Introduction
In the horse, successful in vitro fertilization (IVF) is apparently limited by current methods of in vitro maturation (IVM) of equine oocytes; these methods are less successful than those used in other species. Furthermore, the limited supply of abattoir-derived equine ovaries limits refinement of IVM and IVF protocols. Based on several reports, immature equine oocytes are capable of completing meiosis in vitro, but subsequent fertilization and embryonic development are suboptimal (Dell'Aquila et al., Reference Dell'Aquila, Fusco, Lacandra and Mariato1997; Goudet et al., Reference Goudet, Bezard, Duchamp, Gerard and Palmer1997; Zhang et al., Reference Zhang, Muzs and Boyle1990).
In other mammals, it is clearly recognized that IVM of oocytes require both nuclear and cytoplasmic maturation to achieve normal embryonic development (Damiani et al., Reference Damiani, Fissore, Cibelli, Long, Balise, Robl and Duby1996; Eppig, Reference Eppig1996; Ogushi et al., Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008). The migration of cortical granules (CG) is a critical event that has been used to assess cytoplasmic maturity and organelle organization (Ducibella et al., Reference Ducibella, Kurasawa, Duffy, Kopf and Schultz1993; Carneiro et al., Reference Carneiro, Liu, Hyde, Anderson, Lorenzo and Ball2002; Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009). In that regard, migration of CG to the oocyte periphery and the presence of chromosomes aligned at the metaphase plate are indicators of complete cytoplasmic and nuclear maturation, respectively. In most of these studies, the endpoint was nuclear maturation rate recorded as percentage of oocytes that achieved metaphase II (MII). However, the ability of fertilized oocytes to develop depends upon normal nuclear and cytoplasmic oocyte maturation and, both are essential for an oocyte to develop the capacity for fertilization and normal embryonic development. Identification of CG migration among treated oocytes in vitro has been used as one potential parameter to evaluate cytoplasmic maturation using laser-scanning confocal microscopy (Ducibella et al., Reference Ducibella, Anderson, Albertini, Aalberg and Rangarajan1988, Reference Ducibella, Duffy and Buetow1994; Liu et al., Reference Liu, Mal, Miao, Liu, Bao and Tan2005).
Previous studies have demonstrated the critical roles of growth hormone (GH), GH receptor (GH-R) and insulin-like growth factor-I (IGF-I) in mediation of cell growth (Liu & LeRoith, Reference Liu and LeRoith1999; Lupu et al., Reference Lupu, Terwilliger, Lee, Segre and Efstratiadis2001). Furthermore, GH enhances growth by facilitating cellular proliferation in terminally differentiated cells (Huang et al., Reference Huang, Kim, Jiang and Frank2003). The actions of GH are mediated by binding to the transmembrane GH-R, which is present on the surface of most cells. Following binding, the GH/GH-R complex induces activation of the cytoplasmic tyrosine kinase-2, which is required for tyrosine phosphorylation of GH-R and to interact with other intracellular signaling mechanisms (Lobie et al., Reference Lobie, Breipohl, Aragon and Waters1990; Fernandez et al., Reference Fernandez, Flores-Morales, Lahuna, Sliva, Norstedt, Haldosen, Mode and Gustafsson1998; Bevers & Izadyar, Reference Bevers and Izadyar2002). Insulin-like growth factor-I is an important effector peptide, which functions primarily as a circulatory second messenger to the GH in the somatomedin hypothesis (Le Roith et al., Reference Le Roith, Bondy, Yakar, Liu and Butler2001). Communication between GH and IGF-I is initiated by the binding of GH to its cell-surface receptor and is rapidly propagated to the nucleus to stimulate transcription of the IGF-I gene (Zapf & Hunziker, Reference Zapf, Hunziker, Baxter, Gluckman and Rosenfeld1994).
