Se intake varies markedly among different countries, mainly owing to the variation in its content in soil (Combs & Combs, Reference Combs and Combs1986; Johnsson et al. Reference Johnsson, Åkesson and Alexander1997). Considerable changes in Se intake have occurred with time; for example, in the UK it decreased from 60 to 34 μg/d in 20 years mainly owing to a decreased import of wheat from North America (Rayman, Reference Rayman1997), which can be compared with the Nordic Nutrition Recommendations (2004) for Se intake of 40 μg/d for women and 50 μg/d for men. Different methods of providing selected populations with increased amounts of Se have been elaborated. In Finland Se has been added to fertilizers since 1985 resulting in an increased intake from 40 to 125 μg/d, a more than 3-fold increase in milk Se content (Ekholm et al. Reference Ekholm, Ylinen, Eurola, Koivistoinen and Varo1991a) and also an increased mean serum Se concentration from 0·9 to 1·5 μmol/l (Aro et al. Reference Aro, Alfthan and Varo1995). An alternative way is to add Se directly to animal feed (Ekholm et al. Reference Ekholm, Varo, Aspila, Koivistoinen and Syrjälä-Quist1991b; Johnsson et al. Reference Johnsson, Åkesson and Alexander1997). Globally the Se content of bovine milk varies widely (Alaejos & Romeo, Reference Alaejos and Romeo1995) and there is also some variation according to season and lactation stage (Ekholm et al. Reference Ekholm, Ylinen, Eurola, Koivistoinen and Varo1991a; Van Dael, Reference Van Dael1992; Lindmark-Månsson et al. Reference Lindmark-Månsson, Fondén and Pettersson2003). In Sweden, milk products contribute approx. 20% of the daily intake of Se (Becker, Reference Becker2000).
Studies on Se supplementation of animal diets have shown that selenomethionine caused a higher increase in the concentration of Se in milk, compared with inorganic Se (Knowles et al. Reference Knowles, Grace, Wurms and Lee1999; Ortman & Pehrson, Reference Ortman and Pehrson1999; Givens et al. Reference Givens, Allison, Cottrill and Blake2004) but for economic and legal reasons selenomethionine is not widely used in practice at present. Apart from its significance for human consumption, Se in milk can also affect milk quality for instance its oxidative stability, but this aspect has not been much studied (Nicholson et al. Reference Nicholson, St-Laurent, McQueen and Charmley1991; Stagsted et al. Reference Stagsted, Hoac, Åkesson, Nielsen, Lyons and Jacques2005). Some studies have demonstrated a positive effect of Se supplementation on udder health (Weiss et al. Reference Weiss, Hogan, Smith and Hoblet1990) and other studies have shown Se to be associated with other trace elements in milk e.g., Cu and Zn (Perrone et al. Reference Perrone, Di Palma, Di Toro, Gialanella and Moro1994; Brätter et al. Reference Brätter, Blaso, Negretti de and Raab1998; Kantola & Vartiainen, Reference Kantola and Vartiainen2001). The aims of the present work were to study the initial kinetics of Se appearance and disappearance in milk after the initiation and cessation of Se supplementation, respectively, and to study the effects of supplementation on the distribution of Se, Zn and Cu in bovine whey and plasma. Another aim was to prepare and characterize a highly Se-enriched milk to be used as a tool in further scientific investigations, such as human dietary studies, since several long-term trials have shown anticarcinogenetic effects of Se supplementation (Blot et al. Reference Blot, Li, Taylor, Guo, Dawsey, Wang, Yang, Zheng, Gail, Li, Yu, Liu, Tangrea, Sun, Liu, Fraumeni, Zhang and Li1993; Clark et al. Reference Clark, Combs, Turnbull, Slate, Chalker, Chow, Davis, Glover, Graham, Gross, Krongrad, Lesher, Park, Sanders, Smith and Taylor1996; Meyer et al. Reference Meyer, Galan, Douville, Bairati, Kelge, Bertrais, Estaquio and Hercberg2005; Trumbo, Reference Trumbo2005).
