Introduction
Capitellid polychaetes are ubiquitous worldwide in polluted and organically enriched marine and estuarine sediments and have often been referred to as bioindicators (e.g. Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Méndez et al., Reference Méndez, Romero and Flos1997, Reference Méndez, Flos and Romero1998). Capitella capitata was long regarded as a cosmopolitan species, until Grassle & Grassle (Reference Grassle and Grassle1976) demonstrated that it consists of multiple lineages which are distinct in their allozyme electrophoresis patterns as well as in their life histories. Following Grassle & Grassle's seminal paper, other studies have detected additional sibling species using reproductive, developmental, ecophysiological and, more recently, DNA sequence data (Wu et al., Reference Wu, Qian and Zhang1991; Gamenick et al., Reference Gamenick, Abbiati and Giere1998a, Reference Gamenick, Vismann, Grieshaber and Giere1998b; Hilliard et al., Reference Hilliard, Hajduk and Schulze2016; Tomioka et al., Reference Tomioka, Kondoh, Sato-Okoshi, Ito, Kakui and Kajihara2016; Silva et al., Reference Silva, Seixas, Barroso, Di Domenico, Amaral and Paiva2017). Méndez et al. (Reference Méndez, Linke-Gamenick and Forbes2000) reviewed the reproductive and developmental characteristics of 50 morphologically similar sibling species, but for many additional lineages developmental data are not available. Genetic data, on the other hand, can identify cryptic lineages fairly easily, but the DNA based clades usually cannot be linked to previously characterized species because the life history characteristics of the sequenced samples are unknown.
The genus Capitella mostly includes opportunistic species that have been described as classic r-selected species able to produce either planktotrophic or lecithotrophic larvae (Grassle & Grassle, Reference Grassle and Grassle1974, Reference Grassle and Grassle1976; Wu et al., Reference Wu, Qian and Zhang1991; Gamenick, Reference Gamenick1997; Gamenick et al., Reference Gamenick, Abbiati and Giere1998a, Reference Gamenick, Vismann, Grieshaber and Giere1998b; Méndez et al., Reference Méndez, Linke-Gamenick and Forbes2000, Méndez, Reference Méndez2002a, Reference Méndez2006, Reference Méndez2016; Adkins & Schulze, Reference Adkins and Schulze2011). The most important life history characteristics that distinguish different Capitella lineages include planktotrophic, lecithotrophic or direct development, poecilogony, hermaphroditism, size and duration of the stages, number of brooded embryos, and ciliation patterns in metatrochophores.
Female Capitella spp. construct brood tubes (open at both ends) with mucus, faecal materials and sediment particles. The fertilized eggs are placed around the inner tube surface, where they remain until developing into trochophores (Tsutsumi & Kikuchi, Reference Tsutsumi and Kikuchi1984). Females irrigate the brooding tubes with periodic body undulations (Reish, Reference Reish, Buikema and Cairns1980). Trochophore larvae are characterized by an oval shape without obvious segmentation, two ciliary rings and two eyes (Reish, Reference Reish, Buikema and Cairns1980). The ciliary rings allow them to move inside the tube or in the water column after release. Trochophores can reach the metatrochophore stage either inside the tube or in the water column. Metatrochophores have 13 segments and a visible ventral stomodeal cavity, not connected with the gut (George, Reference George and Hutchings1984; Seaver et al., Reference Seaver, Thamm and Hill2005). Most species have two well-defined ciliary rings allowing them to swim (Reish et al., Reference Reish, Piltz, Martin and Word1974; George, Reference George and Hutchings1984; Méndez et al., Reference Méndez, Linke-Gamenick and Forbes2000; Méndez, Reference Méndez2002a, Reference Méndez2006, Reference Méndez2016), while others lack such rings, suggesting that movements are achieved by muscular longitudinal contractions (Warren, Reference Warren1976; Méndez, Reference Méndez1995). Metatrochophore chaetal arrangements always consist of capillary chaetae in the first three chaetigers and hooded hooks in subsequent ones (George, Reference George and Hutchings1984). Metamorphosis of the metatrochophores to the juvenile stage involves the loss of cilia (when present) and increased peristaltic movements. The larvae will stop swimming and either burrow into the sediment (indirect developers) or settle inside the brood tube (direct developers). The juvenile stage of Capitella spp. has a vermiform shape, complete segmentation, a clear distinction between thorax and abdomen, and a chaetal arrangement identical to the metatrochophore stage (George, Reference George and Hutchings1984; Méndez, Reference Méndez1995). As they grow, the thoracic hooded hooks are gradually replaced by capillaries until a maximum of seven thoracic chaetigers with capillaries is reached. Individuals are considered to be immature adults when they have an elongated prostomium (with or without eyes) and at least five capillary thoracic chaetigers (Méndez, Reference Méndez2016). At this stage, females contain yellowish ovaries in the mid-ventral region. Females are mature when they have free-floating, white, intra-coelomic oocytes (Eckelbarger & Grassle, Reference Eckelbarger and Grassle1983), and males when they bear genital spines on the 8th and 9th chaetigers (Reish, Reference Reish, Buikema and Cairns1980).
The objectives of this paper are to describe the early life history stages of two Capitella species from the Gulf of Mexico (Tampa, Florida and Tamiahua, Veracruz) and to examine whether they match any previously characterized Capitella species. The concept of ecological indicator species is only meaningful if a species is clearly identifiable and if its responses to environmental disturbance are well understood. An additional objective was therefore to examine the development under undisturbed and disturbed conditions in a controlled laboratory setting.
Materials and methods
Sampling locations and methods
One population of Capitella sp. (hereafter Capitella sp. TF, after Tampa, Florida) was sampled at the Apollo Beach Preserve in Tampa, Florida (27°47′34.0044″N 82°25′3.8928″W) near a red mangrove (Rhizophora mangle) stand. The site was mostly sandy with organic deposits dominated by leaf litter near the mangroves (sediment organic matter content: 5.55%; salinity: 26 psu). The worms were collected from shallow grooves (up to ~1 m depth) with accumulated organic matter resulting from water run-off from the mangroves. Sediment was collected with a shovel (max depth of 15 cm). Some of the collected sediment was sieved through a 0.5 mm hard-plastic mesh while the rest was sieved through a sport mesh bag with a 1 mm pore size (done in an attempt to prevent fragmenting worms). All sieved material was pooled together into plastic bottles for processing in lab. Worms were kept with ambient sediment and water for transport to Texas A&M University Galveston Campus (TAMUG).
Another population of Capitella sp. (hereafter referred to as Capitella sp. TV) was sampled in February 2018 in Tamiahua City, Veracruz (Boca de Corazones; 21°16′11.0568″N 97°26′37.7268″W) near the shore of the lagoon at 1 m depth. The 15 cm superficial layer of sediment (organic matter content 18.78%) was collected with a shovel and was sieved through a 0.5 mm mesh. The retained worms were collected with forceps and placed in a 50 ml centrifuge tube with ambient seawater (salinity: 24 psu) and transported to Invertebrados Bentónicos Laboratory in the Unidad Académica Mazatlán, from the Instituto de Ciencias del Mar y Limnología, UNAM.
Culturing methods
Capitella sp. TF were reared in 473.2 ml plastic containers with ~45 ml of cleaned sediment. Sediment was collected from Galveston, Texas (29°16′21.9072″N 94°52′52.5828″W), sieved at 0.5 mm, soaked in tap water overnight, dried at room temperature, and frozen-thawed-frozen at −80°C to render it azoic before use. Filtered seawater (FSW) was obtained from the TAMUG Sea Life Facility (SLF) and was maintained at ~27 psu. Animals were fed by the weekly addition of 0.1 g of aquarium fish flakes (Wardley) ground with mortar and pestle. The specimens were reared under natural light conditions at room temperature for eight months prior to the observations. The early development of Capitella sp. TF was observed under disturbed and undisturbed conditions. Both scenarios used the same cleaned sediment and filtered seawater as described for the culturing methods.
Capitella sp. TV were kept in rearing tanks (20 × 12 cm) containing 100 g (dry weight) of clean sediment, from the Hotel Las Flores beach in Mazatlán, which had previously been dried (60°C) and sieved to a grain size lower than 250 µm in diameter and frozen. Each tank also contained 1.5 l of filtered (<30 µm) aerated seawater (salinity 24 psu), and was maintained at 23 ± 1°C in the dark. Animals were fed 0.5 g of aquarium fish flakes (Wardley) weekly. The food items were dried, ground and sieved to less than 250 µm. The specimens were reared under such conditions for two months prior to the observations. Females (with white oocytes or yellow ovaries) and males (bearing genital spines) were sorted from the stock cultures. Couples were maintained in the dark at 23 ± 1°C and were each placed in dishes containing 100 g of experimental sediment, 0.5 g of food and 7 ml of filtered seawater. Broods were removed from the cultures and placed individually in dishes with the same culturing conditions. Juveniles and adults were fed by the weekly addition of 0.001 g (dry wt) of artificial food (Méndez, Reference Méndez2002a).
For both species, larvae and juveniles were observed and measured daily. The duration of developmental stages and their size were recorded for each brood. Larvae inside the brood tube were observed through the transparent mucus lining of the brood tube after removal of some of the surrounding sediment particles.
