with t tests. Infant smiling, a dichotomous categorical variable, was analyzed using chi-square with adjusted residuals. Maternal attachment groups, spanning the antepartum to postpartum, were created by first distributing scores into high and low antenatal and postpartum groups based on antenatal (mean MAAS) and postnatal (MPAS) scores. These were then combined into a single categorical variable containing four groups. ANOVA was used to evaluate associations with antenatal and postnatal measures with post hoc contrasts (Tukey HSD, p < .05).
Missing data
Maternal and fetal physiological variables during the positive and negative videos were subject to instances of missing data due to signal artifact, predominantly in fetal data. However, the maternal RSA variable had the most missing data due to difficulties introduced by the use of respiratory bellows with the pregnant body habitus. Computation of AUCi values further affected missing data due to missing data in one of the three FFSF episodes. As a result, df values are somewhat variable throughout. At the postpartum visit, several MPAS (n = 3) and MES (n = 4) questionnaires were not completed. FFSF data were not available for 11 of the 140 mother–infant dyads. Reasons for missing values include excess infant fussiness upon being placed in the infant seat or inadequate views during behavioral coding.
Results
Maternal attachment and affective valence during pregnancy and early motherhood
Pregnancy
Maternal feelings of attachment to the fetus (MAAS) increased significantly from 24 to 36 weeks, F (2, 272) = 66.64, p < .001 while maternal affective appraisal of the positive (uplifts) and negative (hassles) aspects of pregnancy (PES) remained constant during this period, Table 1. PES intensity and frequency ratios (uplifts ÷ hassles) resulted in values that were greater than 1.0 for nearly all participants (90% to 95% for intensity, 87% to 93% for frequency). MAAS scores showed robust intra-individual consistency during this time, rs ranged from .80 to .88; mean values were used in subsequent analyses. There was also strong intra-individual stability in PES intensity and frequency values across the three reports, rs range from .62 to .84. To consolidate analyses, mean values during pregnancy are used from this point forward, based on weighted (i.e., intensity × frequency) scores for hassles and uplifts, separately. Maternal attachment was significantly correlated with the degree to which women experienced pregnancy as uplifting and positive, but was unrelated to the degree to which women experienced pregnancy as hassling and negative, Table 2.
Table 1. Means (SD) for maternal attachment and affective valence during pregnancy and early motherhood (N = 158)
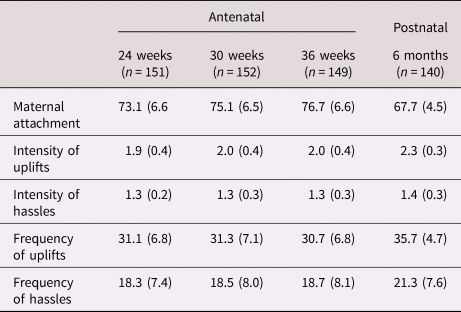
Table 2. Antenatal and postnatal intercorrelations of maternal attachment and affective valence

Note. Bolding signifies stability over time. ns = 158 and 137 for antenatal and postnatal measures, respectively.
*p < .01. **p < .001. MAAS = Maternal Antenatal Attachment Scale; PES = Pregnancy Experience Scale; MPAS = Maternal Postnatal Attachment Scale; MES = Maternal Experience Scale
During the 36-week maternal interview, top nominations for the most undesirable feature of pregnancy were: physical discomforts (60%), worrying about the fetus/baby (19%) and pregnancy constraints on activities (18%). Responses regarding the best feature of pregnancy were more idiosyncratic; the most frequent contenders were feeling the fetus move (19%) and preparing for the baby's arrival (9%). Two examples below illustrate of the breadth of perceptions offered by women.
“I get to feel his every movement and he's like mine right now … I already feel there is such a special little bond going … Nobody else but me can experience that right now … I know his little isms already, I know when he'll get hiccups, I know when he's going to be moving, or when he'll keep me up at night … I love it, before long I'm not going to have that feeling anymore.”
“I can't walk as fast, I'm not comfortable. Our house is a mess and I don't have the energy to clean it. I run marathons which is why this [pregnancy] is so hard … it's annoying. And I've been having lots of pelvic pain, it's like with this pregnancy the baby's head went right down there and started making trouble right away. Whatever he's doing down there right now sucks so bad … I'm just saying. Like I feel like he's trying to get out.”
