Introduction
Sluzhenikinite, Pd15(Sb7–xSnx) with 3 ≤ x ≤ 4, is a new platinum-group mineral (PGM) discovered in the pegmatitic galena–chalcopyrite massive ore from the Oktyabrsk mine (schaft No 1), Oktyabrsk deposit of the Noril`sk deposits, Russia (coordinates: 69°32′10″N, 88°27′17″E). Pegmatoidal schlieren (with up 30 vol.% of galena) occur in the upper-contact of Cu-rich orebodies of the Oktyabrsk mine and in the southern orebody in the eastern part of the Komsomolsky mine (Sluzhenikin and Mohkov, Reference Sluzhenikin and Mokhov2015). Galena forms symplectitic intergrowths with chalcopyrite; pentlandite, cubanite and fine-grained pyrrhotite are less abundant. The schlieren vary from centimetres to a few decimetres in size and have sharp boundaries with the host ore. Their shape is variable from irregular to spherical. This type of ore is rich in rare minerals such as argentopentlandite, parkerite, Pb–Tl-rich djerfisherite, thalfenisite, thalcusite, stannite, cassiterite, tellurobismuthite and breithauptite. Sluzhenikinite forms intergrowths with Sb-sobolevskite, Bi–Te geversite, Te-insizwaite, maslovite, stannopalladinite, paolovite, Sb-paolovite, altaite, hessite, native Ag and Ag–Au alloy. The size of aggregates of platinum-group minerals reach up to 4 cm in size. These types of ores are formed from residual sulfide solutions, rich in copper and volatile components (Sluzhenikin and Mohkov, Reference Sluzhenikin and Mokhov2015).
A preliminary description of a mineral having a similar composition to sluzhenikinite as an unnamed phase Pd4SnSb was given by Sluzhenikin (Reference Sluzhenikin2011), Sluzhenikin and Mokhov (Reference Sluzhenikin and Mokhov2015) and Spiridonov et al. (Reference Spiridonov, Kulagov, Serova, Kulikova, Korotaeva, Sereda, Tushentsova, Belyakov and Zhukov2015). The unnamed mineral UM1992–32-Sb:PdSn (Grokhovskaya et al., Reference Grokhovskaya, Distler, Klyunin, Zakharov and Laputina1992) listed in Smith and Nickel (Reference Smith and Nickel2007) is most likely to be Sb-rich paolovite.
The new mineral and its name (symbol Szhi) were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (CNMNC IMA) as proposal 2020-089 (Vymazalová et al., Reference Vymazalová, Welch, Laufek, Kozlov, Stanley and Plášil2021). The mineral is named after Sergey Fedorovich Sluzhenikin (b. 1943) (Сергей Фёдорович Служеникин), research geologist at the Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry of the Russian Academy of Sciences, Moscow, Russia, for his contributions to the ore mineralogy and mineral deposits of platinum-group elements, particularly from the area of the type locality, the Noril`sk deposits.
Holotype material (polished section and the single crystal used in the structure determination) is deposited in the mineralogical collection of the Natural History Museum, London, United Kingdom, catalogue number BM 2020,20 and co-type material (polished section) is deposited in the Fersman Mineralogical Museum, Moscow, Russia, under catalogue number 5691/1.
Appearance, physical and optical properties
In polished sections, sluzhenikinite forms euhedral elongate lamellar crystals (100–150 μm long and 10–50 μm wide) associated with with Au–Ag alloy, insizwaite and myrmekitic intergrowths of Pt–Pd minerals (stibiopalladinite, maslovite and sobolevskite), in close association of sperrylite and base-metal sulfides (galena, chalcopyrite, cubanite and pentlandite). Reflected-light and back-scattered-electron images of sluzhenikinite and its associations are shown in Fig. 1 and 2, respectively. Other associated minerals are geversite, paolovite, Sb-paolovite, altaite, hessite, argentopentlandite, bornite, and pyrrhotite.