Measurements
For size measurements, specimens were photographed through a camera mounted on a dissecting microscope. From each picture, body length and area (based on the contour of entire worms) were calculated using the image analysis software ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). Body volume (mm3) was calculated assuming a cylindrical shape (Forbes et al., Reference Forbes, Forbes and Depledge1994) as V = π A 2/4L, where A is area and L is length. Three pictures with their respective measurements were taken per individual and averaged to obtain their length and volume. A maximum of 10 individual larvae inside the tube, 10 larvae outside the brood and 10 juveniles were measured each census day to estimate average length and volume. Some specimens were relaxed before photography with a 1:1 ratio of 7% magnesium sulphate:filtered seawater.
Calculations of growth rates
For all observations, larval and juvenile length and volume data were pooled to analyse stage-specific growth. Growth rates (mm day−1 and mm3 day−1) were calculated as differences in mean length and mean volume between one census day and the subsequent one, and a global estimate was derived from the mean values calculated for each developmental stage. Growth models of each developmental stage were obtained through the relationships between age and size (length and volume) by the adjustment to linear, exponential or power functions, according to the highest correlation coefficient (r) in Microsoft Excel. The significance values were obtained through the Student's t-test, in which P < 0.05 represented significant correlations.
Scanning electron microscopy
Fifteen metatrochophore larvae of Capitella sp. TF were observed and photographed with a Hitachi scanning electron microscope (SEM) (TM 3000). Two days after hatching, metatrochophores were relaxed with MS:FSW and fixed in 1 part of 25% glutaraldehyde, 4 parts 0.34 M NaCl, and 5 parts Millonig's phosphate buffer (0.46 M sodium phosphate monobasic, pH 7.4) at 4°C overnight. Fixation was followed by three 5-minute washes in a rinse buffer of 1 part Millonig's phosphate buffer and 1 part 0.6 M NaCl. Samples were then dehydrated in an ethanol series (30% for 10 min, 50% for 10 min, 2 × 70% for 10 min, 2 × 80% for 10 min, 2 × 95% for 10 min and 2 × 100% for 10 min) and dried with hexamethyldisilizane. Larvae were mounted on SEM stubs using carbon tabs and sputter-coated with gold/palladium. No SEM observations were performed on Capitella sp. TV due to lower productivity of the cultures and lack of access to an SEM at the Invertebrados Bentónicos Laboratory in Mazatlán.
Disturbance experiments
