Introduction
Rice (Oryza sativa L.) is the staple food crop for more than half of the world’s population and an important crop for achieving global food security (Subudhi et al. Reference Subudhi, Sasaki and Khush2006). In the United States, rice is predominantly produced in Arkansas, Mississippi, Missouri, Louisiana, Texas, and California (USDA-ERS 2017). Weeds are among the major causes of rice yield losses worldwide and are becoming a bigger threat as growers shift from transplanted to direct-seeded rice due to water and labor limitations (Chauhan Reference Chauhan2012; Rao et al. Reference Rao, Johnson, Sivaprasad, Ladha and Mortimer2007). The most problematic weeds in southern U.S. rice fields (Arkansas and Mississippi) are barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], sprangletops (Diplachne spp.), weedy rice (Oryza spp.), northern jointvetch [Aeschynomene virginica (L.) Britton, Sterns & Poggenb.], and Palmer amaranth (Amaranthus palmeri S. Watson) (Norsworthy et al. Reference Norsworthy, Bond and Scott2013). In Arkansas, season-long weed interference can reduce rice yield from 10% with eclipta [Eclipta prostrata (L.) L.] to 82% with Oryza spp. Yield losses vary depending on weed species and density (Smith Reference Smith1988; Smith et al. Reference Smith, Flinchum and Seaman1977).
Oryza spp. are conspecific, highly competitive, and aggressive weeds of cultivated rice (Vaughan et al. Reference Vaughan, Ottis, Prazak-Havey, Bormans, Sneller, Chandler and Park2001). It has evolved through hybridization among and within cultivated and wild rice and has diverse genetic, physiological, and morphological characteristics compared with cultivated rice (Londo and Schaal Reference Londo and Schaal2007; Shivrain et al. Reference Shivrain, Burgos, Agrama, Lawton-Rauh, Lu, Sales, Booyett, Gealy and Moldenhauer2010a, Reference Shivrain, Burgos, Scott, Gbur, Estorninos and McClelland2010b). Oryza spp. vary widely in terms of hull type (blackhull, strawhull, brownhull, goldhull), awn color (black, straw, pink), awn length (long awn, short awn, awnless), plant height (76 to 190 cm), number of tillers, panicle length, and seed production potential (Shivrain et al Reference Shivrain, Burgos, Scott, Gbur, Estorninos and McClelland2010b). Oryza spp. have vigorous vegetative growth, high tillering, large biomass production, high nitrogen consumption, variable seed germination and maturity timings, high seed shattering, differential dormancy, and high seed longevity, which collectively make it highly persistent and more competitive than cultivated rice (Burgos et al. Reference Burgos, Norman, Gealy and Black2006, Reference Burgos, Shivrain, Scott, Mauromoustakos, Kuk, Sales and Bullington2011; Constantin Reference Constantin1960; Diarra et al. Reference Diarra, Smith and Talbert1985; Fogliatto et al. Reference Fogliatto, Vidotto and Ferrero2011; Tseng et al. Reference Tseng, Burgos, Shivrain, Alcober and Mauromoustakos2013). Pantone and Baker (Reference Pantone and Baker1991), through weed–crop competition modeling, demonstrated the dominant nature of Oryza spp. and showed that the competitive ability of one Oryza spp. was equivalent to that of four cultivated rice plants. Oryza spp. have very high interference potential, with an average density of 1 plant m−2 causing 100 to 755 kg ha−1 yield loss of rice cultivars (‘CL161’, ‘Cocodrie’, ‘LaGrue’, ‘Lemont’, and ‘XL8’) (Ottis et al. Reference Ottis, Smith, Scott and Talbert2005).
The aggressive and persistent nature of Oryza spp. makes it one of the most troublesome weeds in rice fields. However, the ability of Oryza spp. to tolerate various biotic and abiotic stress conditions makes it an important resource for plant breeders. Liu et al. (Reference Liu, Qi, Gealy, Olsen, Caicedo and Jia2015) identified 28 novel blast-resistance quantitative trait loci (QTL) in two U.S. Oryza spp. lines. Oryza spp. respond positively to elevated carbon dioxide concentrations, suggesting potential to thrive under climate change and global warming (Ziska and McClung Reference Ziska and McClung2008).