Materials and Methods
Chemicals
Palladium nitrate and tris(hydroxymethyl)-aminomethane were obtained from Merck (64293 Darmstadt, Germany). Superdex 200 10/300 GL (1 cm×30 cm, fractionation range for globular proteins 10–600 kDa) and PD-10 columns were purchased from Amersham Biosciences AB (75184 Uppsala, Sweden). Superdex columns were calibrated as described elsewhere (Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007). For the identification of the separated fractions orotate, urate, selenomethionine, selenocystine, bovine erythrocyte superoxide dismutase (Zn, Cu), rabbit metallothionein-II, bovine β-lactoglobulin, bovine α-lactalbumin and bovine ceruloplasmin (Sigma Chemical Co, St Louis MO 63178, USA) were used as reference compounds. Seronorm and Medisafe sera were purchased from Sero AS (1375 Billingstad, Norway) and Medichem (71144 Steinenbronn, Germany), respectively.
Feeding procedure
At the Research Centre Foulum, two feeding trials were conducted and the same standard mineral supplement was used in both trials (Mn (as MnO) 17·3; Cu (as CuSO4) 2·4; Zn (as ZnO) 70; Se (as Na2SeO3) 0·16; Mg (as Mg3(PO4)2) 500 and Co (as CoCO3) 0·07 mg/kg dry matter feed). In Experiment 1, six Danish Holstein cows in mid-lactation were fed a basal feed, containing 0·16 ppm Se (approx 3 mg Se/d) as sodium selenite in the mineral supplement described. They were randomly assigned to two groups in a 4-week cross-over study. At the start of the experiment, group A was in addition given an oral pill (25 g Se yeast containing 25 mg Se/d (at least 75% selenomethionine); Sel-Plex™, Alltech, Nicholasville KY 40356, USA) and the other group (B) continued on the basal feed, and after 2 weeks the groups were switched. Milk and blood were sampled at different time intervals. In Experiment 2, sixteen cows were randomly assigned to two groups in a 1-week study. One group of eight cows received the four-fold higher dose of 100 mg of yeast Se/d in addition to the basal feed, and the other group was given the basal feed. No exclusion criteria were applied regarding animals. The average feed consumption was 20 kg dry matter/d and the average daily milk volume was 40 l.
Sample preparation
Aliquots of milk samples from cows in each group taken at the same time point were pooled. Samples were defatted by centrifugation at 4000 g (J2-21, Beckman, Palo Alto CA 94304, USA) at 4°C for 30 min. Casein was precipitated at pH 4·6 by the addition of 10% (v/v) lactic acid, and the whey supernatant was obtained by centrifugation at 5000 g (Beckman GPR) at 4°C for 30 min and its pH readjusted to 6·7. A portion of the defatted milk samples were desalted using PD-10 columns, and the high-molecular-weight fraction was recovered for analysis. Plasma was obtained from blood collected in heparinized tubes (BD Vacutainer, Franklin Lakes NJ 07417, USA) after centrifugation at 1500 g at room temperature for 30 min. Milk, whey and plasma were stored at −80°C until analysis.
Se analysis by atomic absorption spectrometry
Se in bovine plasma was measured by graphite furnace atomic absorption spectrometry (Aanalyst 800, PerkinElmer, Waltham MA 02451, USA) as described by Borglund et al. (Reference Borglund, Åkesson and Åkesson1988). Briefly, a matrix modifier containing Pa and Mg was added to the samples, and the mixture was then dried, charred and atomized according to the following temperature programme: 110, 130, 1400, 2150 and 2500°C with ramp times of 1, 20, 10, 0, and 1 s, respectively, and hold times of 30, 30, 20, 4 and 5 s, respectively. Absorbance was measured at 196 nm using the Zeeman background correction. Seronorm and Medisafe sera were used as standard and control, respectively. Se concentration of Medisafe serum was measured as 49·7 (sd 0·9) μg/l, and the target value was 50·0 μg/l. The detection limit (3×sd/slope) was measured as 1·3 μg/l, and the limit of quantification (10×sd/slope) was 4·4 μg/l.
