Innovative medical devices offer solutions to medical problems, but can also reduce of the length of hospital stay or improve the quality and safety of care. The International Information Network on New and Emerging Health Technologies (EuroScan International Network) provides the following definition of innovative medical devices: “emerging technologies are technologies that are not yet adopted by the health care system. [. . .] Medical devices will be prior to marketing, or within 6 months of marketing, or marketed but less than 10 percent diffused or localized to a few centers”. Nevertheless, emerging technologies are one of the major cost drivers of healthcare expenditure worldwide. In this context, many countries have implemented formal policies promoting the development of health technology assessment (HTA). The purpose of HTA is to support the decision-making process in healthcare policy, by providing high-quality information about the clinical efficacy, cost-effectiveness, and broader impact of drugs, medical devices, and technologies (Reference Drummond, Schwartz and Jönsson1). HTA is designed for use at a macroscopic level, by national or regional decision makers. Hospital managers are faced with similar issues when they decide whether to introduce a particular new device into a specific clinical department. In this context, the European Network for HTA (EUnetHTA) has developed a toolkit facilitating the adaptation of HTA reports (Reference Chase, Rosten, Turner, Hicks and Milne2). However, hospital managers do not have the time and resources necessary for large HTA projects; instead, they require more locally focused HTA, at the hospital level. Hospital-based HTA is thus relevant for university or teaching hospitals considering the introduction of innovative technologies.
In 2008, the Hospital-Based Health Technology Assessment Sub-Interest Group of the Health Technology Assessment International (HTAi) Society published a worldwide survey of hospital-based HTA (Reference Cicchetti, Marchetti, Dibidino and Corio3). Between April 2007 and May 2008, a questionnaire was sent to sixty-four members of the Hospital-Based HTA Interest Sub-Group (fifty different healthcare organizations). The overall response rate was 66 percent. This questionnaire was designed so as to identify characteristics common to responders in terms of organizational structures, processes, skills, and outputs. The survey also described applications of HTA methods and tools within hospitals, providing evidence for managerial decision making and to support effective clinical practice. The authors of the survey highlighted two organizational models of hospital-based HTA for managerial decision-making purposes: a mini-HTA model and an HTA unit model. Mini-HTA is a decision-support tool featuring various questions exploring the prerequisites for and the consequences of using a specific health technology. Mini-HTAs and similar tools were initially developed in Danish hospitals and have since been designed and adapted by other organizations worldwide (Reference Ehlers, Vestergaard and Kidholm4,5). By contrast, HTA units are formal organizational structuresemploying HTA specialists on a full-time basis (Reference Gagnon, Lepage-Savary and Gagnon6,Reference Juzwishin, Olmstead and Menon7). HTA units produce more complete assessment reports, but can also provide mini-HTAs in situations in which a comprehensive HTA is not required. According to the results of the worldwide survey, only thirteen teaching hospitals have an organizational model (nine HTA units, three internal committees, and one mini-HTA), but it seems unlikely that so few teaching hospitals have adopted some kind of model for hospital-based HTA. Indeed, the experiences of many teaching hospitals in hospital-based HTA have been published in recent years.
Moreover, many different healthcare professionals, including clinicians, managers, health economists, epidemiologists, and biomedical engineers, are involved in hospital-based HTA. Hospital pharmacists are not included in the list of key professional profiles of the Hospital-Based HTAWorldwide Survey. However, hospital pharmacists are the chief professionals responsible for the purchase, management, and dispensing of sterile medical devices in many European hospitals, including university hospitals (8). Hospital pharmacists already manage implantable and critical devices, for which innovation is frequent. Thus, hospital-based HTA is clearly an area in which hospital pharmacists can make a useful contribution, as they already have the skills required.
We review here the hospital-based HTA models for university (or teaching) hospitals reported in previous studies, highlighting the role of hospital pharmacists through international experiences and discussing the contribution that these professionals could potentially make, in terms of their skills and knowledge.
METHODS
We used data from the Hospital-Based Health Technology Assessment Worldwide Survey as the starting point for this study (Reference Cicchetti, Marchetti, Dibidino and Corio3). We then searched for international reports of experiences with hospital-based HTA, focusing on two organizational models: mini-HTAs and HTA units. We searched for articles, letters, and reports published in English, French, or Italian and included in specialist databases (Health Technology Assessment database, MEDLINE, and EMBASE). The search was conducted in July 2011, with various combinations of terms, including “local health technology assessment”, “hospital-based HTA”, “mini-HTA”, or “HTA unit”. These searches identified 163 articles on 61 organizations performing hospital-based HTA.