To examine the effects of physical disturbance, we followed the protocols of Adkins & Schulze (Reference Adkins and Schulze2011) for Capitella sp. TF. Due to fungal and protozoan growth in the cultures of Capitella sp. TV, we were not able to perform the same experiments on this species. As an undisturbed control, 30 brood tubes of Capitella sp. TF were removed from the stock culture and placed individually in dishes (2.5 cm diameter) with 1.5 ml FSW. Daily observations were performed for 15 days until hatching. Once larvae hatched, adults were removed from the dishes and about 0.01 g of experimental sediment mixed with artificial food was added to each dish. Larvae and adults (including reproducing and non-reproducing individuals from the culture) were measured daily and durations of all life history stages were recorded.
To simulate physical disturbance, 6 broods were cut transversally in the middle of the tube and the 12 pieces were transferred to individual dishes of 6-well culture dishes with 7 ml FSW. Larvae were forced out from one half (dissected larvae) while the other half of the tube remained intact with the larvae enclosed. Developmental stages including embryos, larvae and juveniles were measured daily for 12 days.
Results
Capitella sp. TF from Tampa, Florida
Undisturbed conditions
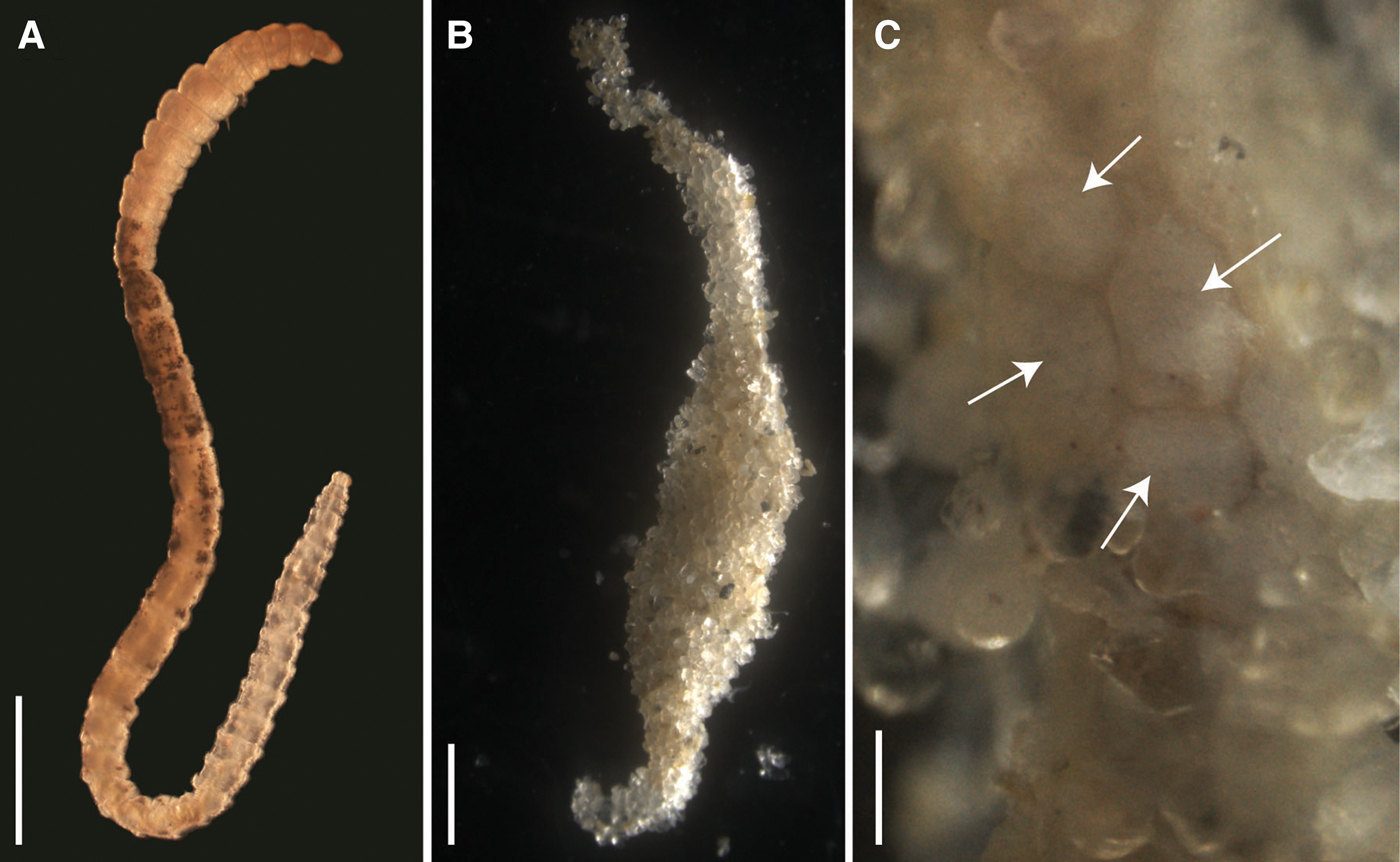
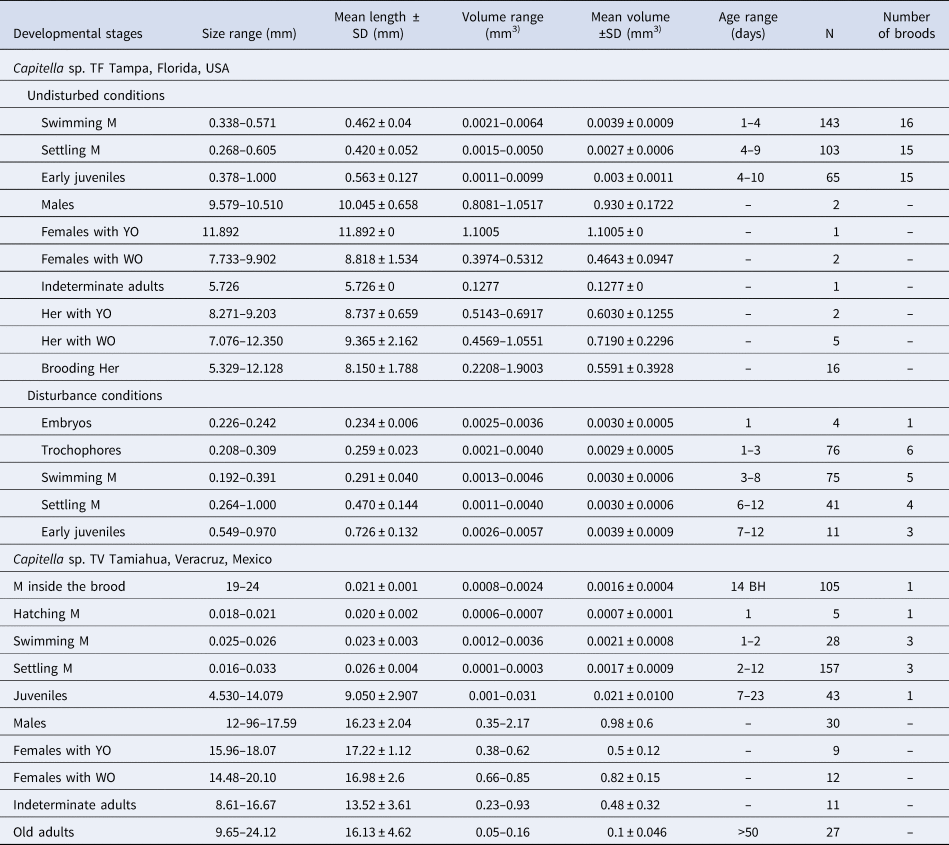
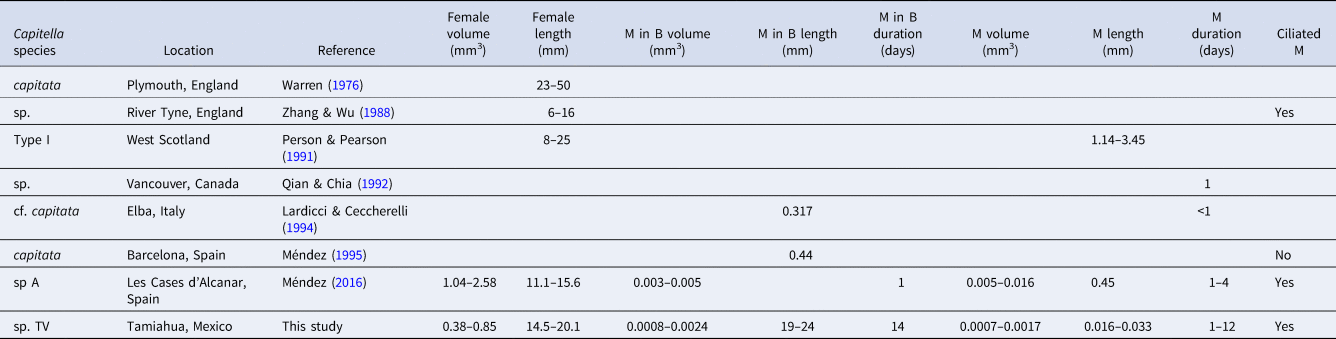
The Capitella sp. TF culture included males, females and hermaphrodites. Adults lacked the intense red colour typical for most Capitella species but had a pattern of brown/black pigmentation spots on the dorsal side (Figure 1A). Most adults without brood tubes that were randomly picked from the culture were hermaphrodites bearing genital spines and yellow ovaries (2 individuals) or white ovaries (5 individuals). Only 2 males and 3 females of variable size were observed (Table 1). Only one indeterminate specimen with characteristic pigmentation but without sexual structures and smaller than males and females was observed, indicating that it was an immature specimen. All adults with brood tubes were hermaphrodites without evident yellow ovaries or white oocytes. The brood tubes were spindle-shaped, with a thickened central region (Figure 1B). Removal of the sand revealed that it only loosely adhered to the tube and the majority of the embryos/larvae resided in this region (Figure 1C). Of the 30 isolated brood tubes, larvae from 17 broods hatched during the 15-day observation period after brood isolation (2 after 2 days, 14 after 6 days and 1 after 9 days). The 13 remaining brood tubes did not hatch during this period.
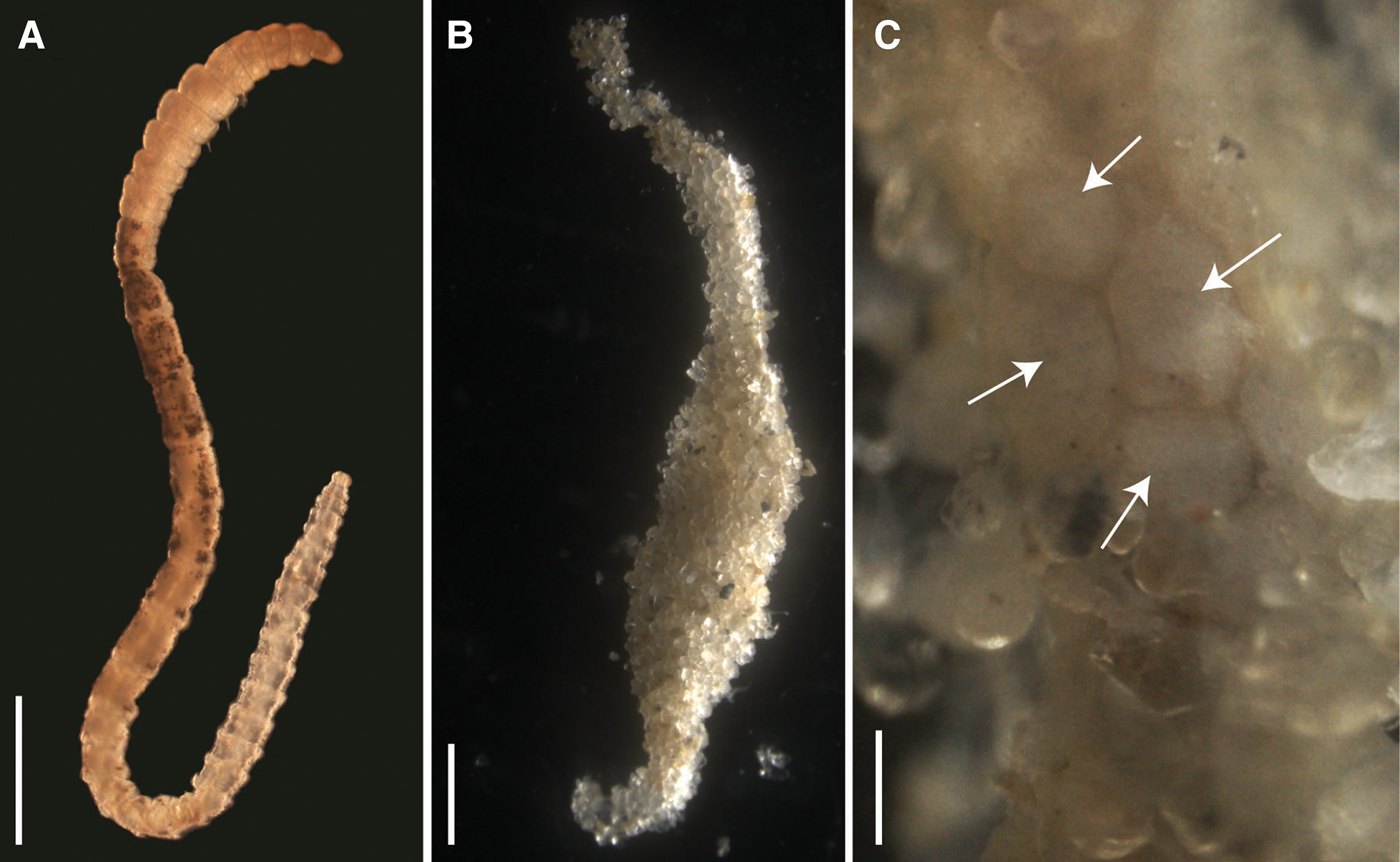
Fig. 1. Light micrographs of the Capitella sp. TF culture; (A) Adult specimen, anterior is up, sex undetermined; note the pale colouration and dark pigmentation in mid-body region; scale bar: 1 mm; (B) Typical shape of brood tube; scale bar: 1 mm; (C) trochophore larvae inside brood tube (arrows), photographed through the mucus lining of the tube after removal of some of the sediment grains; scale bar: 500 µm.