Trace element analysis by inductively coupled plasma-mass spectrometry
Trace elements in whey and milk were quantified by a quadropole ICP-MS instrument (Thermo X7, Thermo Elemental, Winsford, CW7 3GA, UK) equipped with a conical glass nebulizer (Glass Expansion, Melbourne Vic 3003, Australia) with 1 ml/min uptake and a Peltier-chilled conical impact bead spray chamber (Thermo Elemental). Gas flows were 13 l/min for the cooling gas, 1·1 l/min for the auxiliary gas and 0·93 ml/min for the nebulizer gas. Samples were analysed in peak-jumping mode for 45Sc, 65Cu, 66Zn, 79Br, 82Se and 89Y (1 point per peak, 70 sweeps and 10 ms dwell time for Sc, Y, Cu and Zn and 30 ms for Br and Se). Interference corrections were made for 82Se for the spectral overlap of brominehydride. Preparation and introduction of samples were performed according to Bárány et al. (Reference Bárány, Bergdahl, Schütz, Skerfving and Oskarsson1997). Analytical accuracy was checked against reference material (Seronorm trace elements serum; batch JL4409). Results obtained were: for Cu, 1·1 (0·04) mg/l (mean (sd)) v. recommended 1·0–1·1 mg/l; for Zn, 1·1 (0·06) mg/l v. 0·77–0·93 mg/l; and for Se, 67 (5·9) μg/l v. 67–79 μg/l.
Distribution of trace elements by size-exclusion chromatography-ICP-MS
Plasma and whey samples were diluted 1:8 and 1:2, respectively, with the eluent (20 mm Tris-acetate–0·15 m-ammonium acetate–3% methanol (v/v), pH 6·7) and filtered through a 0·22-μm filter before injection into a 250-μl sample loop (Valco Instruments Co. Inc., Houston TX 77255, USA). Sample and eluent were pumped by a Shimadzu LC-10AD pump (Kyoto, Japan) through a Superdex 200 column with a flow rate of 0·7 ml/min. Trace elements in the eluates were detected by the ICP-MS instrument connected on-line to the SEC equipment. The eluate was analysed in the peak-jumping mode for 77Se, 82Se, 65Cu and 66Zn (3 points per peak, 30 ms dwell time) in the time-resolved analysis mode. 77Se was used to confirm the spectral overlap of BrH on 82Se and peaks detected at 82Se but not at 77Se were excluded (Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007).
Statistical analysis
The statistical significance of differences in a trace element in samples from different groups was tested using the Mann-Whitney U method.
Results
Effect of Se supplementation on the Se content in bovine milk, whey and plasma
After the switch from the basal feed to a Se-enriched feed (25 mg Se/d extra) the Se content in both defatted (Fig. 1A) and desalted milk increased markedly within a few days and in 1 week reached a plateau approx. 10-fold higher (134 μg/l) than the control value (P=0·001). For whey samples the increase in Se was also approx. 10-fold up to 30 μg/l compared with the control (P=0·001). In plasma the maximal Se level attained at 2 weeks after the start of supplementation (Fig. 1B) was similar to that in milk (132 μg/l) but the increase was only approximately 2-fold compared with the control value (P=0·003). After cessation of supplementation, Se concentration in milk of group A decreased with a half-life of 3·5 d, which was more rapid than that in plasma (Fig. 1).

Fig. 1. The concentration of Se in defatted milk and plasma of cows given a Se supplement (Experiment 1). Group A was given a Se supplement (25 mg yeast Se/d) and group B was given a basal feed at the start, and the feeds were switched at day 15. Each point represents a value from three cows. A, defatted milk; B, plasma.
In Experiment 2, supplementation with a higher dose of Se (100 mg Se/d) caused a 40–50-fold increase in Se in whole and defatted milk (up to approx. 500 μg/l) compared with the control (P⩽0·001) after 2 d of supplementation, the increase in whey being approx. 20-fold (P<0·001; Table 1).