The relevant references were screened by two reviewers and were included on the basis of several criteria. For inclusion of the reference concerned, hospital-based HTA had to have been used to support managerial decisions relating to medical devices, and had to be still in use. This choice was made to highlight the potential role of hospital pharmacists in decisions concerning these medical products. We then obtained data complementary to those of the Hospital-Based HTA Worldwide Survey, by focusing specifically on university and teaching hospitals. According to the results of the worldwide survey, hospital-based HTA is usually performed in teaching hospitals (39.4 percent) (Reference Cicchetti, Marchetti, Dibidino and Corio3). We, therefore, excluded governmental and private organizations. We ensured that adequate assessments could be made for the organizations identified, by including only those mentioned in peer-reviewed journals for which enough material was available for analysis. The data available had to provide enough information to determine how the process of hospital-based HTA was conducted and by whom it was carried out. Additional information was obtained from, organization Web sites, congress papers, and, more generally, “gray” literature in English, French, and Italian. On the basis of these inclusion criteria, we included forty-five articles in which twenty-two university (or teaching) hospitals worldwide were identified. From the hospital-based HTA models thus identified, we identified those in which hospital pharmacists were involved. We then explored the role of hospital pharmacists in the various models, based on both our own and international experience.
RESULTS
We included twenty-two university hospitals worldwide with a hospital-based HTA system for managerial decision making in this analysis (Table 1). The following countries were represented: Canada, Denmark, France, Italy, Spain, Sweden, Switzerland, the Netherlands, and the United States. The available data concerning organizational models showed that 21 (95 percent) of these hospitals had a dedicated HTA unit and nine (41 percent) used mini-HTAs (or similar decision-support tools). Seventeen of the twenty-one HTA units (81 percent) were interdisciplinary groups within hospitals including professionals engaged full- or part-time in this activity. We also identified four (19 percent) regional HTA units working with one or several university hospitals. Most of the regional units were not located within university hospital premises, and they produced more comprehensive HTAs. For university hospitals with a mini-HTA model (or a similar model), assessments are carried out by one or a few individuals according to a decision-support tool (form or checklist).
Table 1. Details of 22 University Hospitals Worldwide Performing Hospital-Based HTA for Innovative Medical Devices

aThe sources mentioned above were used as starting point for further investigations.
bHTA unit & Mini-HTA like model.
cHTA unit model only.
dMini-HTA model only.
HTA, health technology assessment.
Nine of the twenty-two organizations (41 percent) had at least one pharmacist among the staff performing hospital-based HTA activities. Only five models in which hospital pharmacists were clearly involved provided enough data to understand and describe their role in the process.
Experiences of Hospital-Based HTA with Hospital Pharmacists
As stated above, few studies have described the role of hospital pharmacists in local HTA, particularly in the assessment of medical devices. Four HTA unit models were identified (two regional units and two units within university hospitals), to which we added our own exclusive mini-HTA model.
One of the regional HTA units consisted almost entirely of hospital pharmacists (Reference Filippi, Berto, Fantelli, Fratucello, Scroccaro and Marini9). Consultants from various medical specialities and health economics experts seemed to make regular contributions to assessments, but were not among the permanent staff members. The unit was initially set up to assess drug efficacy, but its function was subsequently extended to include the assessment of innovative medical devices. This illustrates the feasibility of the experience of hospital pharmacists in of the assessment of scientific evidence relating to drugs being transferred to the assessment of medical devices (Reference Scroccaro10). The hospital pharmacists do not use a specific methodology for the collection and analysis of scientific evidence. Instead, literature reviews are conducted, on the basis of medical databases (Cochrane, Medline, Embase, etc.) and HTA reports from elsewhere in the world (INAHTA, NICE, AHRQ, etc.). The budget impact of new devices must be considered for innovative and costly devices not covered by DRG (Diagnosis-Related Group) funding. The staff of the unit assess this impact by calculating the difference between the costs of current practice and those of the new procedure. The available data suggest that hospital pharmacists play an important role in promoting the HTA culture, by publishing information bulletins, reports, and decisions on the Internet. In this regional unit, the diffusion of hospital-based HTA reports within the organization seems to be essential, making professionals aware of the process and promoting a culture of cost-consciousness.
The other regional HTA unit consisted mostly of physicians, but included one hospital pharmacist (Reference Bodeau-Livinec, Simon, Montagnier-Petrissans, Joël and Féry-Lemonnier11). We interviewed this individual, to obtain more information about the role of the hospital pharmacist in this unit. She works in a university hospital and regularly acts as a consultant, providing expertise relating to medical devices and the medical device market. She also has skills in health economics and is heavily involved in assessment of the financial impact of new devices. In 2006, a study showed that the recommendations of this regional unit had a strong impact on hospital decisions (Reference Bodeau-Livinec, Simon, Montagnier-Petrissans, Joël and Féry-Lemonnier11). Decision makers are seeking objective assessments and data concerning the financial burden of the innovative device. The role of the hospital pharmacist in this multidisciplinary team is clearly to provide information reflecting local priorities. This local knowledge is a major asset in the successful implementation of new medical devices in hospitals. Thus, the hospital pharmacist, by considering the practical details of new device implementation, helps to minimize the risk of failure.