Responses on the MAAS were also reflected in the narrative content. Compared to women with lower MAAS scores, those with higher values spoke less about the constraints imposed by pregnancy, t (94) = 2.03, and more about the fetus itself, t (94) = −2.27, ps < .05, and planning for the baby, t (94) = −1.96, p = .05. Women reporting higher MAAS scores spent more time discussing positive aspects of pregnancy relative to negative ones, t (94) = −2.47, did so with more intense affect, t (94) = −2.10, ps < .05, and required less prompting during the positive interview, t (94) = −2.81, p < .006. MAAS scores were unrelated to the degree of physical discomforts and pregnancy worries elicited by the negative interview, confirming the association between positive pregnancy valence and maternal attachment.
Six months postpartum
Means for maternal postnatal attachment (MPAS) and affective appraisal of motherhood (MES) are included in Table 1. Maternal postnatal attachment was significantly correlated with antenatal attachment, Table 2, highlighted. This association was evident as early as the 24th week antenatal visit, r (129)= .39, p < .001 (not shown). As during pregnancy, women overwhelmingly reported positive affective valence towards early motherhood such that 96% of both intensity and frequency ratios were >1.0. There was strong correspondence in the degree to which women experienced pregnancy and motherhood as uplifting versus hassling, Table 2, highlighted. Associations were also present from the 24th week visit, rs = .62 and .56, respectively, ps < .001 (not shown). Unlike during the antenatal period, postnatal attachment scores were significantly associated with postnatal hassles.
The maternal–fetal dyad: Physiological arousal and regulation
Maternal responsivity
Analyses indicated no order effects for whether women viewed the negative or positive video first, so responses were combined. Significant responsivity indicative of arousal was exhibited in each autonomic measure, Figure 2. This includes the response to the positive video, heart rate, F (2, 194) = 4.73, p = .01, skin conductance, F (2, 194) = 12.34, p < .001, and RSA, F (2, 158) = 5.68, p < .01, and to the negative one, heart rate, F (2, 194) = 9.77, p < .001, skin conductance, F (2, 194) = 12.72, p < .001, and RSA, F (2, 162) = 4.05, p < .05. Within individuals, correspondence between the magnitude of the maternal response for each autonomic measure to the positive video was highly correlated with the magnitude of the response to negative video: heart rate, r (96) = .85; skin conductance, r (96) = .89; and RSA, r (78) = .77, ps < .001, signifying within-individual autonomic reactivity that is independent of valence.

Figure 2. Evoked maternal heart rate, skin conductance and respiratory sinus arrhythmia responses to videos depicting positive, heartwarming aspects of pregnancy and childbirth and more negative, discomforting aspects. All responses were significant, F-values are reported in text.
Fetal responsivity
The fetal response to maternal viewing of positive and negative videos is presented in Figure 3. Fetal heart rate did not change in response to either, F (2,184) = 0.73 and F (2, 180) = 1.05, respectively. There was significant and persistent activation of fetal heart rate variability to the positive, F (2, 184) = 17.14, p < .001, and negative videos, F (2, 180) = 22.32, p < .001. Conversely, fetal motor activity was transiently suppressed during the negative video but rebounded after its termination, F (2, 180) = 3.20, p < .05; the fetal motor response to the positive video was similar but did not attain significance, F (2, 180) = 2.06, p = .13. Fetal responsivity to the positive and negative videos was correlated: heart rate, r (89) = .67; heart rate variability, r (91) = .24; and motor activity, r (89) = .56, ps < .001.

Figure 3. Evoked fetal heart rate, heart rate variability, and motor activity responses to maternal viewing of positive and negative birth videos. Fetal heart rate responses to positive and negative videos overlap. Fetal heart rate variability increased to both stimuli; fetal motor activity was suppressed to the negative video; F-values are reported in text.
Correlations between maternal and fetal physiologic responsivity
Given the correspondence between maternal and fetal responses to positively and negatively valenced videos, AUCi values were averaged for positive and negative videos and means were used in subsequent analyses. Results are presented in Table 3 and reveal modest associations between maternal and fetal responses, primarily in relation to maternal heart rate.