Table 1. Trace element content in selenium-enriched milk prepared in different ways. Cows in the supplemented group were given 100 mg yeast Se/d for 1 week (Experiment 2)
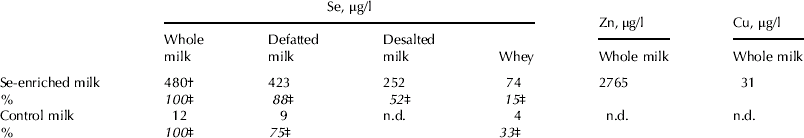
† The coefficient of variation between cows was 3–12% on different days and 20% upon analysis of pooled samples collected on four days at the end of the supplementation
‡ The content of selenium of whole milk was set at 100%
n.d., not determined
Effect of Se supplementation on the distribution of trace elements in whey
Whey prepared from milk samples collected before, during and after the supplementation was subjected to SEC-ICP-MS. Six major peaks at 280 nm were eluted with apparent molecular weights of >600 (7%), 195 (13%), 36 (25%), 14 (14%) and <2 kDa (2 peaks) (36% and 5%). The four latter peaks co-eluted with β-lactoglobulin, α-lactalbumin, orotate and urate, respectively (Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007). Variation in elution times of the peaks between samples was <5% and the distributions of the u.v.-absorbing compounds were similar among samples.
Three major Se peaks were observed in all whey samples (Fig. 2A), eluting at mean apparent molecular weights of 36, 14 and 2 kDa and co-eluting with β-lactoglobulin, α-lactalbumin and selenomethionine (or a similar compound), respectively. No significant difference in molecular weights of the respective Se peaks was found between samples. The proportion of Se in the β-lactoglobulin-α-lactalbumin fraction was significantly higher in whey samples collected in supplementation periods compared with those in non-supplementation periods (P=0·01).

Fig. 2. The distribution of Se and Cu in whey from cows given a selenium supplement for 2 weeks (25 mg/d) in Experiment 1 (group A). Each sample was prepared from a pooled milk sample from three cows. The non-selenium peak eluted at approx. 35 min was excluded. The order of the chromatograms is the same as the order in the legend. The baseline of the curves was adjusted in a stepwise fashion to increase clarity. A, Se. The proportion of Se in peaks I+II in supplemented samples was increased compared with samples from non-supplementation periods (P=0·01). B, Cu. The percentage of Cu found in the void volume peak at 1 week was significantly larger than at 0 d (P=0·05), and the proportions of the 91-kDa and 5-kDa peaks at 1 week were significantly smaller than at 0 d (P=0·046 for both peaks).
Two distinct Zn peaks were found in whey eluting with average calculated molecular sizes of 4 and <2 kDa (8–97% and 3–11% of recovered Zn, respectively). The <2-kDa fraction might be attributed to a Zn-citrate compound but the identity of the 4-kDa fraction is unclear. Distribution of Zn peaks did not differ between samples obtained during supplementation and non-supplementation periods.
Five Cu peaks were observed in whey (Fig. 2B) as found previously (Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007). Peak I eluted at the exclusion limit, peak II migrated close to but not identical with albumin, peak III co-eluted with metallothionein, the identity of peak IV is unclear, and peak V eluted at an apparent molecular weight of <2 kDa, probably a complex with citrate. The first four peaks were of similar size but peak V contained only <5% of recovered Cu. Distribution of Cu compounds in whey was essentially unaffected by Se supplementation.
Effect of Se supplementation on the distribution of trace elements in plasma
Three pooled plasma samples were subjected to SEC-ICP-MS. The u.v. absorption pattern in these samples was very similar with three major peaks eluting at the void volume and at mean molecular weights of 210 and 75 kDa, the latter representing mainly immunoglobulin G and albumin, respectively.
Six Se peaks were observed in all plasma samples with calculated molecular weights of >600, 195, 66, 11 and two peaks at <2 kDa (Fig. 3A and Table 2). Peaks III and V co-eluted with bovine serum albumin and selenomethionine, respectively. Peak II might be attributed to selenoprotein P (Borglund et al. Reference Borglund, Åkesson and Åkesson1988; Palacios et al. Reference Palacios, Encinar, Schaumlöffel and Lobinski2006) but some selenoprotein P might also be in the void volume peak since the samples were collected in tubes containing heparin (Åkesson & Mårtensson, Reference Åkesson and Mårtensson1988). Identities of peaks IV and VI are unclear. The albumin peak contributed approx. 30% of the Se and the rest was distributed rather evenly in the other fractions (Table 2). There were only small differences between the samples although there was probably an increase in the proportion of fraction II due to Se supplementation.