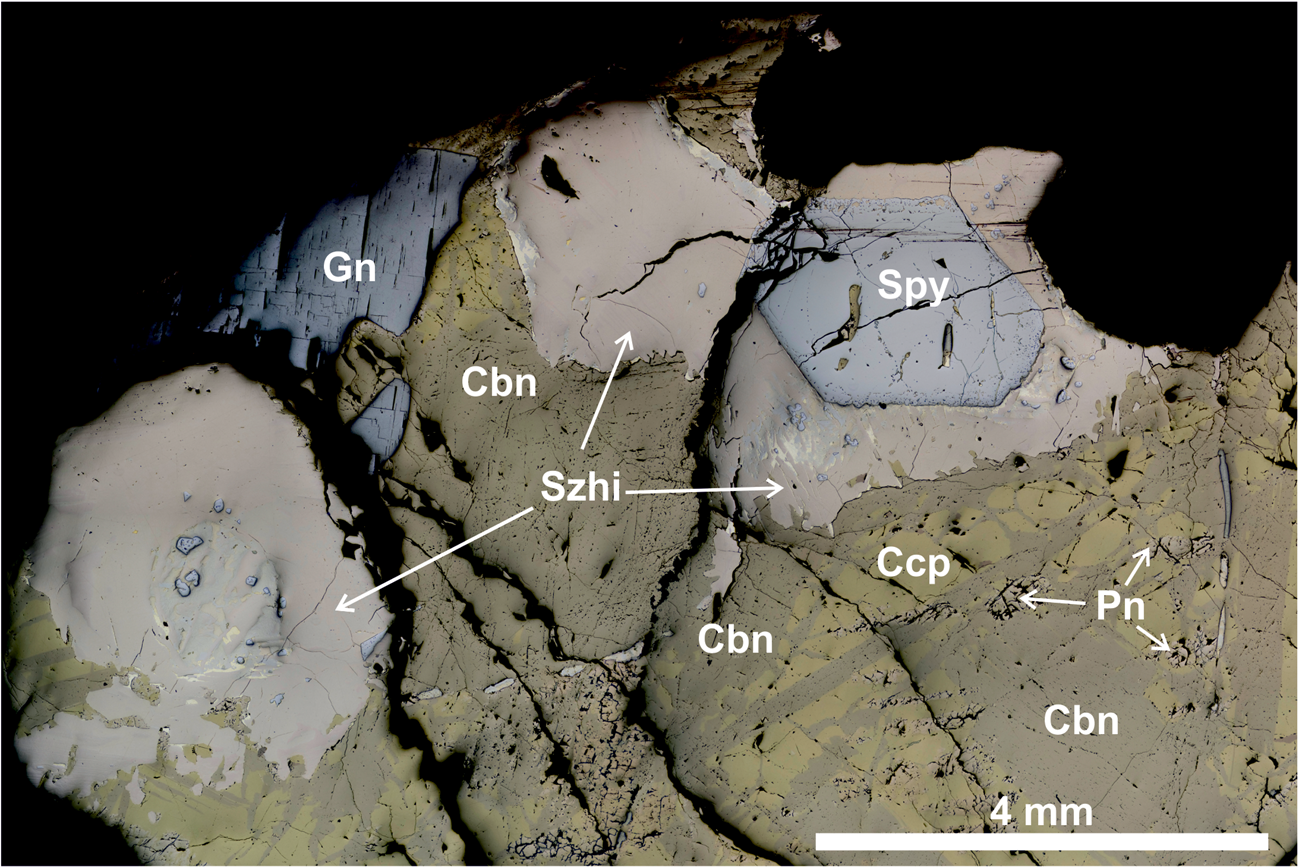
Fig. 1. Reflected-light microphotograph of the sample studied containing sluzhenikinite. Abbreviations: Szhi – sluzhenikinite, Spy – sperrylite, Cbn – cubanite, Gn – galenite and Ccp – chalcopyrite.