Both the local units included hospital pharmacists as permanent staff members (Reference Castoro12,Reference Favaretti, Cicchetti, Guarrera, Marchetti and Ricciardi13). They contribute by providing exepertise concerning medical devices and by collecting requests from physicians. In European hospitals, physicians often initially approach hospital pharmacists when they wish to obtain new medical devices. Thus, hospital pharmacists can provide feedback to HTA units, improving the definition of local needs. The data suggest that hospital pharmacists interact closely with other health professionals, playing a key role in these processes. In terms of organization, the hospital pharmacist is a key go-between who interacts with all the stakeholders, not only physicians and hospital managers, but also biomedical engineers. For example, some innovative medical devices require workstations for which technical details can be obtained only from biomedical engineers. As stated above, in many European hospitals, sterile medical devices are purchased and managed by hospital pharmacists. Thus, hospital pharmacists need to discuss matters with biomedical engineers, to ensure that they purchase appropriate devices. This ability to interact with other disciplines is a major asset in hospital-based HTA activities.
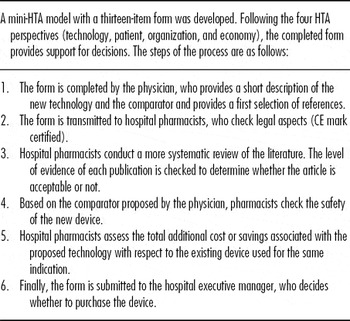
Finally, our own mini-HTA model relies principally on hospital pharmacists (Table 2). In this process, physicians initiate the request by completing a thirteen-item form and hospital pharmacists carry out a systematic literature review to evaluate the device concerned. Based on their knowledge of the medical device market, hospital pharmacists can also propose equivalent devices. This process has been established through close collaboration between the hospital pharmacy and hospital management. In terms of quality, hospital pharmacists must ensure that the applicants complete the forms correctly and that all the legal and safety requirements are satisfied for purchase of the device. Quality control is based principally on the accuracy of clinical evidence based on international standards, such as the Consolidated Standards of Reporting Trials (CONSORT) Statement extension for non-pharmacologic treatment. Finally, hospital pharmacists must correctly collect, summarize, and analyze all the relevant information, to produce a comprehensive report for decision makers.
Table 2. Mini-HTA for Innovative Medical Devices at the European Georges Pompidou Hospital

DISCUSSION
University (or teaching) hospitals are faced with increasing demands for innovative medical devices to improve the efficiency and quality of healthcare. Several models for hospital-based HTA have emerged worldwide, to provide decision makers with information about the possible impact of new technologies. In 2008, Cicchetti, et al. (Reference Cicchetti, Marchetti, Dibidino and Corio3) reported that thirteen teaching hospitals had adopted hospital-based HTA to manage decision making. We show here that there are now many more university hospitals performing local HTA around the world (twenty-two university hospitals identified). The difference between our findings and those of the 2008 survey may reflect some of the limitations mentioned by Cicchetti, et al. (Reference Cicchetti, Marchetti, Dibidino and Corio3). Indeed, the questionnaire used by Cicchetti, et al. was sent only to members of the Hospital-based HTA Interest Sub-Group, who may not have been entirely representative of those involved in hospital-based HTA worldwide. In addition, new hospital-based HTA systems may have emerged since 2008. The present study does not pretend to be exhaustive, but it adds to the overall picture generated by the Hospital-Based Health Technology Assessment Worldwide Survey. We have almost certainly not included all the available international experiences of hospital-based HTA in university hospitals, and further investigation is required to identify the remaining systems. Furthermore, we focused on only two organizational models of hospital-based HTA, because we wanted to explore support for managerial decision making. Two other organizational models—the ambassador and internal committee models—have been defined by the Hospital-Based Health Technology Assessment Sub-Interest Group, but these models focus to a large extent on clinical practice. Furthermore, these models seem to be less widespread in university hospitals, according to the survey.
Our analysis of international experiences and our own experience with HTA demonstrate that hospital pharmacists already contribute to hospital-based HTA activities. Within HTA units, they can extend the interdisciplinary approach, promoting hospital-based HTA and providing feedback about local needs. In exclusive mini-HTA models, they can take responsibility for the organization of HTA and for quality assurance for this process. However, if they are to fulfill this role, hospital pharmacists must have developed skills in reading and analyzing medical literature, and amassed knowledge concerning medical devices. Furthermore, the hospital pharmacists identified in our review also had training in pharmacoeconomics and they applied this expertise to their evaluations of medical devices. Nevertheless, it should be borne in mind that these statements were developed on the basis of only a small number of experiences.