Growth factors play critical roles in normal physiology; IGF-I increased embryo cleavage rates in several species, including rabbits (Lorenzo et al., Reference Lorenzo, Rebollar, Illera, Illera, Illera and Alvarino1996), cattle (Rieger et al., Reference Rieger, Luciano, Modina, Pocar, Lauria and Gandolfi1998), sheep (Guler et al., Reference Guler, Poulin, Mermillod, Terqui and Cognie2000), and humans (Magier et al., Reference Magier, van der Ven, Diedrich and Krebs1990). The addition of IGF-I to the maturation medium increases significantly the proportion of oocytes that reach MII and cleavage rates of equine oocytes after pathenogenic activation (Carneiro et al., Reference Carneiro, Lorenzo, Pimentel, Pegoraro, Bertolini, Ball, Anderson and Liu2001). The presumptive interaction between GH and IGF-I could be important for several reproductive processes, as IGF-I has an important role in ovarian function in both animal and human models (Erickson et al., Reference Erickson, Garzo and Magoffin1989; Adashi et al., Reference Adashi, Resnick, Hurwitz, Ricciarelli, Hernandez, Roberts, Leroith and Rosenfeld1991) and GH has an important role in preantral follicle growth and differentiation and development of small antral follicle to gonadotrophin-dependent stages (Silva et al., Reference Silva, Figueiredo and van den Hurk2009). The effect of GH on the oocyte maturation in vitro of bovine and rat oocytes is a direct action of the hormone itself, and it is not mediated by IGF-I (Apa et al., Reference Apa, Lanzone, Miceli, Mastrandrea, Caruso, Mancuso and Canipari1994; Izadyar et al., Reference Izadyar, Colenbrander and Bevers1997a, Reference Izadyar, Van Tol, Colenbrander and Beversb). However, the mechanisms by which GH affects oocyte maturation and early embryo development in the horse is still unknown, as there are apparently no reports regarding nuclear and cytoplasmatic maturation of equine oocytes matured in vitro after the addition of GH and its interaction with IGF-I.
The overall goal of our work is to develop an effective and consistent method of maturing equine oocytes in vitro in order to facilitate and improves successful IVF procedures in the horse. The specific objective of this study was to test the hypothesis that the addition of equine growth hormone (eGH) and IGF-I to serum-free maturation medium enhances nuclear and cytoplasmic maturation of equine oocytes.
Materials and methods
This experiment was replicated at two locations, southern Brazil at the EMBRAPA Research Center located in Pelotas, RS and the Agriculture and Agri-Food Canada Research Centre in Lethbridge, AB, Canada. At each location, ovaries were collected after slaughter during the physiologic breeding season and the protocols were identical. All chemicals used were purchased from Sigma Chemicals Company, St. Louis, MO, USA, unless otherwise indicated in the text.
Collection of cumulus–oocyte complexes
Equine ovaries were obtained from both abattoirs located approximately 30 min from each respective laboratory. Immediately after collection, ovaries were placed in warm (25–30°C) saline solution (0.9% NaCl) that contained 1000 IU/ml of penicillin and 500 μg/ml of streptomycin for transport. The visceral peritoneum that covers the ovary was removed to reduce contamination and to facilitate identification of follicles. Selected follicles (<25 mm in diameter), were aspirated with an 18-gauge needle connected to a 35-ml syringe. To enhance oocyte recovery, a scraping motion was performed with the needle during aspiration and follicular fluid was flushed in and out of follicles two to four times. The follicular fluid from each follicle aspirated was placed into 15-ml tubes and, after allowing 20 min for sedimentation to occur, the pellet was collected using a Pasteur pipette and placed in 100-mm Petri dishes to locate the cumulus–oocyte complexes (COCs) and evaluate morphology and structural integrity under a stereomicroscope. The COCs were classified as compact (CP; tight, complete, compact cumulus with a distinct, smooth hillock), expanded (EX; granular or expanded cumulus), or denuded (D; partial cumulus or only corona radiata present). As oocytes show compact cumulus cells and homogeneous cytoplasm have a lower mitotic competence in comparison with expanded oocytes (Hinrichs & Williams, Reference Hinrichs and Williams1997), we used only compact oocytes in this experiment in order to test if different supplementation of maturation medium would be effective on the improvement of maturing equine oocytes in vitro. The interval between recovery of ovaries and oocyte culture ranged from 3 to 5 h.