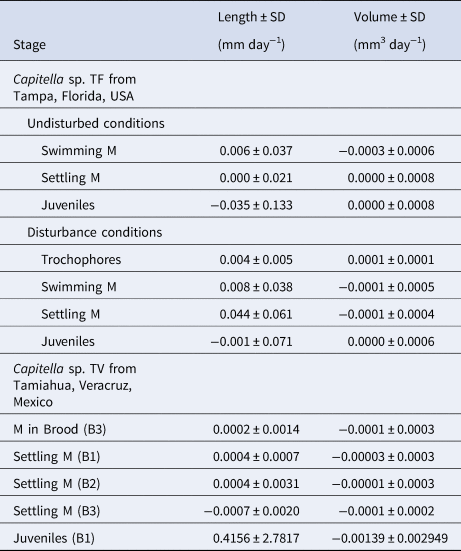
Table 1. Duration, length and volume of the different developmental stages of Capitella sp. TF and Capitella sp. TV (N, number of observations; M, metatrochophores, YO, yellow ovaries; WO, white oocytes; Her, hermaphrodites; BH, before hatching)

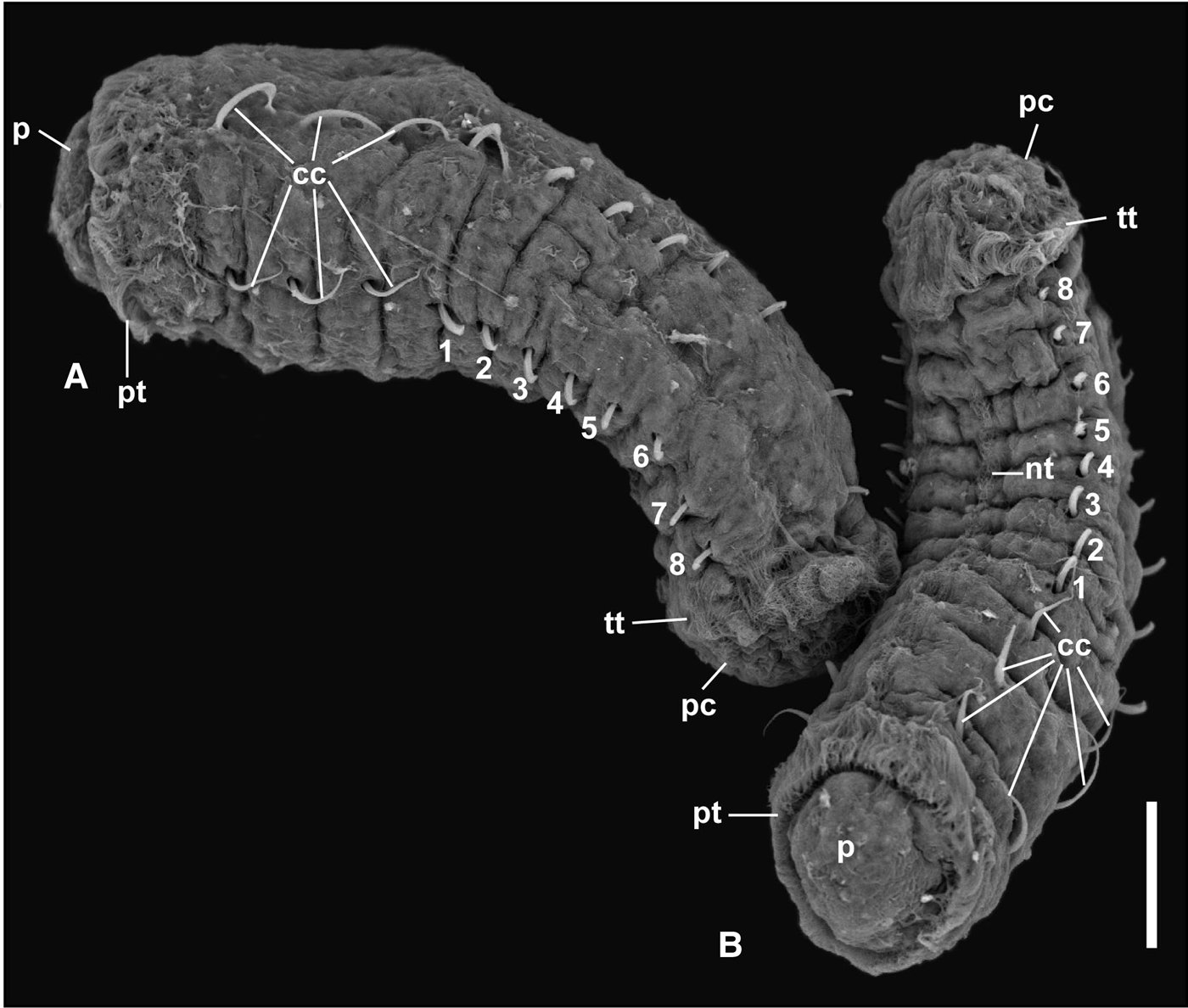
Development was lecithotrophic (see Supplementary material). A variable number of metatrochophore larvae (from 17 to 35) hatched and swam actively until 2 days post hatching, reducing swimming speed and approaching the bottom on day 3. Only larvae from one brood swam for 4 days. Early juveniles from 15 broods were evident 4 days post-hatching and were observed until 6 (14 broods) and 10 (1 brood) days post-hatching. Their length and volume were variable (Table 1). SEM images of the metatrochophores (Figure 2) show that the three anterior chaetae are long capillaries, followed by 8 (N = 8) or 9 (N = 1) hooded hooks. The last hooded hooks of some of the latter individuals were extremely small. The gut was visible under transmitted light before fixation.
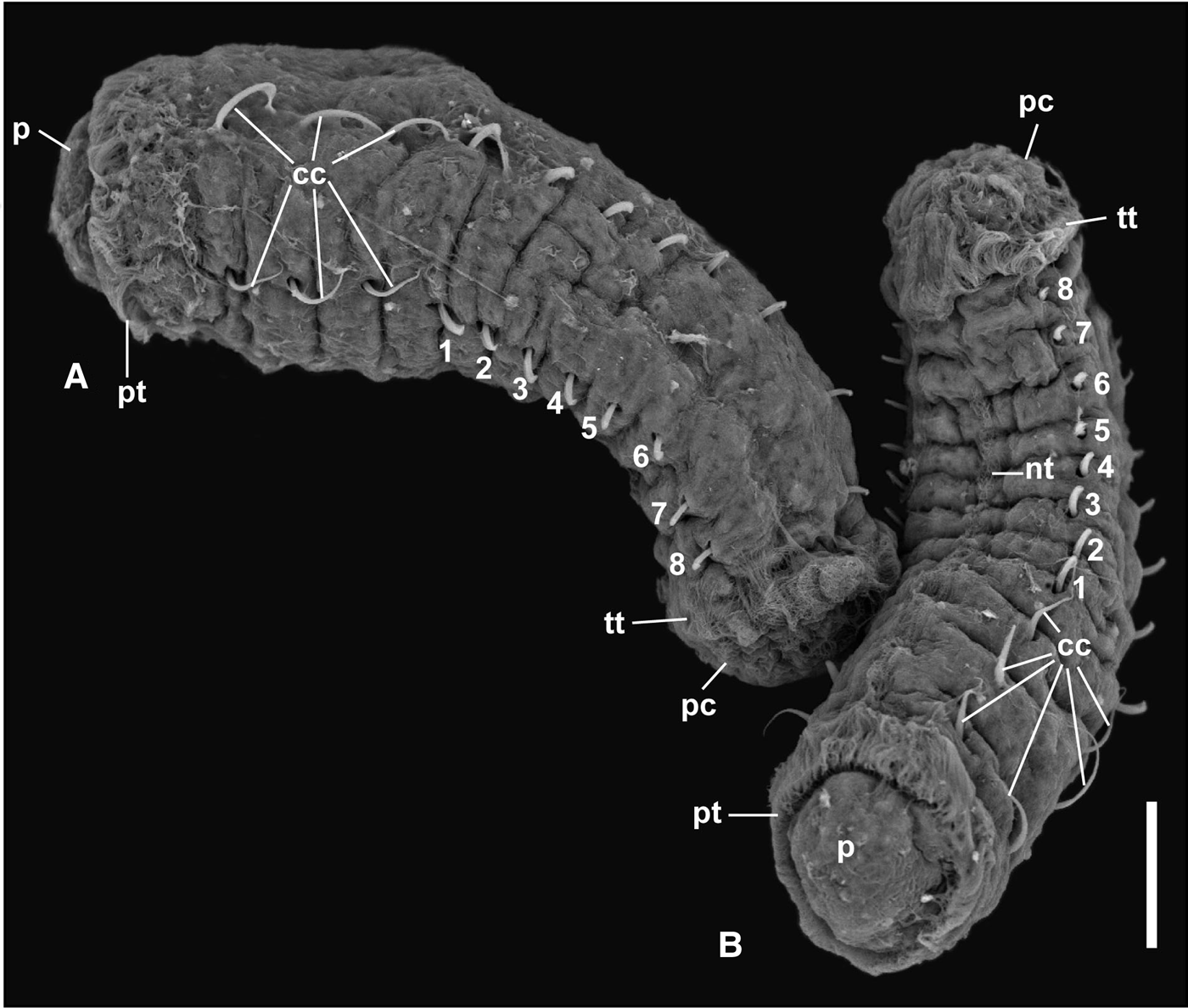
Fig. 2. Scanning electron micrograph of two metatrochophores of Capitella sp. TF 2 days after hatching from the brood tube. (A) Lateral view, anterior towards the left, ventral towards the bottom; (B) ventral view, anterior at the bottom. Abbreviations: cc, capillary chaetae; nt, neurotroch; p, prostomium; pc, pygidial ciliary bands; pt, prototroch; tt, telotroch; 1–8, hooded hooks, numbered from anterior to posterior. Scale bar: 50 µm.