Table 3. Correspondence between evoked mean maternal and fetal responsivity to videos

Note. ns range from 72 to 93 due to missing values. RSA = respiratory sinus arrhythmia
*p < .05. **p < .01.
Antenatal contributors to dyadic social communication during the Face-to-Face Still-Face protocol
Maternal antenatal attachment and affective valence
MAAS and PES scores were unrelated to FFSF social communication behaviors, with one exception: infants of women who viewed pregnancy more negatively were less likely to use smiling as a social bid, t (115) = 2.09, p < .05.
Maternal antenatal autonomic arousal/regulation
Infants of women who reacted with suppression (i.e., AUCi < 0) of RSA to the videos while pregnant (n = 40) expressed less positive affect, t (64) = −2.19, p < .05, and more negative affect, t (64) = 2.82, p < .01, during the FFSF than those whose mothers reacted with RSA activation (n = 26), Figure 4. In addition, those infants were less prone to use smiling as a social bid, χ2 (1, N = 55) = 3.94, p < .05. Maternal electrodermal activity responsivity was unrelated to FFSF behaviors.

Figure 4. Evoked maternal respiratory sinus arrhythmia (RSA) and fetal heart rate variability (HRV) responses to maternal viewing of negative video in relation to dyadic social communication behaviors during Face-to-Face Still-Face (FFSF). Suppressors exhibited a withdrawal of parasympathetic tone in response to stimulation ([AUCi] ≤ 0); activators exhibited an increase (AUCi > 0). Values reflect the mean proportion of time behaviors were expressed during the FFSF protocol. *p < .05. **p ≤ .01.
Fetal autonomic arousal/regulation
Fetuses that responded with suppression (n = 14) in fetal heart rate variability as opposed to activation (n = 62), expressed, as infants, more positive affect during the FFSF, t (74) = 2.25, p < .05, less negative affect, t (74) = −2.22, p < .05, more matched positive affect with mothers, t (74) = 2.64, p < .01, and looked away less, t (74) = −2.56, p < .01, Figure 4. Fetal motor reactivity was unrelated to FFSF behaviors.
Antenatal contributors to maternal ratings of infant temperament
Antenatal attachment and affective valence
Women reporting lower antenatal attachment rated their 6-month infants as more fussy/difficult and unpredictable, rs (134) = −.19, p < .05. Similarly, higher negative pregnancy valence, as measured by weighted PES hassles score, was associated with higher ratings of fussy/difficultness, r (134) = .22, p < .01 and unpredictability, r (134) = .23, p < .01.
Maternal autonomic arousal/regulation during pregnancy
Maternal electrodermal or RSA suppression or activation to the videos during pregnancy were unrelated to temperament ratings.
Fetal autonomic arousal/regulation
Fetal heart rate variability responsivity was unrelated to temperament ratings, but fetuses that responded to maternal viewing of the videos with increased motor activity (n = 40) versus suppression (n = 47) were rated by mothers as more fussy/difficult, t (85) = −2.21, p < .05.
Contemporaneous associations between maternal ratings of infant temperament and observed behaviors
Correlations between the four ICQ factors and infant positive affect, negative affect, look away, and matched maternal positive affect were not significant, all rs < .13. Infants rated as more fussy/difficult and unpredictable were less likely to use smiling as a social bid, t (107) = 2.17 and t (107) = 2.34, ps < .05.