Plants, especially weedy species, show variable response to herbicides (Brown et al. Reference Brown, Chandler and Bridges1987). Oryza spp. have differential tolerance to imazethapyr and glyphosate (Burgos et al. Reference Burgos, Shivrain, Scott, Mauromoustakos, Kuk, Sales and Bullington2011; Kuk et al. Reference Kuk, Burgos and Shivrain2008). The exposure of Oryza spp. to glyphosate has increased with the standard practice of planting glyphosate-resistant soybean [Glycine max (L.) Merr.] in rotation with rice in the southern United States and the use of glyphosate to desiccate vegetation preplant or to manage weeds postharvest of rice. Thus, the tolerance level of Oryza spp. to glyphosate may have increased with time. With the anticipated commercialization of ProvisiaTM rice, with its tolerance to a strong, selective grass herbicide (quizalofop), selection for acetyl-CoA carboxylase–tolerant ecotypes is expected. In field tests, all Oryza spp. accessions can be controlled with a sequential application of quizalofop, although some accessions are more difficult to control than most (NR-B, unpublished data). One herbicide that is relatively new in rice production is the protoporphyrinogen oxidase (PPO) inhibitor saflufenacil. While rice has some commercial tolerance to this herbicide, it is not tolerant to another chemical family in this group, represented by flumioxazin. Thus, Oryza spp. can be controlled by flumioxazin applied in fall or spring, no sooner than 1 mo before planting rice. Glufosinate, a broad-spectrum contact herbicide, can also be used as an alternative tool to control Oryza spp. outside the rice crop cycle in preplant burndown or in glufosinate-resistant soybean grown in rotation. To ascertain the utility of these herbicides for Oryza spp. management, their efficacy on a wide range of Oryza spp. accessions needs to be evaluated. Corollary to this objective, it may be possible to find herbicide-tolerant accessions that can be used for rice crop improvement. Thus, we aimed to (1) test a diverse germplasm of Oryza spp. collected between 2008 and 2009 for response to glyphosate, glufosinate, and flumioxazin; and (2) determine whether the morphological trait (hull color) of Oryza spp. is associated with response to herbicides.
Materials and methods
Seed collection
Over 200 Oryza spp. accessions were collected from all rice growing counties in Arkansas in 2008 to 2009. These accessions were grown at the Rice Research and Extension Center, Stuttgart, AR, and characterized in terms of plant height, tillering, days to flower, hull color, culm length, panicle length, grain shattering, and grain yield (Tseng Reference Tseng2013). Using these morphological data, 54 accessions deemed to be most competitive were selected based on key weedy traits, including high tillering capacity, high seed shattering, and tall culm length; these traits are generally linked to aggressiveness and persistence (Delouche et al. Reference Delouche, Burgos, Gealy, Martin, Labrada, Larinde and Rosell2007). These 54 accessions were tested for response to glyphosate, glufosinate, and flumioxazin in this study.
Bioassay in the greenhouse
Experiments were conducted in 2016 and 2017 in two runs in the greenhouse at the RR Foil Plant Science Research Center, Mississippi State University. The minimum and maximum temperatures were set at 25 and 35 C, respectively, with 70% relative humidity. Two rice cultivars, ‘CL163’ and ‘REX’, were included as reference lines for comparison. Seeds were pre-germinated in 0.3% agar medium. After 1 wk, pre-germinated seeds were transferred to 50-cell trays filled with Sunshine® #1 (Sun Gro Horticulture Canada, Vancouver, Canada), an all-purpose potting medium with sphagnum peat moss, perlite, dolomitic limestone, long-lasting wetting agent, and RESiLIENCE® (Silicon). Two accessions were planted per tray at 25 plants per accession. Plants were sprayed with glyphosate, glufosinate, or flumioxazin, in separate treatments. Data were recorded for individual plants, with 100 data points for each accession. Information on the products and doses used are presented in Table 1. Treatments were applied at the 3- to 4-leaf stage in a spray chamber equipped with TP8002-VS VisiFlo Flat Spray Tip (TeeJet®, Spraying Systems, P.O. Box 7900, Wheaton, IL 60187) calibrated to deliver 187 L ha−1 at 275 kPa. Because smaller plants are more sensitive to herbicides than larger plants (Norsworthy et al. Reference