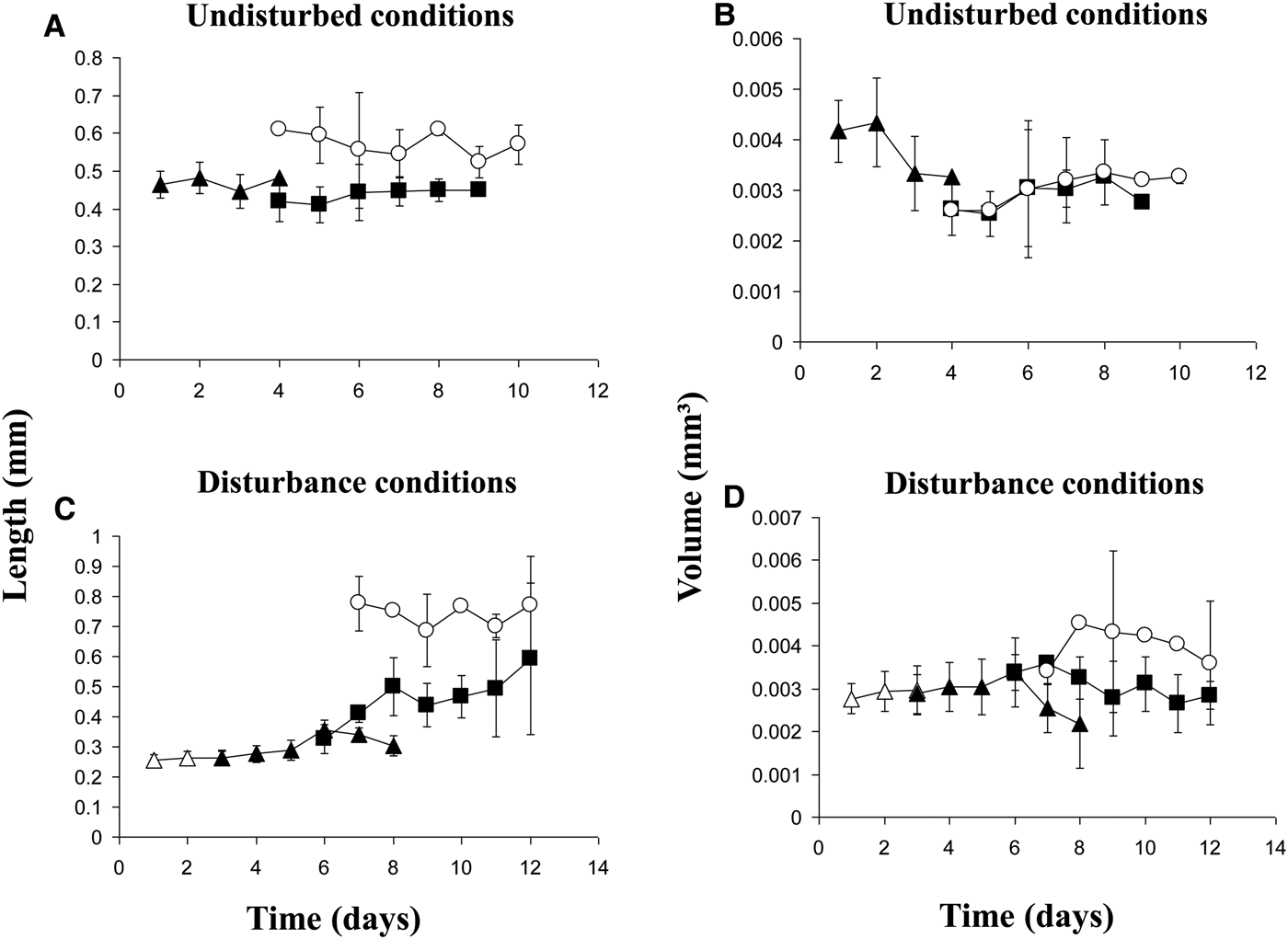
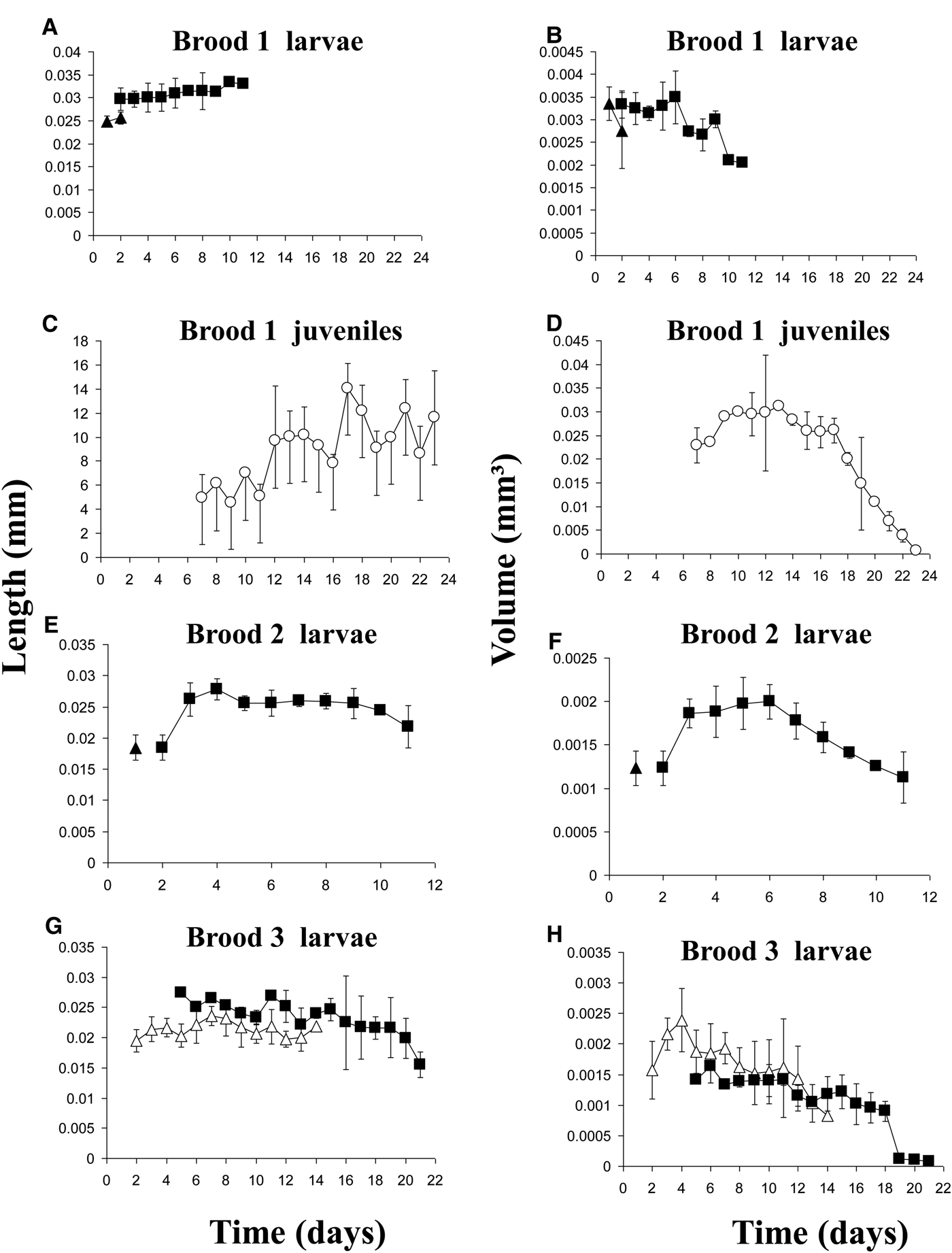
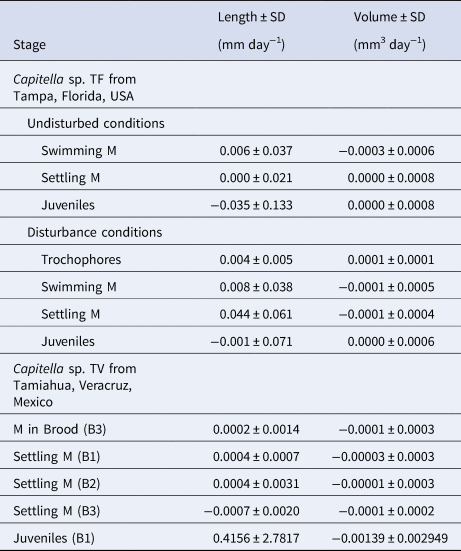
Except for volumes of the swimming metatrochophores, the general growth trend was to increase length and volume over time (Figure 3A, B). Growth rates of the different stages were minimal (Table 2). The exponential functions Length = 0.4782e−0.0187age (r = 0.169; N = 143; P < 0.05) and Volume = 0.0048e−0.1184age (r = 0.500; N = 143; P < 0.01) described the growth models of swimming metatrochophores, with a general trend towards a negative growth, as shown in Figures 3A, B. The relationships between age and size did not show a significant correlation for settling metatrochophores and juveniles. The exponential functions Length = 0.4369e0.014age (r = 0.167; N = 311; P < 0.01) and Volume = 0.0042e−0.0731age (r = 0.492; N = 311; P < 0.001) explained the global growth of early stages of Capitella sp. TF under undisturbed conditions.

Fig. 3. Average size±SD of individuals of Capitella sp. TF from Tampa of (A–B) the undisturbed conditions and (C–D) the disturbance conditions (empty triangles = trochophores; solid triangles = swimming metatrochophores; solid squares = settling metatrochophores; empty circles = juveniles).
Table 2. Mean growth rates (mm day−1 and mm3 day−1) of the different stages observed in the broods of Capitella sp. TF (pooled data) and Capitella sp. TV observed in each brood (M, metatrochophores; B, brood)
Disturbance conditions
Larvae of the 6 half-tubes that were re-sealed hatched 1–3 days post isolation and settled 6–12 days post isolation. All larvae that were released from their brood tubes survived until the end of the observation period on day 12. Some larvae did not survive past the trochophore (one dish) or metatrochophore stage (one dish). Length and volume of settling metatrochophores were similar to specimens from the undisturbed conditions but some differences in size and duration were observed for swimming metatrochophores and juveniles (Table 1).