Exploratory analysis: Antecedent and contemporaneous contributors to stability and change in maternal attachment from the antepartum to postpartum
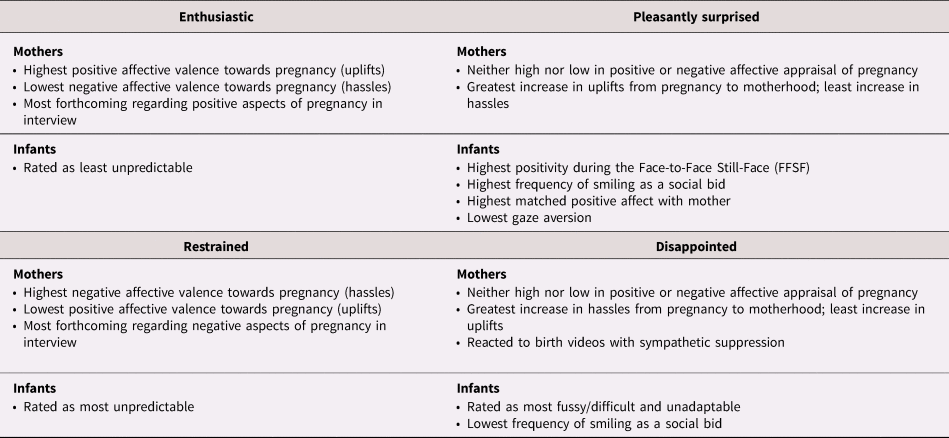
Distribution of women into attachment groups based on their positions above or below antenatal and postnatal sample medians resulted in the following groups: high antenatal/high postnatal or “Enthusiastic” (34%, n = 47); high antenatal/low postnatal or “Disappointed” (18%; n = 25); low antenatal/high postnatal or “Pleasantly Surprised” (15%; n = 20); and low antenatal/low postnatal, or “Restrained” (33%; n = 45).
Maternal affective valence during pregnancy and postpartum
Repeated measures analysis that included both antenatal (PES) and postnatal (MES) values showed significant main effects by attachment group for uplifts, F (3, 131) = 7.22, p < .001, with highest levels of pregnancy/motherhood uplifts in the Enthusiastic group and lowest in the Restrained group; the reverse pattern was observed for hassles, F (3, 131) = 8.24, p < .001. Significant Time × Attachment group interactions indicated differences in trajectory such that the greatest increase in uplifts was reported by the Pleasantly Surprised group and the lowest by the Disappointed group, F (3, 131) = 3.67, p = .01. The converse was true for hassles, F (3, 131) = 3.29, p < .05. Specific content (i.e., types of positive or negative occurrences mentioned) was not associated with attachment categories but women who were more forthcoming about the positive relative to the negative aspects of pregnancy were more likely to be in the Enthusiastic group, while the Restrained group spoke more freely about negative aspects, F (3, 90) = 3.34, p < .05.
Antenatal maternal autonomic arousal/regulation
Pregnant women responding with electrodermal activity suppression to the videos were more likely to be over-represented in the Disappointed group. The Enthusiastic and Pleasantly surprised groups were more likely to show electrodermal activation, χ2 (3, N = 93) = 12.37, p < .01.
Fetal autonomic arousal/regulation
Fetal responsivity to maternal viewing of videos was related to attachment categories.
Infant temperament
Maternal ratings of infant temperament differed on the basis of maternal attachment group for three of four factors, Table 4. The Disappointed group rated infants highest on Fussy/Difficult and Unadaptability, while the Pleasantly Surprised group rated infants as least Unadaptable. Unpredictability ratings were lowest in the Enthusiastic group.
Table 4. Infant Characteristics Questionnaire (ICQ) scale scores by maternal attachment group

Note. Bolding signifies groups that are significantly different (Tukey HSD) from at least one other group in post-hoc contrasts.
*p < .05. **p < .01. ***p < .001.
Dyadic social communication
Maternal attachment groups were significantly associated with infants’ expression of positive affect during the FFSF episodes. Significant ANOVA results included: infant positive affect, F (3, 116) = 3.86, p = .01, such that infants of Pleasantly Surprised mothers exhibited significantly higher positive affect than all other groups; less looking away, F (3, 104) = 2.42, p = .07, and greater matched positive affect between infant and mother, F (3, 114) = 3.02, p < .05; post hoc contrasts are provided in Figure 5. There was a significant association between infant smiling bids to re-engage mothers during the still-face episode and attachment groups, χ2 (3, N = 106) = 8.50, p < .05. Adjusted residuals indicated that this strategy was far more common for infants of women in the Pleasantly Surprised group (65%) than those in the Disappointed group (20%). Analysis of infant negative affect did not attain significance, F (3, 113) = 1.78, p = .15. Thumbnail descriptions of these groups are provided in Table 5.

Figure 5. Associations between maternal attachment categories, reflecting change and continuity from pregnancy to 6 months postpartum, and social communication behaviors. Values reflect the mean proportion of time behaviors were expressed across episodes of the Face-to-Face Still-Face (FFSF) protocol. Bars represent significant Tukey HSD post hoc contrasts between categories, *p < .05.