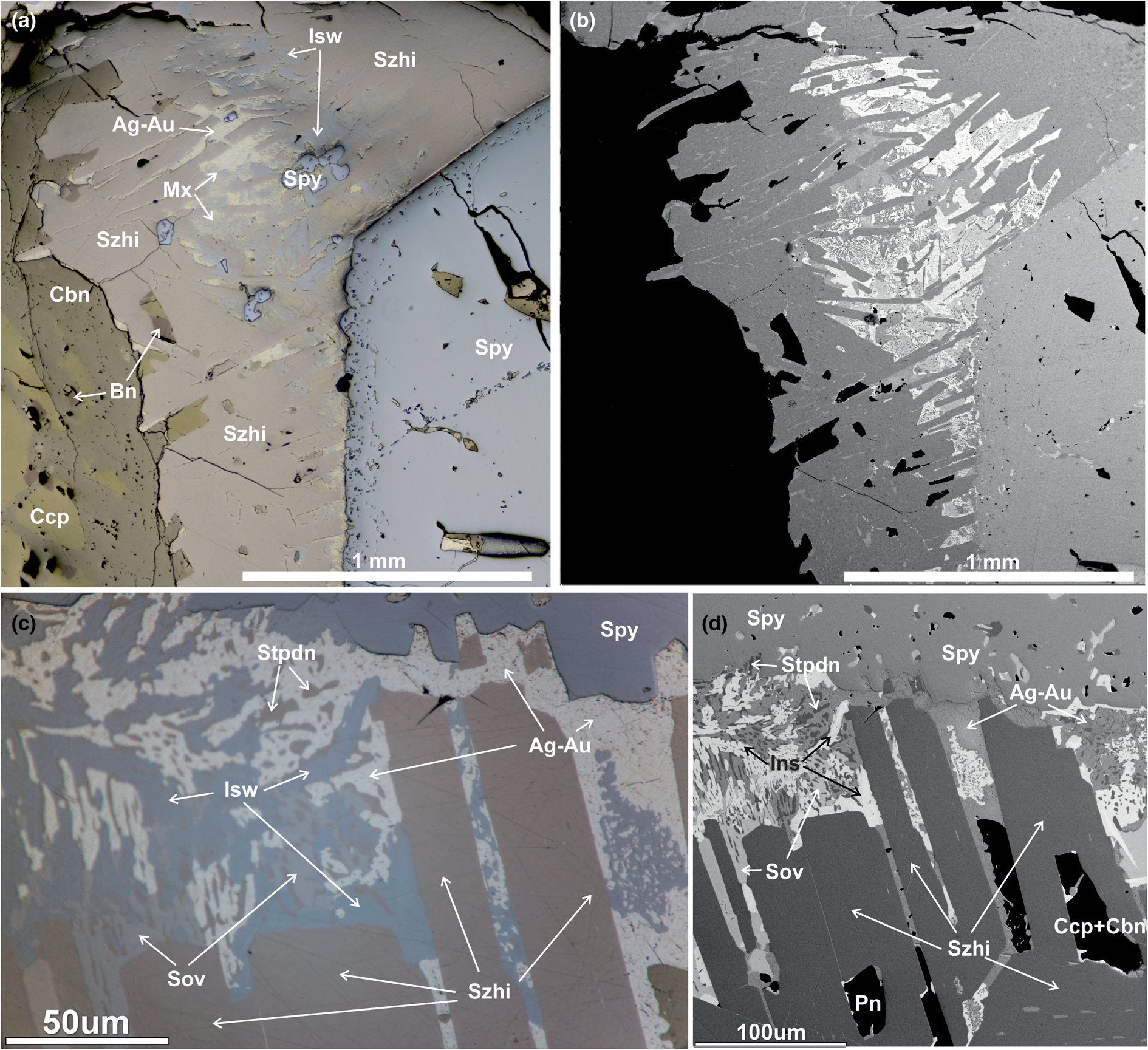
Fig. 2. (a, c) Reflected-light and (b, d) back-scattered electron images showing sluzhenikinite and associated minerals. (c, d) Crystals of sluzhenikinite used for collecting of X-ray data. Abbreviations: Szhi – sluzhenikinite, Isw – insizwaite, Spy – sperrylite, Sov – sobolevskite, Mx – myrmekite mixture of insizwaite, stibiopalladinite and maslovite, Stpdn – stibiopalladinite, Cbn – cubanite, Ccp –chalcopyrite and Bn – bornite.
The density calculated on the basis of the empirical formula and cell dimensions of sluzhenikinite is 11.22 g.cm–3.