Culture of COCs
The basal medium for oocyte washing and maturation was tissue culture medium 199 (TCM-199; containing Earle's salts, HEPES, sodium bicarbonate and without l-glutamine), supplemented with 0.1% of bovine serum albumin (BSA, fraction V), 100 IU/ml of penicillin G, and 50 μg/ml of streptomycin sulfate. The medium was filtered and allowed to equilibrate for 1 h under 5% CO2 in air before use. Equine GH (eGH; Andrology Laboratory, The University of Auckland, Auckland, New Zealand) was diluted in TCM-199 and used at a concentration of 400 ng/ml, determined previously to be optimal for oocyte nuclear maturation (unpublished data). Insulin-like growth factor-I was also diluted in TCM-199 and used at 200 ng/ml, in accordance with Carneiro et al. (Reference Carneiro, Lorenzo, Pimentel, Pegoraro, Bertolini, Ball, Anderson and Liu2001). Before culture, selected COCs were washed three times in basal medium and transferred to 4-well plates (No. 176740; Nunc Intermed), with each well containing 500 μl of basal medium alone (control) or supplemented with 400 ng/ml of eGH alone, 400 ng/ml of eGH + 200 ng/ml of IGF-I, 200 ng/ml of IGF-I alone or 400 ng/ml of eGH + 200 ng/ml of IGF-I + 400 ng/ml anti-IGF-I antibody (Calbiochem, EMD Biosciences Inc.). Complexes were cultured at 38.5 °C in a moisture-saturated atmosphere of 5% CO2 in air for 30 h.
Oocyte fixation and staining
After 30 h of maturation, cumulus cells were removed with 0.1% hyaluronidase solution and stripped mechanically by a microcapillary glass pipette. Zonae pellucidae were removed using 0.1% protease in 300 μl of TCM-199, and oocytes were fixed in 2% paraformaldehyde for 2 h at 5°C, followed by four washes in blocking solution (phosphate-buffered saline solution containing 0.2% sodium azide, 100 mM glycine and 1 mg/ml polyvinyl alcohol). Then, oocytes were individually transferred to 5-ml tubes containing 2 ml of block solution and transported in a thermal container at 5°C to the University of California Davis, USA to be analysed by confocal microscopy. After recovery, from the 5 ml Eppendorf tubes, oocytes were incubated in 10 μg ml−1 fluorescein isothiocyanate-labelled lens culinaris agglutinin (FITC-LCA; Vector Labs, Inc.) for 15 min into a 4-well plate and subsequently washed in blocking solution for 5 min. Then, nuclear status of the oocytes was evaluated by counter staining with 10 μg/ml propidium iodide (PI) for 5 min in the dark. A drop containing three to five oocytes was placed onto a slide in a 25-μl drop of mounting medium (Vector Labs). Oocytes were placed on slides and the space between the coverslip and the slide was filled with anti-fade mounting medium and sealed with nail polish.
Confocal microscopy
Laser-scanning confocal microscopy was performed using a Bio-Rad MRC 1024 ES, equipped with a krypton–argon ion laser for the simultaneous excitation of fluorescein for CG and PI for DNA. Images were achieved on magnetic optical disks and each oocyte was fully observed with optical sections of 0.1–2.0 μm intervals. Oocytes were classified as immature when clusters of CGs were distributed homogeneously throughout the cytoplasm and no chromosomes were visible. Oocytes at metaphase I (MI) stage were characterized by the presence of chromosomes peripherally in the ooplasm and the CGs randomly distributed in the cortex cytoplasm. Oocytes with chromosomes aligned at the metaphase plate and/or evidence of cortical granule migration at the periphery of the oocyte were both classified as metaphase II (MII).
Statistical analyses
A total of eight replicates of the experiment were conducted in this study. The difference in oocyte maturation after treatments groups on IVM was performed by chi-squared test using SAS Software. Fisher exact test were calculated by EpinInfo Statistical Software and used when an expected value cell was less than 5. The level of significance was set at p < 0.05.