Table 5. Summary of maternal and infant characteristics associated with antenatal to postnatal attachment categories
Discussion
The mother–infant social bond is informed by both partners beginning as early as mid-gestation, confirming that it, indeed, does take two to grow a dyad. Individual variation in maternal feelings of attachment to the fetus and affective valence towards pregnancy conveyed to feelings of attachment to infants and affective valence towards motherhood. Attachment patterns characterized by stability from the antenatal to postnatal period were most associated with maternal affective appraisal of pregnancy while attachment shifts were influenced by infant characteristics and maternal physiological responsivity measured during pregnancy. Eliciting maternal physiological arousal through a salient set of pregnancy-related videos evoked a fetal response, with correspondence in the magnitude of response parameters between the maternal–fetal dyad. Patterns of arousal and regulation were associated with subsequent dyadic social communication behaviors, while ratings of infant temperament were most aligned with antenatal maternal affective valence.
From pregnant woman to mother: Maternal attachment and affective valence
The brief interview samples provided at the beginning of the results section illustrate the diversity with which women appraise their pregnancies and relationship to the fetus. Women's overall affective valence to pregnancy and motherhood skewed strongly positive such that nearly all participants expressed a more positive than negative valence towards pregnancy and motherhood on both frequency and intensity measures, although women readily acknowledged and reported the less gratifying aspects of both. Women's feelings of emotional connection to the fetus accelerated as labor and delivery approached, consistent with reports by others (van Bussel, Spitz, & Demyttenaere, Reference van Bussel, Spitz and Demyttenaere2010; Yarcheski, Mahon, Yarcheski, Hanks, & Cannella, Reference Yarcheski, Mahon, Yarcheski, Hanks and Cannella2009). Those expressing higher levels of attachment experienced pregnancy as more uplifting, but attachment was not affected by appraisals of routine hassles of pregnancy, assessed through questionnaire or interview. This changed after birth, such that maternal infant attachment was negatively associated with the hassles of early motherhood, perhaps suggestive of a contribution by actual infant characteristics to maternal mental representations.
The degree of stability in maternal attachment to the fetus and maternal attachment to the infant, reflecting 13% of shared variance, was comparable to that detected in other studies based on different populations and over longer elapsed time periods (de Cock et al., Reference de Cock, Henrichs, Vreeswijk, Maas, Rijk and van Bakel2016; Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021). Positive and negative appraisals of pregnancy and motherhood shared 44% and 40% of variance, respectively, and were conserved from the initial 24-week visit. Thus, the maternal experience of pregnancy and feelings of affiliation toward the fetus may reflect traits focused on more general qualities of women's affective tone and/or general orientation towards caregiving (Walsh, Reference Walsh2010). The strength of associations between maternal attachment and quality of maternal infant interaction does not wane over longer intervals, regardless of methodology, offering additional support (Foley & Hughes, Reference Foley and Hughes2018). Existing analyses based on this sample revealed that continuity in women's affective appraisal of pregnancy and early motherhood is not mediated through more generalized measures of maternal psychological distress (DiPietro, Goldshore, et al., Reference DiPietro, Goldshore, Kivlighan, Pater and Costigan2015b).
Our foray into categorizing relative change and stability in maternal attachment indicates that women who remained consistent in feelings of attachment to fetus and infant, expressing either relatively higher (Enthusiastic) or lower (Restrained) levels of attachment before and after birth, were distinguished by their positive and negative affective appraisal towards pregnancy. By the time infants are 6 months old, the dyad has had experience with one another allowing maternal perspectives to soften or harden and infants to both adapt to and shape maternal behavior. Women in these two groups also rated their infants’ temperaments in ways that reflected their affective stances toward pregnancy and motherhood, suggesting that these mothers may contribute more to the affective valence of the mother–infant relationship than the fetus or infant.