Norsworthy, Scott, Smith and Oliver2008; Shrestha et al. Reference Shrestha, Hembree and Va2007), glyphosate and glufosinate were applied to seedlings with 3 to 4 leaves to observe differences in response among accessions. Three doses (1X, 1.25X, and 1.5 X) of glufosinate were tested, as all Oryza spp. accessions recovered from the 1X dose (Table 1). Flumioxazin was applied PRE. No adjuvants were used with glyphosate and flumioxazin, but ammonium sulfate at the rate of 1,600 g ha−1 was added to glufosinate. Trays were returned immediately to the greenhouse after herbicide application, and watering was resumed after 24 h. Plant injury from glyphosate and glufosinate was evaluated at 3 and 5 wk after treatment (WAT) on a scale of 0% to 100%, where 0% meant no injury, and 100% meant complete plant mortality with no remaining green tissue (Frans et al. Reference Frans, Talbert, Marx and Crowley1986). Visible injury symptoms generally included chlorosis followed by necrosis of leaf tissue, and stunting. For flumioxazin, emergence was evaluated at 1 and 2 wk after planting. Plant height and injury were recorded at 2, 3, 4, and 5 WAT. The degree of stunting relative to nontreated checks was calculated using the following formula:
 $${\eqalign{{\hskip-2pt\rm{Stunting}} \left( \% \right){\rm{ }} \\ \cr \hskip8pt= {{{\rm{Height \ of \ control \ plant}}\left( {{\rm{cm}}} \right){\rm{}} - {\rm{Height \of \ of \ treated \ plant}}\left( {{\rm{cm}}} \right)} \over {{\rm{Height \ of \ control \ plant}}\left( {{\rm{cm}}} \right)}}{\rm{ }} \times 100}}$$
$${\eqalign{{\hskip-2pt\rm{Stunting}} \left( \% \right){\rm{ }} \\ \cr \hskip8pt= {{{\rm{Height \ of \ control \ plant}}\left( {{\rm{cm}}} \right){\rm{}} - {\rm{Height \of \ of \ treated \ plant}}\left( {{\rm{cm}}} \right)} \over {{\rm{Height \ of \ control \ plant}}\left( {{\rm{cm}}} \right)}}{\rm{ }} \times 100}}$$
Table 1. Herbicide information and rates used

Plants showing less than 40% injury at 5 WAT were transferred to larger pots for obtaining grain yield (75–100 days after sowing [DAS]). Nontreated plants were grown until maturity (75–100 DAS) to obtain the full yield potential under this environment.
Dose–response experiment
Owing to large differences in response of Oryza spp. accessions to glyphosate, a dose–response experiment was conducted for three Oryza spp. accessions with less sensitivity to glyphosate (B2, B20, S11). S118 was used as the susceptible standard. Six seeds of each accession were sown in 10-cm-diameter pots and later thinned to 4 plants pot−1. The experimental units (pots) were arranged in a randomized complete block design with three replications for each dose due to seed limitation. Spraying was done at the 3- to 4-leaf seedling stage. Tolerant plants were sprayed with glyphosate at 0, 560, 1,120, 1,680, 1,960, and 2,240 g ae ha−1. The susceptible accession, S118, was sprayed with 0, 140, 280, 560, 840, and 1,120 g ha−1. Visible percent control was recorded at 3 and 5 WAT. The shoot tissues were harvested at 5 WAT, oven-dried for 78 h, and weighed to record aboveground biomass.
Statistical analysis
Oryza spp. accessions and herbicide treatment were considered fixed effects, while replications, runs, and run by accession were random effects (Yang Reference Yang2010).
Normality of the data was investigated by plotting QQ [quantile-quantile] plots for residuals of visual injury and plant height in JMP v. 13 (SAS Institute, SAS Campus Drive, Cary, NC 27513). The QQ plots showed normal distribution of data.
Data were subjected to ANOVA using PROC MIXED METHOD=TYPE3 with accessions and runs as classification variables (SAS v. 9.4, SAS Institute), and mean values were separated using Fisher’s Protected LSD at an alpha level ≤ 0.05. The hierarchical clustering method of JMP v. 13 (SAS Institute) statistical software was used to group accessions into different clusters based on their injury to glyphosate at 5 WAT. Clustering was not performed for flumioxazin and glufosinate, as results did not seem to form distinct clusters based on tolerance. In this approach, the mean injury of each accession is considered as a single point initially, and the two closest points are combined, with the process continuing until all points join to form a single cluster. Ward’s minimum variance is used to group accessions into different clusters, wherein the ANOVA sum of squares between the two nearest clusters added up over all variables is the distance between two variables.