In plane-polarised light, sluzhenikinite is pale brown with weak bireflectance, imperceptible pleochroism, and weak anisotropy with straw yellow to deep blue rotation tints; it exhibits no internal reflections. The reflectance measurements were made in air relative to a WTiC standard on sluzhenikinite using a J & M TIDAS diode array spectrometer attached to a Zeiss Axiotron microscope. The results are presented in Table 1 and illustrated in Fig. 3.
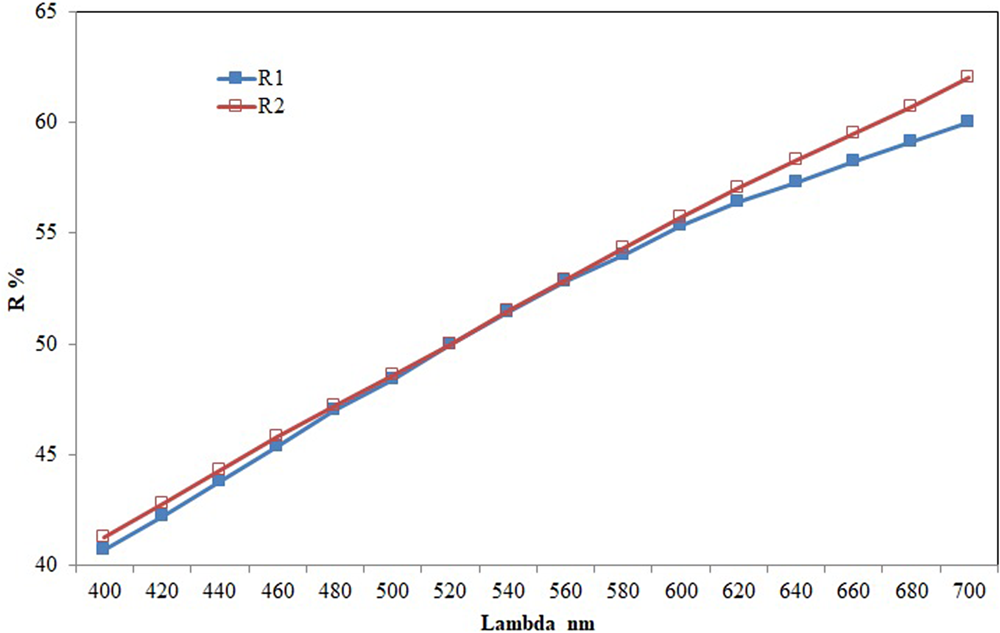
Fig. 3. Reflectance data of sluzhenikinite.
Table 1. Reflectance data for sluzhenikinite.
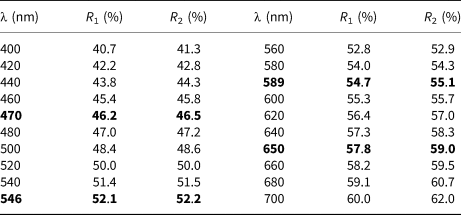
Note: The values required by the Commission on Ore Mineralogy are given in bold.
Chemical composition
Electron microprobe analyses (EMPA) were performed with a CAMECA SX-100 electron probe microanalyser in wavelength-dispersive mode using an electron beam focused to 1–2 μm. Pure elements were used as standards. Concentrations were quantified on PdLα, SbLα and SnMα with an accelerating voltage of 15 kV and a beam current of 10 nA on the Faraday cup. Other elements were below the detection limit. The EMPA data were collected on four different grains of the type material (n = 13). Additional energy dispersive spectroscopy (EDS) analyses (n = 22) were collected on co-type material, using an EDS Aztec Energy X-Max 150 (Oxford Instruments) installed on a SEM Lyra 3GM (Tescan). The following conditions of measurements were used: voltage of 20 kV, a beam current of 0.5 nA with 500,000 counts collected for every spectrum, XPP matrix correction and pure Pd, Sn, Sb and Pt as standards. Chemical analyses for sluzhenikinite are summarised in Table 2.
Table 2. Chemical data (in wt. %) for sluzhenikinite.