Affective appraisal of pregnancy was less relevant for women who expressed a change from below to above average attachment (i.e., Pleasantly Surprised). Instead, their infants exhibited higher levels of positivity than any other group, low levels of gaze aversion, high matched positive affect with mothers, and used smiling as a social bid most frequently during maternal disengagement. This constellation suggests temperamental qualities and social communication strategies in the infant that may elicit stronger feelings of attachment in the mother. Women who shifted from above average attachment during pregnancy to below average after birth were also unremarkable in terms of affective appraisal of pregnancy but more likely to exhibit a paradoxical response of sympathetic (i.e., electrodermal) suppression to the arousing birth video. Their infants were least likely to use smiling as a social bid. Presuming that there is some degree of within-individual stability in physiological response tendencies over time, this may signify a different physiological response pattern to the demands of infant care in general, and to a less socially engaging infant in particular, dampening maternal feelings of affiliation and establishing a cycle of dyadic disengagement.
The emerging dyad: Implications of arousal and regulation
This report contributes to the limited knowledge base regarding the dyadic physiological relationship between the fetus and pregnant woman. Maternal viewing of positive and negative videos evoked responses that were consistent with orienting (i.e., transient reduction in heart rate) and escalating sympathetic tone that did not return to baseline within the observation period. The parasympathetic response was more complex and differed somewhat by affective valence but ultimately marked by withdrawal of vagal tone. In turn, the maternal response generated a fetal response characterized by transient elevation of fetal heart rate variability and motor suppression. This pattern has been previously observed in response to induced maternal arousal (DiPietro et al., Reference DiPietro, Costigan and Gurewitsch2003) and relaxation (DiPietro, Costigan, Nelson, Gurewitsch, & Laudenslager, Reference DiPietro, Costigan, Nelson, Gurewitsch and Laudenslager2008a). Although there were several significant correlations between some maternal and fetal response parameters, associations, when detected, were modest at best and likely cannot explain the magnitude of the fetal response. The specific mechanisms through which maternal physiological reactivity is contemporaneously transduced to fetal autonomic and behavioral functioning remain poorly understood and findings are inconsistent (DiPietro et al., Reference DiPietro, Caulfield, Irizarry, Chen, Merialdi and Zavaleta2006; DiPietro, Costigan, et al., Reference DiPietro, Costigan and Voegtline2015a; Fink et al., Reference Fink, Urech, Isabel, Meyer, Hoesli, Bitzer and Alder2011; Monk et al., Reference Monk, Myers, Sloan, Ellman and Fifer2003). Women viewed the videos while wearing headphones to preclude any inadvertent audio stimulation of the fetus. It is possible that the fetus is reacting to sensory changes within the uterine environment that accompany abrupt changes in maternal autonomic systems, such as alteration in cardiovascular sounds (Porcaro et al., Reference Porcaro, Zappasodi, Barbati, Salustri, Pizzella, Rossini and Tecchio2006). There are also reported fetal responses to maternal arousal that are not measured here, such as altered blood flow in the fetal brain (Hassidov et al., Reference Hassidov, Asher, Ben-Ami, Keselman, Sabri and Haddad2018), as well as processes that remain unidentified.
Within-individual consistency in the magnitude of the maternal physiological responses to the positive and negative videos, regardless of order of presentation, provides support for the notion that maternal arousal and regulation is a stable response characteristic of individual women. This has implications for the threshold and intensity of maternal responses to quotidian events experienced during pregnancy that may elicit arousal. The fetus does not differentiate the affective valence of the maternal response but response tendencies characterized by differences in rise and recovery provide variation in the intrauterine milieu. This affects the fetal environmental context in a manner that is amplified over cumulative exposures, or lack of them, throughout gestation and continuing beyond our assessment at 30 weeks gestation.
As hypothesized, fetal suppression of heart rate variability was associated with indicators of better emotional regulation during the FFSF – more positivity, less negativity and gaze aversion, and greater dyadic matched positive affect. As noted earlier, suppression of vagal tone has been frequently linked to adaptive and regulatory behaviors in infants and young children (Porges, Reference Porges2007); here we find fetal origins of this association. Maternal suppression of RSA was also associated with infant behaviors but in the opposite direction – less infant positivity and more negativity. Given that no associations were detected with maternal sympathetic (i.e., electrodermal) responsivity, this implies that an intrauterine environment characterized by parasympathetic innervation provides a less disruptive environment to the fetus which may foster development of regulatory stability. A prior report of contemporaneous postnatal associations between physiological and behavioral responses during the FFSF in this sample echoes these findings: lower maternal RSA was associated with less synchronous mother-infant interactions, but electrodermal activity was unrelated (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019).