Fig. 3. The distribution of Se (A) and Cu (B) in plasma from cows given a Se supplement (25 mg/d) for 2 weeks in Experiment 1 (group A). Each sample was pooled plasma from three cows. The plasma sample at day 0 was not available.
Table 2. The percent distribution of Se, Zn and Cu in bovine plasma from cows supplemented for 2 weeks with 25 mg yeast Se/d as measured by SEC-ICP-MS. Samples were collected at 1 d, 1 week and 4 weeks after the start of the trial (Group A). The analyses were performed on pooled plasma from three cows
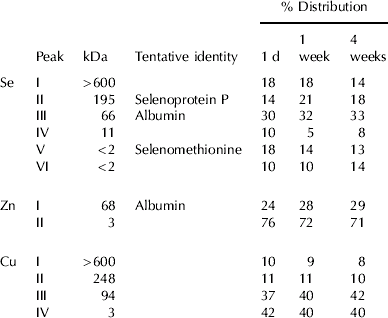
All plasma samples showed similar distribution profiles for Zn and Cu. Two Zn fractions with average calculated molecular weights of 68 and 3 kDa were found (Table 2). The 68-kDa peak containing approx. 20–30% of the Zn was probably albumin (Masuoka et al. Reference Masuoka, Hegenauer, Van Dyke and Saltman1993). Fractionation of Cu in plasma revealed four peaks with mean apparent molecular weights of >600, 248, 94 and 3 kDa (Fig. 3B), and approx. 60% of the Cu was found in the three protein fractions (Table 2). The identity of the Cu peaks is discussed below.
Discussion
Effect of Se supplementation on Se content in bovine milk, whey and plasma
In both experiments the increase of Se in milk and plasma was substantial and dose-dependent which agreed with and extended previous findings regarding the use of a Se yeast supplement (Table 3). The doses chosen in the present study were high since one aim was to produce milk that could be used in a human nutritional trial (Ravn-Haren et al. Reference Ravn-Haren, Bügel, Krath, Hoac, Stagsted, Jørgensen, Bresson, Larsen and Dragsted2008) and they exceeded those allowed in practical animal production (0·5 mg Se/kg dry matter feed, i.e. approx. 10 mg/d). Suitable levels and forms of Se to be used for supplementing animal feed have been much debated (Ullrey, Reference Ullrey1992) and sometimes only inorganic forms of Se were allowed as feed additives. Recently EFSA positively evaluated the use of Se yeast up to 0·5 mg Se/kg dry matter feed according to Regulation 1831/2003 (EFSA, 2006). It was also concluded that a long-term intake of Se yeast of more than 10-times this amount (>100 mg Se/d) may have negative effects. In an earlier study supplementation with 100 mg Se/d resulted in a plasma Se concentration of 275 μg/l (Stowe & Herdt, Reference Stowe and Herdt1992).
Table 3. The concentration of selenium in milk and plasma before and after Se supplementation of cow feed as reported in various studies. The data in parentheses indicate the increase in Se content after supplementation expressed as the fold change relative to the respective value before the supplementation

† These values refer to blood
Interestingly the present results also showed that the changes in Se concentration in milk occurring after starting or stopping supplementation were quite rapid. This indicated that milk Se was provided by pools with a short turnover, as shown by the short calculated half-life (3·5 d) of milk Se after cessation of supplementation. The transfer efficiency of Se to milk is influenced by several factors, such as Se intake and nutritional Se status and approx. 5% of added selenite in a Se-deficient diet was transferred to milk, but only 1% when a Se-adequate diet was used (Conrad & Moxon, Reference Conrad and Moxon1979). We calculated that at both dosages of yeast Se, approx. 20% of supplemental Se was recovered in milk. This agrees with findings in man showing that a higher proportion of administered selenomethionine was excreted in milk compared with selenite (Mangels et al. Reference Mangels, Moser-Veillon, Patterson and Veillon1990). Selenomethionine has also been found to have a higher bioavailability than selenite as assessed by biomarkers of Se status in human blood (Xia et al. Reference Xia, Hill, Byrne, Xu and Burk2005).