Glyphosate dose–response data for biomass were analyzed using nonlinear logistic regression to determine the effective dose of glyphosate that causes 50% biomass reduction. This analysis was performed in R v. 3.4.3 (R Development Core Team 2017) with the drc package. A three-parameter log-logistic model was used, which took the following form:
where the parameter d is the upper limit, b is the slope of the curve, x is the herbicide dose, e is the effective dose that causes 50% biomass reduction, and the lower limit is set at 0, which corresponds to the lowest herbicide dose used in the experiment.
Results and discussion
Response of accessions to glyphosate, glufosinate, and flumioxazin
We expected differential sensitivity to herbicides among Oryza spp. accessions due to their genetic and morphological variation. Data from the experiment supported this assumption, as 54 Oryza spp. accessions showed differential response to glyphosate and flumioxazin (P < 0.0001). However, the accessions did not vary in terms of their response to glufosinate. Glufosinate applied at 874 g ai ha−1 (1X) and 1.25X did not cause any significant injury to either cultivated or Oryza spp., whereas glufosinate applied at the 1.5X rate killed all accessions. Similar results were obtained by Pearson et al. (Reference Pearson, Burgos, Scott, Bullington, Shivrain, Norman, Meullenet and Moldenhauer2006), who found glufosinate to be the weakest herbicide compared with glyphosate and imazethapyr, providing only 49% control of Oryza spp. when applied at the full rate. Glufosinate applied alone for Oryza spp. control is not very effective, and 92% control was obtained only when a high rate of 1,100 g ha−1 (1.8X) was applied (Sankula Reference Sankula1997). Glufosinate is primarily effective on broadleaf weeds; however, the efficacy of this herbicide on grasses can be enhanced by mixing it with other herbicides such as acifluorfen (Sankula Reference Sankula1997).
Oryza spp. injury from glyphosate applied at the rate of 1,120 g ha−1 ranged from 30% (B20) to 97% (S118) at 5 WAT, with B20 being the accession most tolerant to glyphosate and S118 being the most sensitive (Figure 1). The cultivated rice lines CL163 and REX were highly sensitive to glyphosate, with injury rates of 97% and 98%, respectively, at 5 WAT (Figure 1). Of the 54 Oryza spp. accessions, three exhibited less sensitivity to glyphosate than others, with less than 40% injury at 5 WAT (shaded bars in Figure 1). We considered these accessions potentially tolerant to glyphosate, as they recovered from glyphosate injury with new growth at 5 WAT compared with 3 WAT. Sensitive accessions and rice cultivars, on the other hand, showed gradual increase in injury from 3 to 5 WAT. Injury of the three most tolerant accessions, B20, B2, and S11, decreased from 36%, 39%, and 49% at 3 WAT to 30%, 34%, and 38% at 5 WAT, respectively, reflecting their potential to recover from injury. On the other hand, injury of the three most sensitive accessions, ALR-1, B44, and S118, increased from 86%, 89%, and 95% to 94%, 94%, and 97%, respectively, from 3 to 5 WAT.

Figure 1. Injury among the Oryza spp. accessions at 3 and 5 wk after treatment (WAT) with glyphosate (1,120 g ae ha−1). Shaded bars represent accessions with reduced sensitivity to glyphosate exhibiting high recovery from glyphosate injury and less than 40% injury at 5 WAT.
Oryza spp. accessions collected from major rice growing counties of Arkansas in 2002 to 2003 were controlled 81% to 100% by the commercial dose of glyphosate (Burgos et al. Reference Burgos, Shivrain, Scott, Mauromoustakos, Kuk, Sales and Bullington2011). The Oryza spp. accessions used in this experiment were collected in 2008 to 2009 from mostly the same regions and were controlled 70% to 98% by glyphosate, excluding three potentially glyphosate-tolerant accessions. Glyphosate was effective on Oryza spp., except for B2, B20, and S11, which showed less than 40% injury. Reduced sensitivity of these accessions to glyphosate may be due to cropping practices and glyphosate use patterns in these rice production areas. Most farmers in the southern United States rotate rice with soybean to control infestation of weeds, including Oryza spp., in rice fields (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Griffin et al. Reference Griffin, Baker, Dunand and Sonnier1986; Smith et al. Reference Smith, Flinchum and Seaman1977). In soybean, glyphosate was initially used as a burndown herbicide to control all emerged weeds before planting. Depending on the time of planting, these preplant weeds could include Oryza spp. Stale seedbed has been a very effective practice to reduce the Oryza spp. population. Any Oryza spp. that emerge during the soybean season can be controlled with selective grass herbicides. Since the commercialization of glyphosate-resistant soybean in 1996, glyphosate has been applied over the top of soybean, so exposure of Oryza spp. populations to glyphosate has increased, and selection for tolerant populations is expected.