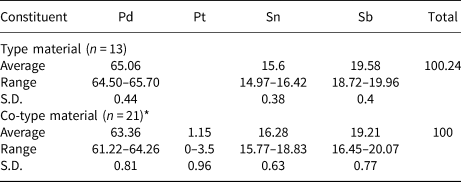
S.D. – standard deviation, *EDS analyses.
The range of compositional variation of sluzhenikinite is shown in Fig. 4. It forms a narrow solid solution along the Pd2Sb–Pd2Sn join indicating substitution of Sb by Sn, while Pd+Pt content remains approximately constant. Hence, the empirical composition of this solid solution can be expressed as Pd15(Sb7–xSnx) with 3 ≤ x ≤ 4, more specifically where 3.06 ≤ x ≤ 3.97. According to our preliminary experimental results in the Pd–Sn–Sb system, there is no complete solid solution between Pd2Sb and Pd2Sn. On the basis of published phase diagrams for Pd–Sb and Pd–Sn binary systems (Massalski et al., Reference Massalski, Okamoto, Subramanian and Kacprzak1990), sluzhenikinite does not have its structural analogue within these binary systems. Our experimental results also show, that both Sn and Sb are essential for sluzhenikinite formation.
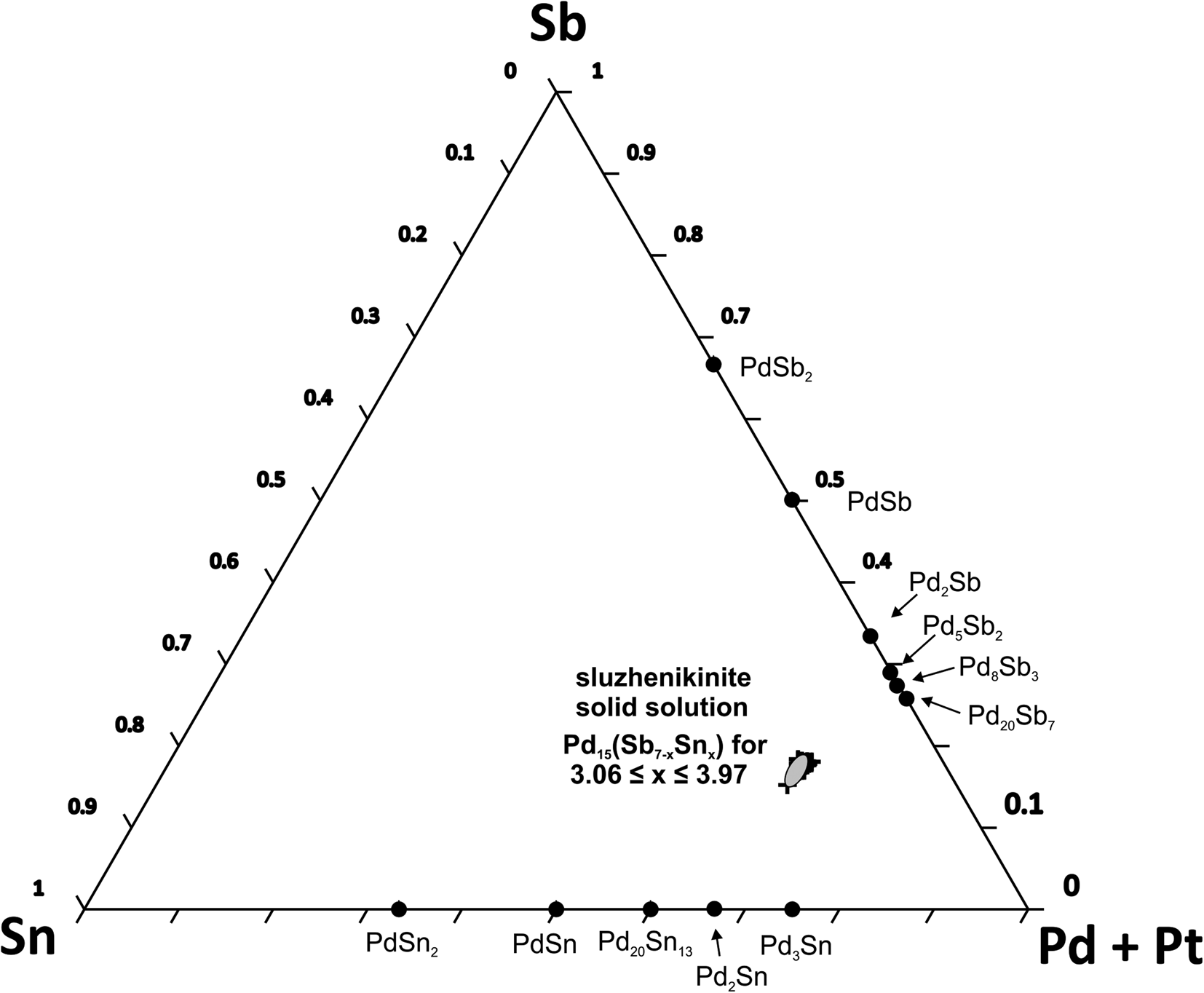
Fig. 4. A ternary diagram of the (Pd+Pt)–Sb–Sn system.