Little is known of the secretion mechanism of Se into milk. A study on transgenic mice lacking selenoprotein P showed that an extra supply of dietary selenite to the dam could provide enough Se to their offspring, but surprisingly the milk Se content was only marginally affected by selenite supplementation (Schweizer et al. Reference Schweizer, Michaelis, Köhrle and Schomburg2004).
Effect of Se supplementation on the distribution of Se in whey
Most of the Se increase in whey due to selenium supplementation was found in the β-lactoglobulin-α-lactalbumin fraction, in agreement with previous findings in whey not enriched with Se (Van Dael, Reference Van Dael1992; Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007). As expected this indicated that non-specific incorporation of selenomethionine, the predominant form of Se in Se-enriched yeast, occurred into methionine-containing proteins in whey, and probably also into caseins. At least five selenoproteins are formed in bovine mammary tissue, but no information on their excretion into milk is available (Bruzelius et al. Reference Bruzelius, Hoac, Sundler, Önning and Åkesson2007). Human and bovine milk are known to contain extracellular glutathione peroxidase (eGSHPx) (Avissar et al. Reference Avissar, Slemmon, Palmer and Cohen1991; Lindmark-Månsson & Åkesson, Reference Lindmark-Månsson and Åkesson2001) but it is uncertain whether any of the small Se peaks at SEC-ICP-MS could represent this enzyme or another selenoprotein. The role of the remaining 20 selenoproteins found in the mammalian selenoproteomes (Kryukov et al. Reference Kryukov, Castellano, Novoselov, Lobanov, Zehtab, Guigo and Gladyshev2003; Gromer et al. Reference Gromer, Eubel, Lee and Jacob2005; Köhrle et al. Reference Köhrle, Jakob, Contempré and Dumont2005; Papp et al. Reference Papp, Lu, Holmgren and Khanna2007) for mammary and milk physiology has so far been studied only to a small extent. It is important to point out that the distribution of Se in whey is distinctly different from those in the soluble fractions from various sources of fish and meat (Daun et al. Reference Daun, Lundh, Önning and Åkesson2004; Önning et al. Reference Önning, Daun, Drevelius, Lindmark, Lundh, Åkesson, Khassanova, Collery, Maynard, Khassanova and Étienne2004).
Effect of Se supplementation on the distribution of Se in bovine plasma
In the present study the increase of Se in plasma was distributed across most Se peaks, although it was more evident in the selenoprotein P fraction. Moreover, in man, supplementation with yeast Se increased Se in all main fractions of plasma (Borglund & Åkesson, Reference Borglund and Åkesson1988) which probably represents both the non-specific incorporation of selenomethionine, and the specific incorporation of selenocysteine into selenoproteins. Recent data on human serum indicated that selenocysteine accounted for approx. 70% of the Se (Ruiz Encinar et al. Reference Ruiz, Schaumlöffel, Orga and Lobinski2004) but no corresponding data for bovine material are available.
The present results seem to be the first ones on the separation of seleno-compounds in bovine plasma by SEC-ICP-MS. The protein pattern with three peaks at the void volume, IgG and albumin was similar to findings for human serum (Borglund et al. Reference Borglund, Åkesson and Åkesson1988; Borglund & Åkesson, Reference Borglund and Åkesson1988). Other chromatographic procedures including assay of selenoproteins have been used to measure Se in three main fractions: albumin, eGSHPx and selenoprotein P (Deagen et al. Reference Deagen, Butler, Zachara and Whanger1993; Awadeh et al. Reference Awadeh, Abdelrahman, Kincaid and Finley1998; Burk et al. Reference Burk, Hill and Motley2001). Awadeh et al. (Reference Awadeh, Abdelrahman, Kincaid and Finley1998) applied one of the procedures to bovine serum but did not observe any significant effects on the distribution of Se after the addition of different amounts and forms of Se to the feed. Regarding the existence of other Se compounds in plasma apart from those mentioned above, one of the three peaks in the low-molecular-weight region migrated as selenomethionine in agreement with previous findings (Palacios et al. Reference Palacios, Encinar, Bertin and Lobinski2005). As suggested by these workers the other peaks (peaks IV and VI in our study) may represent selenite, selenate or fragments of selenoproteins, but this needs to be substantiated.