Oryza spp. have variable germination and dormancy (Tseng et al. Reference Tseng, Burgos, Shivrain, Alcober and Mauromoustakos2013); some Oryza spp. germinate later in the season. Individuals that escape the preplant herbicide application may be exposed to glyphosate during the first POST application in soybean. Late-emerging plants would be exposed to the second application of glyphosate if a farmer applied glyphosate twice. The diverse emergence patterns of Oryza spp. imply that plants of various sizes are exposed to glyphosate, which may favor selection for increased tolerance.
Flumioxazin is used for preplant burndown in rice, cotton (Gossypium hirsutum L.), and soybean. As rice has a 30-d rotational restriction for flumioxazin, Oryza spp. were also expected to be sensitive to PRE application of flumioxazin. Most Oryza spp. accessions were susceptible to flumioxazin, indicating its effectiveness in controlling this weed. With flumioxazin, most accessions were injured more than 80% at 5 WAT; however, B49, B51, and S59 showed lower injury rates of 22%, 30%, and 39%, respectively (Figure 2). Among these three accessions that were less sensitive to flumioxazin, S59 showed a high recovery rate of 33% from herbicide injury, while, B49 and B51 showed slight increase in injury at 5 WAT compared with 3 WAT. Flumioxazin did not affect Oryza spp. emergence, which was greater than 80% in all accessions tested (unpublished data). Herbicides for controlling Oryza spp. in rice fields are limited, and flumioxazin has a 30-d waiting period before rice planting due to potential injury to rice. One PPO-inhibitor herbicide, saflufenacil, is now labeled for use in rice, but could cause some injury under certain conditions and is not effective on Oryza spp. As rice and Oryza spp. are closely related, flumioxazin could be effective for Oryza spp. control if we could develop flumioxazin-tolerant rice cultivars. Oryza spp. accessions with tolerance to flumioxazin can provide tolerance genes to introgress the trait into cultivated rice. Genetic analysis will be conducted to identify QTL associated with tolerance to glyphosate or PPO-inhibitor herbicides.

Figure 2. Injury among the Oryza spp. accessions at 3 and 5 wk after treatment (WAT) with flumioxazin (72 g ai ha−1). Shaded bars represent accessions with reduced sensitivity to flumioxazin and injury of less than 40% at 5 WAT.
Effect of herbicide treatment on plant height and yield of Oryza spp.
Reduction in plant height following glyphosate application ranged from 4% to 40% at 5 WAT compared with control plants. The highest rate of stunting (40%) was observed in ALR-1. The three most tolerant accessions, namely, B20, B2, and S11, showed minimal stunting of 4%, 5%, and 8%, respectively. Due to high sensitivity of most accessions to flumioxazin, stunting was computed only for B49, B51, and S59, and values were 19%, 22%, and 38%, respectively. Glyphosate or flumioxazin treatments did not reduce the grain yield of tolerant accessions compared with nontreated plants. Percentage reduction in grain yield of B2, B20, and S11 with low sensitivity to glyphosate ranged from −6% to 12% compared with the untreated control (Table 2). Accessions S59, B49, and B51, which had low sensitivity to flumioxazin, showed grain yield reduction from −6% to 18%. The yield data infer that these plants have high potential to dominate the soil seedbank in a few generations. Oryza spp. seeds have long dormancy periods and variable emergence and may germinate after several years, causing sudden outbreaks of Oryza spp. in crop fields (Goss and Brown Reference Goss and Brown1939; Tseng et al. Reference Tseng, Burgos, Shivrain, Alcober and Mauromoustakos2013).
Table 2. Grain yield of Oryza spp. accessions with reduced sensitivity to glyphosate and flumioxazin applied at the rate of 1,120 and 72 g ae ha−1 compared with grain yield of respective control plants

a Accessions potentially tolerant to glyphosate.
b Accessions potentially tolerant to flumioxazin.