No clear growth trends were observed in the 6 disturbed broods (Figures 3C, D). Growth rates were also minimal in these broods (Table 2). The relationship between length or volume with time was non-significant in most of the cases, except for swimming metatrochophores length (Length = 0.2132e0.0657age; r = 0.600; N = 75; P < 0.001), and settling metatrochophores; for the latter, length follows the exponential function Length = 0.2495e0.841age (r = 0.460; N = 41; P < 0.001) whereas volume follows the linear function Volume = −0.0001age + 0.0041 (r = 0.354; N = 41; P < 0.02). Global longitudinal growth of the early stages was explained by the expression Length = 0.2158e0.082age (r = 0.822; N = 203; P < 0.001), while volumetric growth was non-significant.
Capitella sp. TV from Tamiahua Lagoon, Veracruz
Twelve days after the installation of two stock cultures in the laboratory, a superficial layer of white fungi covered the sediment of one of the aquaria. About 50 worms, including several in tubes, were transferred to new sediment. After 3 days, the layer of fungi appeared again. Worms were individually extracted and all tubes were removed. Filtered seawater was UV-sterilized for 6 hours prior to establishing the new cultures. Females with white oocytes and males were observed. The same transfer procedure was repeated on day 19 because some fungi were observed again. Coupling was initiated 26 days later and the cultures were left to acclimatize for 2 months. After the acclimatization period, only four broods were found in the culture and observed daily. Brood tubes 1 and 2 had the female inside, while brood tubes 3 and 4 were found without the female but with live larvae. The four brood tubes were isolated from each other in individual dishes. The larvae were lecithotrophic and hatched from the tube as ciliated metatrochophores. The duration of each larval stage was highly variable (Table 1).
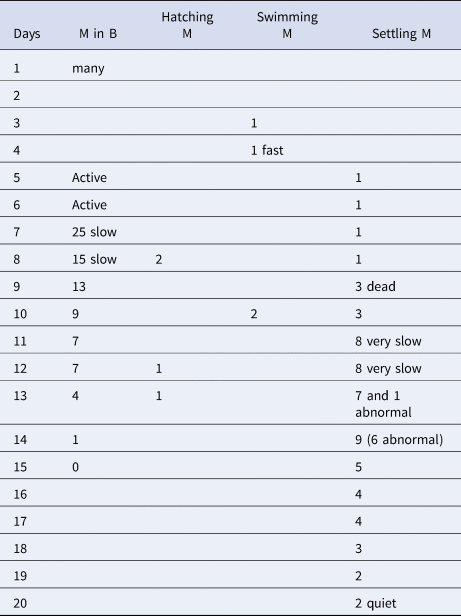
In all four dishes fungi re-appeared to various degrees. In addition, ciliated protozoans of the genus Euplotes became increasingly more common. In the two dishes with a female ventilating the brood tube (1 and 2), metatrochophore larvae (33 and 100, respectively) hatched and swam for 2 days, then settled. Mortality after settling was high. In brood 1, two juveniles were observed on day 7. They remained transparent with eyes and the first three chaetigers with capillary chaetae until day 19 and died on day 23. In brood 1, some larvae remained in the tube and were transferred to a new dish, but never hatched. In brood 2, none of the settled metatrochophores reached the juvenile stage. In one of the dishes without a female (brood 3), the larvae remained active inside the tube. Although some swimming metatrochophores were observed on days 3 and 4, most seemed to remain in the tube until about day 8 and then settled within a short time after hatching. On day 8, two metatrochophores hatched, swam and settled in 1–2 minutes. On day 13, a settling metatrochophore had an abnormal shape, with two rounded protuberances on each side of the body (Figure 4). Additional abnormal larvae were observed until day 20. The settling larvae became increasingly slower until all of them were dead on day 20. On day 15, all the larvae inside the brood had died (Table 3). In brood 4, some metatrochophores developed inside the brood tube, but none hatched and all were dead by day 3. The dish had a considerable amount of fungi and Euplotes.

Fig. 4. Abnormal settling metatrochophore from brood 3 of Capitella sp. TV after 13 days of hatching in dorsal view (scale bar: 100 mm).
Table 3. Number of live metatrochophores observed inside and outside brood 3 of Capitella sp. TV, and observations of the different stages (M in B, metatrochophores inside the brood; M, metatrochophores)
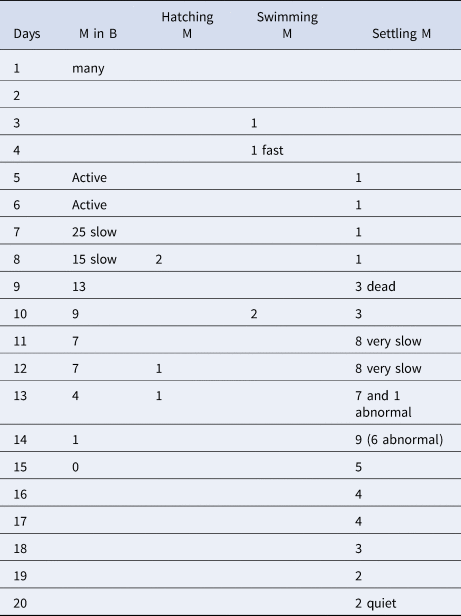
Metatrochophore length and volume varied among broods (Figure 5). Their size ranges and mean values are summarized in Table 1 and the growth rates in Table 2. The linear function Volume = −9E05age + 0.0023 (r = 0.805; N = 13; P < 0.01) describes their negative volume growth inside the tube (Figure 5H). The relationship between length and age was non-significant. For hatching (Table 1) and swimming (Figure 5) metatrochophores, too few measurements were obtained to calculate growth rates. The sizes and growth rates of settling metatrochophores are depicted in Tables 1 and 2. Using the exponential function Length = 0.0292e0.0192age (r = 0.503; N = 37; P < 0.01) and the linear function Volume = −6E0.5age + 0.0014 (r = 0.384; N = 37; P < 0.01) their growth rates can be described as negative (Figures 5B, E–H). Likewise, using the exponential function Length = 5.358e0.588age (r = 0.739; N = 15; P < 0.01) and the linear function Volume = −0.0017age + 0.0351 (r = 0.776; N = 15; P < 0.001), at least one of the juveniles from brood 1 showed negative growth (Table 1; Figure 5D).
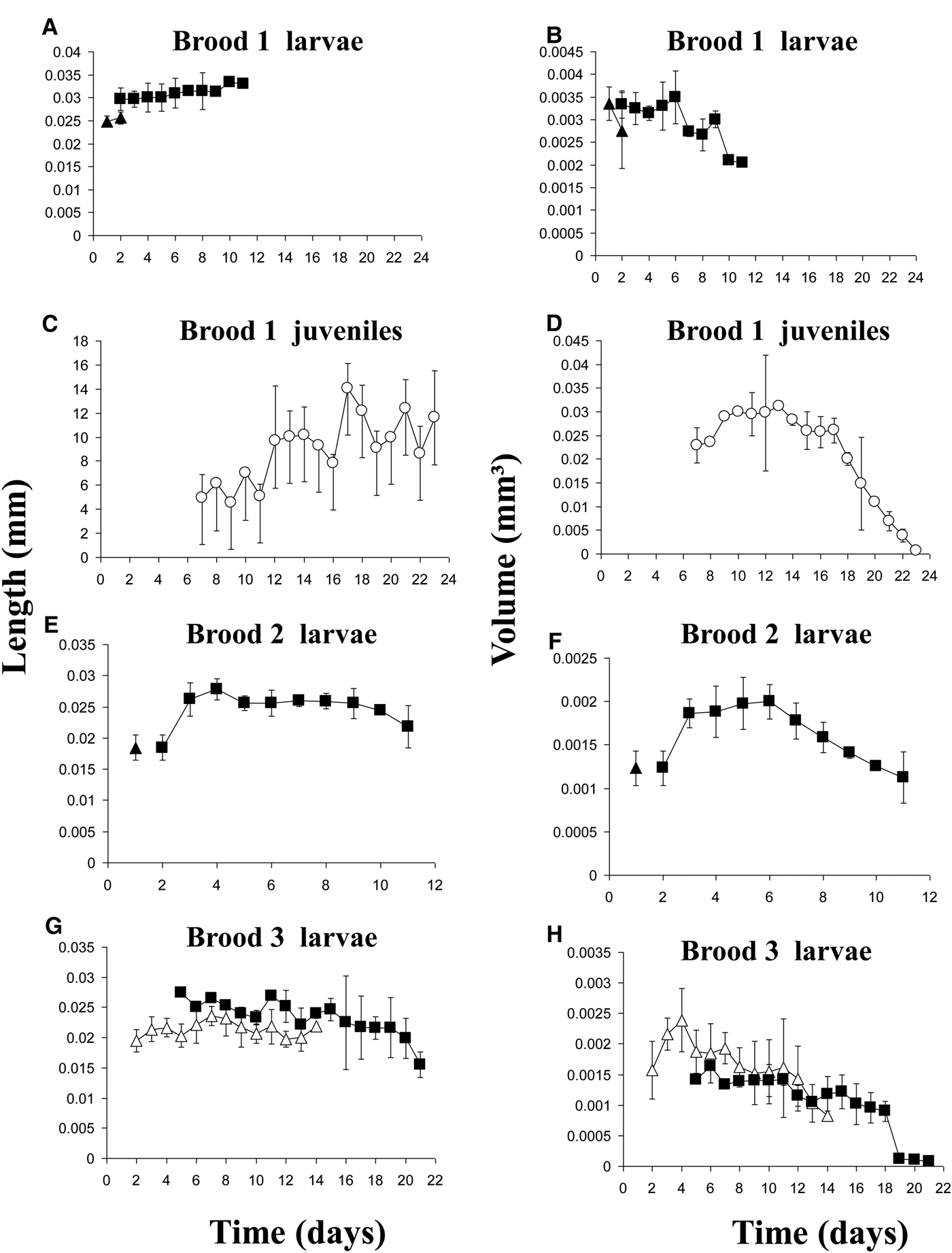
Fig. 5. Average size±SD of individuals from broods 1 to 3 of Capitella sp. TV from Tamiahua (empty triangles = brooding metatrochophores; solid triangles = swimming metatrochophores; solid squares = settling metatrochophores; empty circles = juveniles).