We were surprised to find only a few examples of hospital-based HTA involving hospital pharmacists. Indeed, publications about hospital-based HTA deal more with the results of HTA than with the process or actors involved. In addition, the available data remain limited, because hospital-based HTA is relatively new. We believe that the published data may not necessarily reflect the reality on the ground, and it seems likely that hospital pharmacists play a more important role. One of the other two organizational models, the internal committee model, involves hospital pharmacists and could be investigated in future studies.
We limited our study to university hospitals, because our own model was established in a university hospital and we wanted to compare it with others in a similar context. The teams responsible for most of the twenty-two hospital-based HTA systems, consisted of physicians and surgeons. Many studies have demonstrated the benefits of integrating hospital pharmacists into clinical departments, but hospital pharmacists are not experts in all fields of medicine (Reference Horn and Jacobi14). Some innovative medical devices are used in very specific medical applications requiring detailed knowledge of the medical speciality. Due to this lack of expertise, hospital pharmacists cannot always provide a critical view of requests from physicians, on whom they may be too reliant for information.
Acquired experience in pharmacoeconomics may gradually be transferred to medical devices, but hospital pharmacists often lack knowledge and experience in health economics (Reference Scroccaro10). In a multidisciplinary HTA unit, deficiencies may be compensated by specialists in these fields, but this raises questions about the efficacy of models based essentially on hospital pharmacists. Furthermore, hospital pharmacists have other responsibilities and cannot be assigned full-time to hospital-based HTA activities. They are, therefore, no substitute for a dedicated HTA unit. The pros and cons of hospital pharmacist involvement in hospital-based HTA are summarized in Table 3.
Table 3. Pros and Cons of the Involvement of Hospital Pharmacists in Hospital-Based HTA

HTA, health technology assessment.
Finally, given the acknowledged expertise of hospital pharmacists, we suggest three possible roles that these professionals could fill in HTA. First, the importance of involving patients in local HTA has been highlighted in a recent publication (Reference Gagnon, Lepage-Savary and Gagnon6) that has suggested that comprehensive coaching is required to guide patients or their representatives through the HTA process. A member of the HTA team could provide accessible information to patients and support them. This role could be fulfilled by the hospital pharmacist. Indeed, several studies have shown that hospital pharmacists have close enough interactions with both clinical staff and patients to understand their needs, but are sufficiently detached to be objective (Reference Horn and Jacobi14,Reference Bremberg, Hising, Nylén, Ehrsson and Eksborg15). The ability of hospital pharmacists to advise patients has been clearly demonstrated in previous studies and could be applied to the context of local HTA (Reference Chisholm-Burns, Kim Lee and Spivey16).
Combination products are becoming increasingly available and are of growing importance in health care. According to the FDA definition, combination products are therapeutic and diagnostic products that combine drugs, devices, and/or biological products. For example, drug-eluting stents or catheters with antimicrobial coatings may be considered combination products. HTA stakeholders must develop “dual expertise” if they are to deal with these new products. Due to their training, hospital pharmacists are drug experts, but they are developing skills and knowledge concerning medical devices (Reference Bremberg, Hising, Nylén, Ehrsson and Eksborg15).
The demands for evidence-based information must originate from the hospital management if they are to have a significant impact on decision making (Reference Bodeau-Livinec, Simon, Montagnier-Petrissans, Joël and Féry-Lemonnier11,Reference Gallego, Fowler and van Gool17). Hospital managers must identify staff members who will support the process and ensure its quality. The World Health Organization has recently introduced the concept of “HTA focal points” (18). These “HTA focal points” are individuals who could develop HTA capacity within a designated framework and help decision makers to disseminate HTA information. We think that hospital pharmacists could take on this responsibility and serve as HTA mobilizers within university hospitals.
CONCLUSION
This is the first study, to our knowledge, to investigate the role of hospital pharmacists in the context of hospital-based HTA. We are convinced that hospital pharmacists could make a major contribution to the development of local HTA in university hospitals. They already promote safety, efficacy, and cost-effectiveness for medicines and could extend these functions to medical devices. Nevertheless, hospital pharmacists would need to attend courses and training programs on HTA and health economics, to be effective. As stated above, they could act as “HTA focal points,” developing and supporting hospital-based HTA activities.
CONTACT INFORMATION
Dr Nicolas Martelli, PharmD (nicolas.martelli@egp.aphp.fr), Pharmacy, Hôpital Européen Georges Pompidou, Assistance Publique-Hôpitaux de Paris, Paris, France
Dr Anne-Sophie Lelong, PharmD; Pr Patrice Prognon, PhD; Dr Judith Pineau, PharmD, Pharmacy, Hôpital Européen Georges Pompidou, Assistance Publique-Hôpitaux de Paris, Paris, France
CONFLICTS OF INTEREST
The authors report they have no potential conflicts of interest.