Temperament as a dyadic measure
Maternal ratings of infant temperament are influenced, to some degree, by infant behavior but also by maternal perceptions mediated by a host of maternal characteristics (Hane, Fox, Polak-Toste, Ghera, & Guner, Reference Hane, Fox, Polak-Toste, Ghera and Guner2006; McGrath, Records, & Rice, Reference McGrath, Records and Rice2008). In this study, maternal ratings of aspects of difficult infant temperament were negatively associated with maternal feelings of attachment and positively associated with negative affective appraisal of pregnancy. This implies that perception of infant temperament has its roots in the overall affective appraisal of pregnancy and affiliative tendencies towards the fetus. However, the possibility that maternal impressions are shaped by fetal signaling is suggested by the finding that fetuses reacting to the videos with motor activation were rated as more fussy/difficult. Evoked fetal motor activity stimulates an almost immediate maternal sympathetic response (DiPietro et al., Reference DiPietro, Voegtline, Costigan, Aguirre, Kivlighan and Chen2013); fetuses that respond to perturbation in this manner may affect maternal autonomic balance and thereby alter maternal perceptions. Fussy/difficultness is also a signifier of heightened temperamental reactivity and diminished regulatory capacity so the fetal response may portend actual infant behavior that is detected by mothers. Studies that have documented more extensive associations between maternal (Ostlund et al., Reference Ostlund, Vlisides-Henry, Crowell, Raby, Terrell, Brown and Conradt2019) or fetal reactivity (DiPietro, Ghera, et al., Reference DiPietro, Ghera and Costigan2008b; Werner et al., Reference Werner, Myers, Fifer, Cheng, Fang, Allen and Monk2007) to evoked maternal arousal with subsequent infant temperament have measured observed behavior in infants as opposed to relying on maternal reports.
In the postnatal period, maternal ratings of infant temperament were orthogonal to observed infant dyadic behaviors with the exception of the specific behavior of infant smiling, which was associated with significantly lower ratings of infant fussy/difficultness and unpredictability. Infant smiling as a bid to re-engage mothers during the still-face episode is a highly distinctive behavior and connotes significant regulatory control on the part of the infant. Infants who employ this strategy are more likely to be securely attached at 12 months (Cohn, Campbell, & Ross, Reference Cohn, Campbell and Ross1991) and exhibit lower levels of externalizing behaviors later in infancy (Moore, Cohn, & Campbell, Reference Moore, Cohn and Campbell2001). We suggest that this is a particularly salient behavior to mothers and/or is representative of a constellation of other behaviors implicated in self-regulation. Yet, the role of maternal affective tone in fostering this behavior cannot be discounted; infants of women reporting more hassles during pregnancy were less likely to use this strategy during the still-face segment.
The RDoC framework
The construct of maternal attachment and its variations in terminology (e.g., bonding) are most frequently found in biomedical research, whereas the construct of mother–infant attachment has a long history in developmental research. Attachment as conceptualized by the RDoC framework is inherently a social bond involving another individual, despite the fact that it is often measured in terms of behaviors of only one individual (e.g., infant behavior in response to maternal separation and reunion). Maternal attachment, as operationalized in this study, provides recognizable shorthand for the affective engrossment that generally originates during pregnancy and accompanies the transition to motherhood and that, inherently, involves another individual. When measured in the antepartum, that individual is an imagined future infant. When measured in the postpartum, maternal attachment must now accommodate the mother's interactions with the actual infant, which may contribute to a change in the degree or quality of maternal attachment.
We acknowledge that our measures of positive and negative valence are not traditional measures of response to threat or reward as described within current RDoC constructs. We suggest that they may be appropriate applications given that pregnancy and early motherhood reflect unique, intense and transient periods during which the stakes are high and the potential for reward and threat are particularly salient. Interpretation of pregnancy and motherhood experiences as either positive or negative reflects an individual's valuation of pregnancy and motherhood in terms of either an ongoing rewarding (i.e., affirming) or aversive (i.e., threatening) experience, or combination of both. Maternal reactivity to stimuli that depict narratives about labor, delivery and the initial experience of motherhood reflect the response to an anticipated reward (i.e., desired birth outcome) or a potential threat (i.e., traumatic birth experience).