The 21 adults that survived in the cultures were 10 males, 4 females with white oocytes, 3 females with yellow ovaries and 4 indeterminate adults (without evident sexual structures). These were coupled in seven dishes with 1 male and 1 female, two dishes with a male and an indeterminate adult, and one dish with 1 male and 2 indeterminate adults. Indeterminate adults were smaller in length and volume than males and females, which suggest that they were immature worms (Table 1). No hermaphroditic individuals were observed. The mucus produced by worms was very thick and viscous, making it difficult to break using forceps and a scalpel.
During the coupling period, fertilization and brood tube building did not occur in any of the 10 dishes. Euplotes were abundant in all dishes. On day 50 after the observations started, only 9 worms survived and all had a greyish red colour, typical of old worms. Length was maintained in a similar range as that observed in the beginning of the observation, while volume was considerably reduced (Table 1). On day 58, the 9 individuals were dead and covered by Euplotes.
Discussion
Unique traits of Capitella sp. TF and Capitella sp. TV
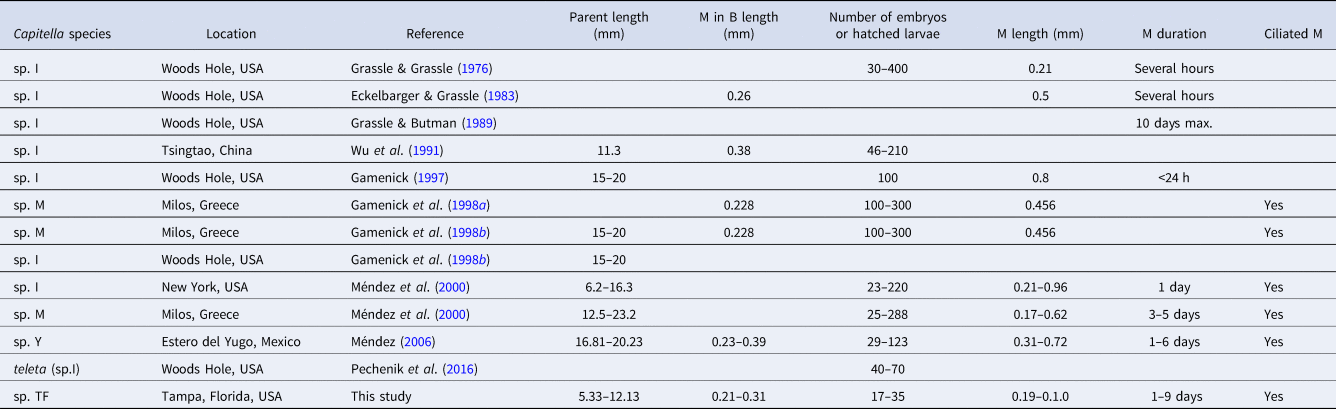
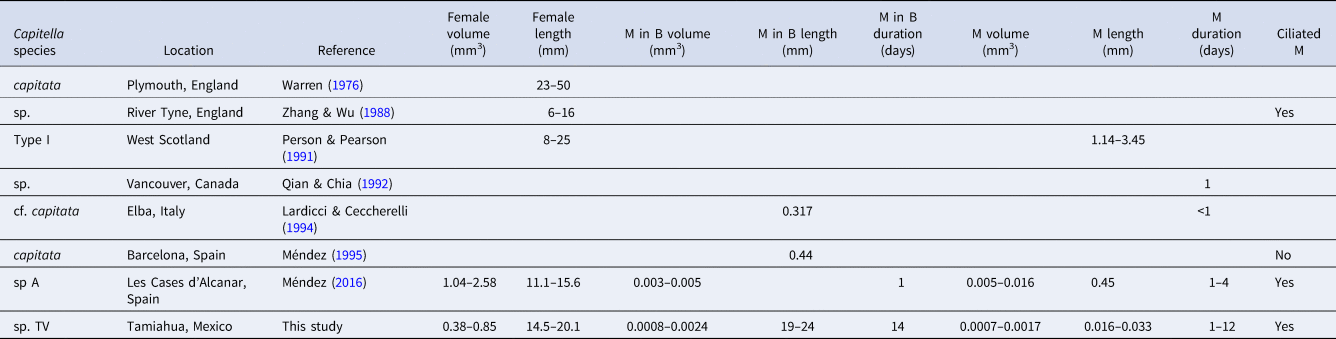
Both new species can be distinguished from other Capitella species by unique combinations of life history traits (Tables 4 and 5). While adult Capitella sp. TV display the typical traits of the genus and do not show any obvious distinguishing features, Capitella sp. TF has a distinctive colour pattern (Figure 1A) and produces uniquely shaped brood tubes (Figure 1B). The primary distinguishing feature between Capitella sp. TF and Capitella sp. TV is the presence of hermaphrodites in the former. The most distinctive feature of Capitella sp. TV is the small size of the metatrochophores, both free-swimming and inside the brood tube (where reported), compared to those of other species (Table 5).
Table 4. Differences between Capitella sp. TF and the other lecithotrophic hermaphroditic species studied around the world (M in B, larvae in broods; M, metatrochophores)
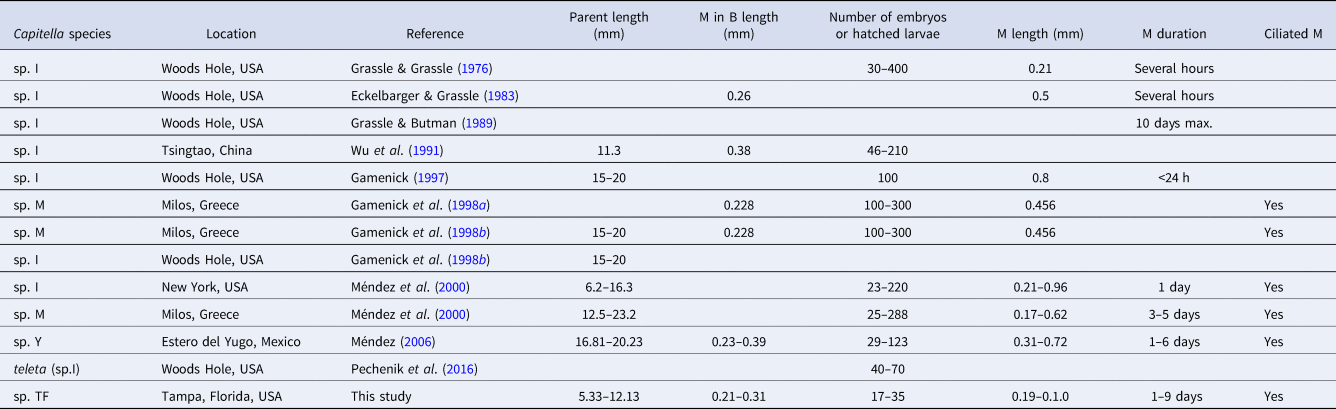
Table 5. Differences between Capitella sp. TV and the other lecithotrophic non-hermaphroditic species studied around the world (M, metatrochophores; M in B, metatrochophores in broods)
Comparison with other Capitella species
A commonality of the life cycle in both species is that the hatching stages are lecithotrophic metatrochophore larvae. The Capitella species complex contains at least 16 lecithotrophic species, three of them with hermaphrodites (compilation in Méndez et al., Reference Méndez, Linke-Gamenick and Forbes2000 and Méndez, Reference Méndez2016; Table 4). Comparisons of early developmental features of Capitella sp. TF with the other three hermaphroditic and lecithotrophic Capitella species indicate some common features such as the size of larvae inside the brood tube and the size of metatrochophores (Table 4). Some descriptions of Capitella spp. metatrochophores indicate the presence of 13 segments with capillary chaetae in the first three segments (Méndez, Reference Méndez1995; Adkins & Schulze, Reference Adkins and Schulze2011) and hooded hooks in the subsequent 10 segments (George, Reference George and Hutchings1984). Our SEM images of metatrochophores show the presence of 8 or 9 segments bearing hooks in Capitella sp. TF. Additional hooks may have been embedded in the tissue, impeding their observation. Detailed SEM studies have not been performed with metatrochophores from the other hermaphroditic species, which prevents comparisons at this level.