Results
A total of 443 ovaries were processed and 753 oocytes were recovered. From recovered oocytes, 530 (70.3%) oocytes were classified as compact, 144 (19.3%) degenerated, and 77 (10.3%) expanded. Only 45.6% (242) of the in vitro matured oocytes were stored and stained to evaluate the presence of nuclei and cortical granule migration by confocal microscopy. Oocytes not used in this experiment were preserved in block solution and designated to another design study.
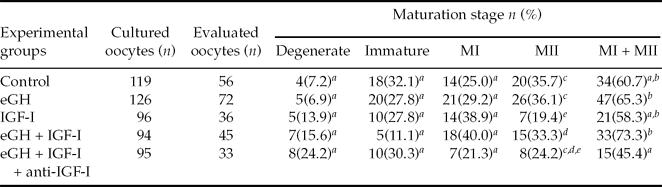
The proportion of immature oocytes that developed to the MII stage in the group treated with eGH + IGF-I (15 of 45; 33.3%) was greater than the proportion that were treated only with IGF-I (7 of 36; 19.4%, p = 0.03), as showed by Fischer Exact Test analyses. Oocytes that reached MII in the control group (20 of 56; 35.7%) showed a tendency to be different when compared with eGH + IGF-I group (15 of 45; 33.3%, p = 0.08). We also observed similar oocyte maturation rates at the MII stage of development when we compared the control (20 of 56; 35.7%) with eGH (26 of 72; 36.1%) and IGF-I (15 of 45; 33.3%) treated groups (p > 0.05). When data were combined (MI + MII), the treated group that contained anti-IGF-I (15 of 33; 45.4%) showed a decrease in the number of oocytes that reached any stage of development when compared with eGH (47 of 72; 65.3%) and eGH + IGF-I (33 of 45; 73.3%) treated groups (p = 0.05), as showed by chi-squared test analyses. Maturation data are shown in Table 1.
Table 1 The effect of equine growth hormone and insulin growth factor-I on the nuclear and cytoplasmic maturation of equine oocytes in vitro after 30 h of culture
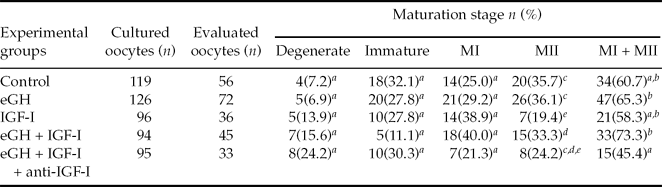
Metaphase I (MI); Metaphase II (MII); Equine growth factor (eGH); Insulin growth factor-I (IGF-I).
a–eDifferent letters in the same column indicate that values differ significantly (a vs.b; p = 0.05, cvs.d; p = 0.08, c, dvs.e; p = 0.03).
Data were pooled from eight replicates.
Overall, 63 (26.03%) oocytes had no evidence of development (immature oocyte; Fig. 1A), whereas 74 (30.6%) reached MI and 86 (35.5%) reached MII (MII oocyte; Fig. 1B). In that regard, chromosomes aligned at the spindle equator (indicating nuclear maturation) and the migration of CG (indicating complete cytoplasmic maturation) was designated as MII (Fig. 1C,D).

Figure 1 Equine oocytes evaluated after 30 h of incubation using confocal microscopy. The images were taken at the middle section. (A) Immature oocyte: cortical granules (CG) are distributed homogeneously throughout the cytoplasm. Bar represents 30 μm. (B) Oocyte at metaphase II (MII) stage of development and the CG released to the periphery after oocyte maturation. Bar represents 30 μm. (C) Closer view of the chromosomes aligned at the metaphase plate. Bar represents 60 μm. (D) Closer view of a CG cluster formation located at the periphery of the oocyte. Bar represents 60 μm.