Results highlight the integral role of Social Processes in other RDoC domains of Arousal/Regulation and Positive/Negative valence systems. We conceptualize the mother–child relationship as a developmental and multilevel set of processes with precursors in the antenatal period, including maternal and fetal arousal and regulatory systems, which have intra-individual continuity into the postpartum period and are associated with social communication and affiliation behaviors during mother - infant interaction. Maternal arousal systems both reveal maternal characteristic patterns of reactivity and regulation but also provide variation in the intrauterine milieu experienced by the developing fetus. This environment both elicits and helps shape the substrate for temperamental attributes related to infant reactivity and regulation. Variation in response tendencies and attributes of both the fetus and mother serve as scaffolding for dyadic processes which change in dynamic ways over time and ultimately lead to mutual engagement or dysfunction within maternal–infant dyads.
Limitations and other considerations
The principal limitation of this report is that it is based on a sample of primarily well-educated women with wanted pregnancies and lifestyles permitting them to attend repeated laboratory-based visits. Maternal attachment is often found to be unrelated to sociodemographic factors (Van den Bergh & Simons, Reference Van den Bergh and Simons2009) although when associations are detected, more educated women tend to report lower levels of antenatal and postnatal attachment (Ertmann et al., Reference Ertmann, Bang, Kriegbaum, Væver, Kragstrup, Siersma and Smith-Nielsen2021; Lindgren, Reference Lindgren2001; van Bussel et al., Reference van Bussel, Spitz and Demyttenaere2010). Although not an exclusion criteria per se, the sample does not include women under treatment for significant mental health issues that may jeopardize psychological well-being during pregnancy. Mean MAAS (antenatal attachment) values recently reported from two large, population-based cohorts in Denmark (Ertmann et al., Reference Ertmann, Bang, Kriegbaum, Væver, Kragstrup, Siersma and Smith-Nielsen2021) and Australia (Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021) were very similar to those found in our United States-based sample, although MPAS (postnatal attachment) values in our sample were considerably lower than in Australia (Le Bas et al., Reference Le Bas, Youssef, Macdonald, Mattick, Teague, Honan and Hutchinson2021). An interesting cross-cultural study provides evidence for measurement equivalence of the construct of antenatal attachment across eight low- and middle-income countries and found no differences in mean antenatal attachment levels among six of them (Foley et al., Reference Foley, Hughes, Murray, Baban, Fernando, Madrid and Eisner2021). Nonetheless, the current study went beyond the construct of maternal attachment and the fairly narrow sociodemographic profile of participants requires expansion to inform generalizability.
Non-pregnancy specific features of maternal psychological functioning, such as state or trait anxiety and depressive symptoms, were not included in this analysis. These characteristics have been frequently measured and reported in other studies. The positive emotional valence of pregnancy and motherhood and escalating feelings of affiliation toward the fetus and infant, observed here and elsewhere, is not widely reflected in the research literature. Viewing pregnancy and motherhood through a lens of psychological distress and strain has implications for public perceptions, and for women in particular (Matthey, Reference Matthey2010; Oates, Reference Oates2002). Moreover, it can obscure key influences on the shaping of the mother–infant dyad and generate an unbalanced view of pregnancy and motherhood in adult development. Of course, a narrow view of pregnancy using only a positive lens can be equally affecting for women who face pregnancy and motherhood challenges, but the current state of the literature as a whole is not biased in this direction.
Conclusions
In concert, current findings illustrate how variation in arousal and regulatory systems of the pregnant woman and fetus operate within the context of maternal positive and negative valence systems to separately and jointly shape affiliation and attachment during the first 6 months of life. Attachment is inherently a social process and findings suggest that pregnant women are gestating not only a fetus but the seeds of a social bond that arises from their affective orientations towards pregnancy, motivation for affiliation, and caregiving tendencies once the infant is born. Results also underscore the importance of understanding the contribution of each member of the dyad in the developmental process that ultimately yields the mother–infant social relationship.
Acknowledgment
We are grateful for the dedication and generosity of our study families without whom this work would not be possible.
Funding Statement
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) award R01 HD27592 awarded to the first author.
Conflicts of Interest
None.