Response of Oryza spp. accessions to herbicides based on hull color
In the present study, the primary classification of Oryza spp. accessions was based on hull color. The response of accessions to herbicides based on hull color was therefore analyzed to see whether herbicide tolerance was correlated with hull color. Three hull types of Oryza spp. accessions were used in this study, namely, blackhull, strawhull, and brownhull. Cultivated rice, CL163, and REX, were grouped separately for comparison. Even though there was variable response to herbicide within each hull type, blackhull-type plants were the least injured by herbicides than other hull types at both 3 and 5 WAT (Table 3). Injury rates due to glyphosate and flumioxazin for blackhull types were 76% and 89%, respectively, at 5 WAT. Brownhull and strawhull biotypes were controlled 85% to 100% by both herbicides. Rice cultivars were most sensitive, with 98% to 100% injury to both the herbicides. In a similar study by Gealy et al. (Reference Gealy, Dilday, Baldwin, Black, Norman and Johnston1999), blackhull accessions were more tolerant to imazethapyr (Pursuit®) than strawhull accessions. The Oryza spp. accession TX4 showed high tolerance to glufosinate and was not completely controlled at the 1,120 g ai ha−1 rate (Noldin et al. Reference Noldin, Chandler, Ketchersid and McCauley1999). TX4, with low susceptibility to glufosinate, was a blackhull biotype with high dormancy obtained from Texas. Higher herbicide tolerance in blackhull biotypes may be due to the presence of diverse genes. Nei’s genetic distance for blackhull Oryza spp. from Arkansas was 0.76, while that of strawhull Oryza spp. was 0.66 (Shivrain et al. Reference Shivrain, Burgos, Agrama, Lawton-Rauh, Lu, Sales, Booyett, Gealy and Moldenhauer2010a), suggesting higher genetic diversity in blackhull and thus greater potential to adapt to stresses (Jia and Gealy Reference Jia and Gealy2018). Blackhull and strawhull strains evolved independently, and their genetic basis for weediness varies (Li et al. Reference Li, Li, Jia, Caicedo and Olsen2017). Tseng et al. (Reference Tseng, Burgos, Shivrain, Alcober and Mauromoustakos2013) reported blackhull biotypes have a greater number of dormancy-linked loci than strawhull biotypes, again indicating greater variation in the genetic makeup of blackhull biotypes. Thus, blackhull Oryza spp. infestations in rice fields may pose a bigger threat to rice growers due to this biotype’s differential selectivity with higher tolerance to herbicides and diverse genetic makeup. Identification and control of blackhull accessions in early stages of rice culture can therefore minimize yield reduction in rice. However, it is not practical to distinguish blackhull strains from other strains in the early stage; thus, integrated weed management practices such as fallow tillage, stale seedbed, and crop rotation should be used along with chemicals to suppress Oryza spp. Care should be taken to ensure Oryza spp. biotypes do not escape management tactics, and farmers should be encouraged not to use sublethal doses of herbicides that would promote selection among tolerant Oryza spp. accessions. However, as blackhull showed higher tolerance to glyphosate and flumioxazin than diversity in Oryza spp., and it can be harnessed to improve biotic and abiotic stress tolerance in rice cultivars.
Table 3. Injury of different Oryza spp. accessions based on hull type 3 and 5 wk after treatment (WAT) with glyphosate (1,120 g ha−1) and flumioxazin (72 g ha−1) applied at field rates
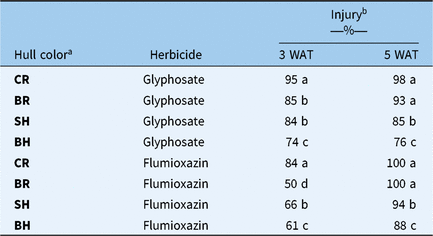
a BH, blackhull; BR, brownhull; CR, cultivated rice; SH, strawhull.
b Means within a column followed by the same letter are not different based on Student’s t-test at α = 0.05.