Discussion
This study was designed to determine the effect of eGH and IGF-I interactions under serum-free culture conditions on maturation of equine oocytes cultured in vitro, effectively ruling out the influence of unknown serum factor(s) that might potentially alter the results in our IVM system. We reported previously that the addition of eGH under serum-free culture in vitro increased significantly progesterone and testosterone production during oocyte maturation, whereas estradiol and androstenedione remained constant (Pereira et al., Reference Pereira, Lorenzo, Carneiro, Bilodeau-Goeseels, Kastelic, Pegoraro, Pimentel, Esteller-Vico, Illera, Silvan, Casey and Liu2006). The addition of IGF-I to an IVM medium that contained hormones did not increase nuclear maturation but promoted cytoplasmic maturation as measured by pathenogenic cleavage, which suggested the presence of a synergistic interaction between IGF-I and gonadotropins in nuclear and cytoplasmic maturation of equine oocytes (Carneiro et al., Reference Carneiro, Lorenzo, Pimentel, Pegoraro, Bertolini, Ball, Anderson and Liu2001, Reference Carneiro, Liu, Hyde, Anderson, Lorenzo and Ball2002). Moreover, results of our study on the in vitro development of equine oocytes, using eGH in combination with IGF-I to a medium under serum-free conditions, suggested that nuclear and cytoplasmic maturation is necessary as a prerequisite in order for oocyte competence to occur. Further investigation is required to determine whether factor(s) in serum could potentially enhance the steroidogenic effect of GH and IGF-I in culture medium.
The insulin growth factors are expressed ubiquitously and regulate cell growth, differentiation and maintenance of differentiated cell function (Gluckman, Reference Gluckman1986; Waters & Kaye, Reference Waters and Kaye2002). Insulin-like growth factor-I has been reported to stimulate bovine oocyte maturation and fertilization in vitro (Herrler et al., Reference Herrler, Lucas-Hahn and Niemann1992), and to promote rabbit blastocyst development (Herrler et al., Reference Herrler, Krusche and Beier1998). We observed a decrease in the overall number of oocytes that reached any stage of development when oocytes were treated with IGF-I. However, only a low number of oocytes was incubated when IGF-I alone was added into our IVM system and this might impair the data observed in our experiment. In contrast, Carneiro et al. (Reference Carneiro, Lorenzo, Pimentel, Pegoraro, Bertolini, Ball, Anderson and Liu2001) observed an increase of nuclear maturation of 60% in oocytes incubated in the presence of 200 ng/ml of IGF-I. Our interpretation was that eGH has an effect on equine oocyte development, and this effect might be accentuated by the addition of IGF-I into the IVM system. The use of GH has been demonstrated to improve nuclear maturation of bovine oocytes, to stimulate subsequent embryonic development, and to reduce apoptosis during early embryo development in vitro (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1996, Reference Izadyar, Van Tol, Hage and Bevers2000; Moreira et al., Reference Moreira, Paula-Lopes, Hansen, Badinga and Thatcher2002).
To our knowledge, this study is the first to evaluate the effect of eGH and its interaction with IGF-I on maturation of equine oocytes cultured in vitro and using CG release as an indicator of cytoplasmic maturation. In the present study, 35.5% of equine oocytes matured in vitro achieved cytoplasmic maturity based on CG migration to the oocyte cortex, which was observed in an area just beneath the oolemma after 30 h of maturation. We infer that during oocyte maturation, not only is nuclear maturation important but cytoplasmic maturation is also required to achieve developmental competence.
The CG distribution has been used as a reliable indicator for the cytoplasmic maturation of oocytes in horses (Carneiro et al., Reference Carneiro, Liu, Hyde, Anderson, Lorenzo and Ball2002), calves (Damiani et al., Reference Damiani, Fissore, Cibelli, Long, Balise, Robl and Duby1996), porcine (Wang et al., Reference Wang, Sun, Hosoe, Shioya and Day1997), and mouse (Liu et al., Reference Liu, Sims, Calarco and Talbot2003), whereas cortical migration is indicated when the CG density decreases significantly in the central area compared with the periphery as maturation proceeds. In this sense, we also observed that the cytoplasmic pattern during oocyte maturation becomes more evident when we used eGH in combination with IGF-I after 30 h of IVM. This positive effect of eGH on oocyte maturation, whether exerted directly on oocyte or indirectly through cumulus cells, has yet to be determined in equine species. It may be that the lack of optimal development of horse oocytes in vitro could be attributable to impaired cytoplasmatic maturation. Former studies in our laboratory have suggested that eGH has an effect on the nuclear and cytoplasmic maturation to achieve complete maturation competence (Pereira et al., Reference Pereira, Lorenzo, Carneiro, Bilodeau-Goeseels, Kastelic, Pegoraro, Pimentel, Esteller-Vico, Illera, Silvan, Casey and Liu2006).