The size of females of Capitella sp. I (now Capitella teleta; Blake et al., Reference Blake, Grassle and Eckelbarger2009) and the duration of metatrochophores of Capitella sp. M and Capitella sp. Y are in the same order of magnitude as those from Capitella sp. TF (Table 4). In contrast, the number of larvae inside broods and the duration of the metatrochophore stage vary among the species. Capitella sp. TF can also be distinguished from C. teleta by the unique shape of the brood tubes in the former species. Pechenik et al. (Reference Pechenik, Chaparro, Pilnick, Karpi, Acquafredda and Burns2016) described the brood tubes of C. teleta as generally about 8–15 mm long and about 0.6–1.3 mm wide, and did not mention any peculiarity in the shape of the brood tube (like sand accumulation) as observed in Capitella sp. TF (Figure 1B).
The small size of metatrochophores in Capitella sp. TV is remarkable (Table 5). Inside the brood tube, the metatrochophores are only roughly half the length of those of C. capitata (Méndez, Reference Méndez1995) and about one order of magnitude smaller in volume than Capitella sp. A (Méndez, Reference Méndez2016). Likewise, free-swimming metatrochophores are more than one order of magnitude smaller than in any other species for which metatrochophore size has been reported. Zhang & Wu (Reference Zhang and Wu1988) did not report metatrochophore size for Capitella sp. from the River Tyne, but reported oocytes of 0.159–0.189 mm diameter which is an order of magnitude larger than the metatrochophores of Capitella sp. TV. Furthermore, Capitella sp. TV can be distinguished from Capitella sp. (Qian & Chia, Reference Qian and Chia1992) by the longer duration of the metatrochophores inside the brood tube, from C. capitata (Warren, Reference Warren1976) by the smaller size of the female and from C. capitata (Méndez, Reference Méndez1995) by the absence of ciliary rings in the metatrochophore.
Disturbance effects
Observation of embryonic and larval development inside the brood tubes is challenging due to crowding and lack of transparency of the tubes. More detailed observations of the early stages were possible for Capitella sp. TF larvae that had been forcibly removed from the tubes for the disturbance experiments. These experiments also presented an opportunity to compare the results with those of previous studies on specimens from Galveston, TX (Adkins & Schulze, Reference Adkins and Schulze2011). We demonstrated that larvae can survive until metamorphosis when forced from the tube as trochophores or metatrochophores, but not before reaching trochophore stage. Offspring that were forced out of the tubes as embryos (before the appearance of cilia or eyes) did not survive more than 3 days. When released at the metatrochophore stage, larval duration was similar to the undisturbed broods. When larvae were forced to emerge from the tube in the trochophore state, they grew to the normal size, but developmental timing increased, suggesting that physical disturbance of the brood tubes slows their normal growth rates. This flexibility in the life cycle is consistent with Adkins & Schulze's (Reference Adkins and Schulze2011) conclusion that plasticity in developmental timing is an adaptation to living in frequently disturbed environments, as common in the Gulf of Mexico.
Although we were unable to perform the same controlled experiments with Capitella sp. TV due to the culturing problems we encountered, the presence of fungi and ciliated protozoans (Euplotes) provided another source of environmental disturbance that was probably detrimental to the survival of the cultures. The fungi were apparently introduced to the lab from the sediment in Tamiahua Lagoon. The experimental sediment was from another location and was clean of fungi and protozoans because it was previously dried and frozen to eliminate any live organism. However, fungal spores adhering to the worms or transported in their digestive tract may have been accidentally introduced into the experimental dishes. The protozoans were observed in the dishes during the first part of the experiment. Euplotes spp. feed on bacteria and typically aggregate in biofilms (Lawrence & Snyder, Reference Lawrence and Snyder1998). The bodies of the polychaetes and the mucus they produce may therefore present ideal substrate for their proliferation. Fungi and protozoans were particularly abundant in specimens which were close to death, sometimes surrounding them like a layer of cotton. We assume that the observed production of thick mucus, delay in metamorphosis, juvenile mortality, small size of worms and failed coupling is at least partially attributable to the presence of fungi and Euplotes. Personal observations failed to see thick mucus in other species of Capitella, which suggests that Capitella sp. TV produces thicker mucus to protect itself from fungi and protozoans.
When female Capitella sp. TV remained within the brood tubes (broods 1 and 2), the larval duration was 12 days, while larvae from brood 3 (which was abandoned by the female) lasted 21 days. During hatching, some metatrochophores from brood 1 remained inside the tube and died there. Maybe fungi and protozoans produce toxins that affect growth, such as the delay of metamorphosis, as demonstrated in previous studies with cadmium, copper and teflubenzuron that can have similar effects on metamorphosis in Capitella spp. (Méndez, Reference Méndez2002b, Reference Méndez2005; Méndez & Green-Ruiz, Reference Méndez and Green-Ruiz2006).
The larval duration in brood 3 is very long compared with other lecithotrophic non-hermaphroditic Capitella species (Table 5). It is actually unusual for larvae to survive for such a long time (14 days) inside the brood tube without the presence of the mother. Usually C. teleta females do not leave the tube until the larvae hatch (Pechenik & Cerulli, Reference Pechenik and Cerulli1991; Pechenik et al., Reference Pechenik, Chaparro, Pilnick, Karpi, Acquafredda and Burns2016). The role of the female during the brooding period consists of ventilating the fertilized eggs by periodic body undulations to allow water circulation inside the tube (Reish et al., Reference Reish, Piltz, Martin and Word1974; Reish, Reference Reish, Buikema and Cairns1980; Pechenik et al., Reference Pechenik, Chaparro, Pilnick, Karpi, Acquafredda and Burns2016). Pechenik et al. (Reference Pechenik, Chaparro, Pilnick, Karpi, Acquafredda and Burns2016) report that females of C. teleta abandon the brood tubes under hypoxia, leading to the death of the offspring. Similarly, Méndez et al. (Reference Méndez, Linke-Gamenick and Forbes2000) found that the lack of the female inside the brood tube was the main reason that larvae were not viable. The presence of brood tubes in stock cultures containing embryos without the female has been observed previously, but the larvae had never survived more than one day (N. Méndez, pers. obs.). The long metatrochophore development inside the brood could induce the production of abnormal larvae that survived for 8 days outside the tube. Abnormal larvae from Capitella spp. have been observed when exposed to copper (Reish et al., Reference Reish, Piltz, Martin and Word1974) and fluoxetine (Méndez & Barata, Reference Méndez and Barata2015), but had never been reported in the laboratory under natural conditions.
Field and laboratory studies have demonstrated that Capitella spp. growth rates strongly depend on food availability (Tenore, Reference Tenore1977; Forbes & Lopez, Reference Forbes and Lopez1990; Tsutsumi et al., Reference Tsutsumi, Fukunaga, Fujita and Sumida1990; Qian & Chia, Reference Qian and Chia1992; Linton & Taghun, Reference Linton and Taghun2000; Méndez, Reference Méndez2002a). The possibility that Capitella sp. TV juvenile mortality could be due to starving is discarded because the experimental sediment had enough food, as demonstrated by adults' survival for more than 50 days in the same sediment and by the presence of their faecal pellets in the sediment, which demonstrates that they were feeding normally. Thus, juvenile mortality could be attributable to the presence of fungi and Euplotes.
Growth rates
The documented growth rates in both species show a high degree of variability. Growth of the early stages of Capitella sp. TF was minimal or even negative. This effect was even more pronounced in juveniles (Table 1; Figure 3). For Capitella sp. TV, growth rates of the different developmental stages were positive for length but negative for volume. Volumetric data (Figure 5) show a gradual shrinkage of adults before death and a more pronounced trend for metatrochophores. The positive growth rates of larvae and juveniles calculated with length values, as well as length of old adults, could be explained by the elongation observed in members of C. capitata under stressed situations, such as formalin fixation (Méndez & Cardell, Reference Méndez and Cardell1994) or, in this case, the presence of fungi and protozoans.
Conclusions
This study documents observations concerning the reproductive biology and early development of two Capitella species that have not been previously studied. The differences in developmental modes, size and timing of Capitella sp. TV and Capitella sp. TF indicate that they do not belong to any of the lecithotrophic species described. Formal species descriptions, including DNA sequence data, are currently underway.
Our observations and experiments demonstrate that despite a few fixed life history features (brood tubes, trochophore and metatrochophore larvae), there is remarkable diversity and plasticity in Capitella life cycles. Small changes in life history characteristics (e.g. larval duration inside the tube) could have significant effects on the survival of a population. Environmental disturbance can also be the cause of changes in life histories. The concept of environmental indicators can be meaningful in the general sense that Capitella species tend to be resilient to environmental disturbance where other species may not be. However, responses to specific disturbances are still not very well understood and could vary broadly among species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000687
Acknowledgements
José Salgado Barragán, Sergio Rendón Rodríguez and Zachary Hancock helped with collections in Mexico. Nathan Reed, David Karlen and Marissa Hajduk assisted with collections in the USA. Stephanie Wagner (TAMUG) assisted with maintenance of larval cultures and digital imaging. Candace Grimes and Janelle Goeke assisted with the organic content analysis of the sediment. Thanks are also given to Yosahandy Vázquez Molina for her advice with the software used in Mazatlán. Fernando Pardos Martínez identified the genus Euplotes.
Financial support
This study was performed at the Department of Marine Biology (TAMUG) and the Unidad Académica Mazatlán (ICMyL, UNAM), and was supported by a TAMU-CONACyT Collaborative Grant to AS and NM (project #2017-025).