Hierarchical clustering of Oryza spp. based on injury at 5 WAT with glyphosate
Oryza spp. accessions were divided into four distinct clusters on the basis of their differential sensitivity to glyphosate at 5 WAT (Figure 3). Glyphosate injury was chosen for cluster analysis, as the Oryza spp. accessions showed greatest variation in injury with this herbicide. Hierarchical clustering grouped accessions in response to their mean injury. Accessions B20, B2, and S11, which were considered more tolerant than others, were grouped together in cluster 1 and had mean injury of 36%. Accessions in cluster 1 showed signs of recovery and regrowth from the glyphosate injury at 5 WAT compared with 3 WAT. Cluster 2 consisted mostly of blackhull accessions with mean injury of 73%. Cluster 3 consisted of a mixture of both blackhull and strawhull accessions with mean injury of 88%. Cluster 4 represented the highest injury of 94% and primarily consisted of strawhull accessions. Accessions in cluster 2 were sensitive to glyphosate, and although they showed some signs of recovery from glyphosate injury, remained stunted over time. Accessions in clusters 3 and 4 showed no signs of recovery from glyphosate injury at 5 WAT. Both rice cultivars, CL163 and REX, were part of cluster 4, indicating that commercial rice cultivars are highly susceptible to glyphosate and show high injury symptoms with no recovery when exposed to this rate of glyphosate. Information from the cluster analysis should be considered by weed scientists and plant breeders when developing Oryza spp. management and crop improvement programs, respectively. Further, with commercial rice being highly sensitive to glyphosate, steps should be taken to prevent drift of glyphosate into rice fields.

Figure 3. Hierarchical clustering of Oryza spp. accessions and rice cultivars based on injury ratings at 5 wk after treatment with glyphosate at the rate of 1,120 g ae ha−1. Cluster 1 represents accessions with the lowest injury and cluster 4 represents accessions with the highest injury.
Tolerance level to glyphosate
To further gain insight into the level of glyphosate tolerance among tolerant accessions identified in the screening study, dose–response analysis was conducted for three potentially glyphosate-tolerant accessions (B2, B20, S11). We chose a three-parameter log-logistic model over a four-parameter log-logistic model based on the AIC (Akaike information criterion) and RMSE (root-mean-square error) values.
The AIC values were −1,477.5 for a three-parameter model and −1,446.9 for a four-parameter model. RMSE values were calculated using the Matrices package and were 0.0109 for the three-parameter model and 0.0118 for the four-parameter model. Based on these values, the three-parameter model was considered the best-fit model for the data. Based on biomass reduction, the ED50 (effective dose causing 50% biomass reduction) values of tolerant accessions B2, B20, and S11 were 939, 1,141, and 2,406 g ha−1, respectively (Table 4; Figure 4). The biomass of the susceptible standard S118 was reduced 50% with 290 g ha−1. Tolerance level to glyphosate ranged from 3.3 to 8.3, and hierarchy of clustering was S11 > B20 > B2. Higher tolerance of these accessions was confirmed through dose–response analysis, but the tolerance mechanism is yet to be investigated.
Table 4. Regression parameters for dose response based on biomass reduction of Oryza spp. accessions at 5 wk after treatment with glyphosate

a S118, susceptible Oryza spp. accession identified from the screening study; B2, B20, S11, accessions with reduced sensitivity to herbicides applied at field rate (1,120 g ae ha−1).
b ED50, effective dose causing 50% biomass reduction.
c T/S ratio is calculated as ratio of ED50 of potential tolerant accession to ED50 of susceptible accession (S118).

Figure 4. Shoot dry biomass response of three potential herbicide-tolerant accessions (B2, B20, S11) and a susceptible accession (S118) treated with glyphosate in a whole-plant dose–response experiment.
Oryza spp. accessions from Arkansas exhibited differential tolerance to glyphosate and flumioxazin, and none of the accessions were killed by the 1X rate of glufosinate. Three accessions exhibited reduced sensitivity to glyphosate and another three to flumioxazin. The application of a field rate of these herbicides did not affect the grain yield of respective tolerant accessions. Management of Oryza spp. using these herbicides needs to account for the existence of a few tolerant ecotypes and must integrate various tools to achieve sustainable weed management. The tolerant accessions can, however, be explored for the development of herbicide-tolerant varieties.
Author ORCID
Te-Ming Paul Tseng, https://orcid.org/0000-0002-8102-8149
Acknowledgments
Funding for this project was provided by the Special Research Initiative Grant sponsored by the Mississippi Agricultural and Forestry Experiment Station and is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 230060. The authors would also like to express their appreciation for the assistance provided by Shandrea Stallworth and Ziming Yue for planting and herbicide application. No conflicts of interest have been declared.