In the horse, Marchal et al. (Reference Marchal, Caillaud, Martoriati, Gerard, Mermillod and Goudet2003) demonstrated a significant increase on nuclear maturation of oocytes that reached metaphase II (43%) when eGH and equine luteinizing hormone (eLH) were used in vitro to promote equine oocyte development. We observed that 65.3% of the oocytes reached meiotic resumption when eGH was added into our IVM system. However, we observed a higher maturation rate when eGH was added into the maturation medium with IGF-I (73.3%), which suggested that these oocytes could probably lead to meiotic resumption to achieve oocyte competence in vitro. This relationship between GH and IGF-I is explained by the somatomedin hypothesis in which the signals initiated by the binding of GH to its cell-surface receptor are rapidly propagated to the nucleus to stimulate transcription of the IGF-I gene (Rotwein et al., Reference Rotwein, Thomas, Gronowski, Bichell, Kikuchi, Baxter, Gluckman and Rosenfeld1994; Sirard, Reference Sirard2001). The effect of eGH and its potential mediation by IGF-I in equine oocytes matured in vitro is not well defined.
The source and the amount of GH used in the current study was different from that used in bovine (rbGH, 100 ng/ml), in ovine (roGH, 300 ng/ml) and in porcine (pGH, 500 ng/ml) species (Izadyar et al., Reference Izadyar, Colenbrander and Bevers1996; Marchal et al., Reference Marchal, Caillaud, Martoriati, Gerard, Mermillod and Goudet2003; Shirazi et al., Reference Shirazi, Shams-Esfandabadi, Ahmadi and Heidari2008). Based on our preliminary dose-dependent study, that compared 50, 100, 200, 400 and 800 ng/ml of eGH (unpublished data), 400 ng/ml was the optimal dose to be used in our IVM system. Research by Izadyar et al. (Reference Izadyar, Hage, Colenbrander and Bevers1998) showed high rate of bovine oocytes that reached MII and embryos that developed to the blastocyst stage seems to be significant with the addition of rbGH at 100 ng/ml in vitro (Izadyar et al., Reference Izadyar, Hage, Colenbrander and Bevers1998). Shirazi et al. (Reference Shirazi, Shams-Esfandabadi, Ahmadi and Heidari2008) obtained a positive effect on nuclear maturation when sheep oocytes were matured in the presence of roGH and 0.05 IU/ml of follicle-stimulating hormone (FSH) (88.9%) for 24 h. The same study obtained a signficant increase in blastocyst development in serum-containing medium of roGH (73.4%), FSH (75.7%), and roGH + FSH (73.2%) treated groups when compared with control (42.3%) (Shirazi et al., Reference Shirazi, Shams-Esfandabadi, Ahmadi and Heidari2008). Marchal et al. (Reference Marchal, Caillaud, Martoriati, Gerard, Mermillod and Goudet2003) showed that the addition of pGH in the presence of epidermal growth factor and FSH in the maturation medium had a positive effect on nuclear maturation (89% vs. 78% control); however, no effect on porcine fertilization and embryo development was observed. We have shown in this study that the addition of eGH at 400 ng/ml to IVM medium improves the development of immature equine oocytes to MII (35.1%) when compared with IGF-I (19.4%) cultured oocytes.
The results from this study demonstrate that a significant number of equine oocytes resumes meiosis in the presence of eGH and IGF-I into the IVM system after 30 h of culture, compared with control. These results may provide a physiological and biological insight into the maturation and development of horse oocytes when grown artificially. Further research is warranted to investigate the mechanisms by which the role of GH and its relationship with IGF-I could improve the production of high-quality oocytes.
Acknowledgements
This research was supported by the Del Amo Grant collaborative programme from the University Complutense of Madrid and the University of California Davis. The authors thank Paul Panich and Randy Wilde for technical assistance with retrieving ovaries and Frank Ventimiglia for his assistance with confocal microscopy.