Effect of Se supplementation on the distribution of Zn and Cu in bovine whey and plasma
Previous studies showed that the concentration of Se in human milk was directly correlated with that of Cu and inversely correlated with that of Zn (Perrone et al. Reference Perrone, Di Palma, Di Toro, Gialanella and Moro1994; Brätter et al. Reference Brätter, Blaso, Negretti de and Raab1998; Kantola & Vartiainen, Reference Kantola and Vartiainen2001). Moreover, the distribution of different forms of Zn in milk was found to be related to the maternal Se intake (Brätter et al. Reference Brätter, Blaso, Negretti de and Raab1998). These studies were the background for the present experiments on the effects of Se supplementation on the distribution of Cu and Zn in whey and plasma.
Concerning Cu, more than 95% of it in human blood plasma is bound to ceruloplasmin, and also in cattle there is a high correlation between ceruloplasmin and Cu in plasma (Blakley & Hamilton, Reference Blakley and Hamilton1985). Surprisingly no major peak migrated as standard ceruloplasmin (calculated mass 116 kDa, expected mass 132 kDa), which was previously demonstrated in rat plasma (Linder et al. Reference Linder, Wooten, Cerveza, Cotton, Shulze and Lomeli1998) and human serum (Inagaki et al. Reference Inagaki, Mikuriya, Morita, Haraguchi, Nakahara, Hattori, Kinosita and Saito2000). In the present experiments most of the Cu was instead found in the 94-kDa fraction and in low-molecular-weight compounds. The 94-kDa peak moving close to, but not identical with albumin, may hypothetically represent a degraded or modified form of ceruloplasmin. In the present study of Se supplementation, the distribution of Cu in whey and plasma was essentially unchanged after such supplementation.
All Zn in whey was found in low-molecular-weight fractions as discussed elsewhere (Hoac et al. Reference Hoac, Lundh, Purup, Önning, Sejrsen and Åkesson2007). Regarding the form of Zn in plasma, other workers found that most of it was bound to albumin in human serum (Inagaki et al. Reference Inagaki, Mikuriya, Morita, Haraguchi, Nakahara, Hattori, Kinosita and Saito2000) while with our procedure most of the Zn was in the low-molecular-weight fraction. This may reflect both species variation and differences in methodology. The present results indicated that the distribution of Zn in bovine whey and plasma was not affected by Se supplementation. Brätter et al. (Reference Brätter, Blaso, Negretti de and Raab1998) found an inverse relationship between maternal Se intake and Zn content of breast milk of women living in seleniferous areas and that the citrate-bound Zn fraction in milk decreased with increasing Se concentration, but this was not reproduced in the present study.
In summary, the present study provided new data on the procedures to prepare Se-enriched milk for various purposes e.g., human intervention studies, functional foods for specific populations, and maybe cancer prevention, which can be considered in relation to other alternatives (Johnsson et al. Reference Johnsson, Åkesson and Alexander1997). It is also necessary in this context to consider the recent developments concerning the occurrence of many selenoproteins and their physiological functions (Kryukov et al. Reference Kryukov, Castellano, Novoselov, Lobanov, Zehtab, Guigo and Gladyshev2003; Gromer et al. Reference Gromer, Eubel, Lee and Jacob2005; Köhrle et al. Reference Köhrle, Jakob, Contempré and Dumont2005; Papp et al. Reference Papp, Lu, Holmgren and Khanna2007).
This work is part of the research programme New Antioxidant Strategies for Food Quality and Consumer Health (FOODANTIOX) supported by The Committee for Research and Development of the Öresund region (Öforsk). Additional support was obtained from the Swedish Farmers' Foundation for Agricultural Research, the Faculty of Engineering, Lund University, the Swedish Dairy Association, the Danish Dairy Association and the Lund University Hospital. Biomedical Nutrition is a member of the NoE The European Nutrigenomics Organization (NuGO) and the NoE Environmental Cancer Risk, Nutrition and Individual Susceptibility (ECNIS), which partly supported the study.