X-ray crystallography
Single-crystal X-ray diffraction
A thin, elongate, lamellar crystal (0.145 mm × 0.025 mm × 0.010 mm) was extracted from a polished mount and attached to a 0.01 mm diameter non-diffracting carbon fibre that was glued to a 0.1 mm glass support rod. Data collection used an XcaliburE four-circle diffractometer equipped with an Eos area detector and graphite-monochromated MoKα radiation operated at 50 kV and 40 mA. Raw reflection intensities were corrected for polarisation and Lorentz effects and converted to structure factors using the program CrysalisPro ® (Rigaku Oxford Diffraction). Structure determination used SHELX (Sheldrick, Reference Sheldrick2008; Reference Sheldrick2015); specifically SHELXL version 2018/3. Neutral atomic-scattering factors taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used.
The assignment of atom sites as Pd or Sn/Sb was based upon refined site scattering values (46 e– versus 50.5 e–) and consideration of nearest interatomic distances. The ranges of plausible Pd–Pd and Pd–Sb distances were obtained from the structures of several related Pb–Sb and Pb–Sn minerals and synthetic compounds. Initially, the structure was determined in orthorhombic space group Cmcm and gave acceptable agreement indices, but a high GoF (1.275). A very high value of the second SHELX reflection-weighting parameter (b = 256) also indicated an inadequacy in the Cmcm model. Furthermore, there were 10 strong violators [I/σ(I) > 7, the strongest has I ≈ 28σ(I)] of the c-glide. Consequently, a structure solution was sought in the most closely related sub-group of Cmcm, namely a monoclinic P21/m structure with half the volume of the orthorhombic unit-cell and related to the latter by the transformation matrix [½½0/00$\bar{1}$![]() /½½$\raise2pt{\bar{ }}$
/½½$\raise2pt{\bar{ }}$![]() 0]. The P21/m model led to an excellent refined structure and much lower value of b = 26. This value is, nonetheless, somewhat high and may reflect an inability to distinguish Sb and Sn over the eight non-equivalent Sb/Sn sites in the structure. We also attempted to refine the structure in a monoclinic C2/m derivative of Cmcm, but this model failed to lead to a stable refinement or to give a plausible structure.
0]. The P21/m model led to an excellent refined structure and much lower value of b = 26. This value is, nonetheless, somewhat high and may reflect an inability to distinguish Sb and Sn over the eight non-equivalent Sb/Sn sites in the structure. We also attempted to refine the structure in a monoclinic C2/m derivative of Cmcm, but this model failed to lead to a stable refinement or to give a plausible structure.
A close hexagonal/orthorhombic/monoclinic metric pseudosymmetry is a common feature of this family of Pd–Sb minerals. As there is a very close corresponding hexagonal metric [a = 7.5662(1) Å and c = 29.2932(6) Å], we also carried out data reduction in hexagonal space groups. However, merging of symmetry-equivalent reflections was very poor. For example, we used the space group of stibiopalladinite Pd5Sb2, P63cm (non-centrosymmetric), which has a cell metric (a = 7.598 Å and c = 28.112 Å) near that of sluzhenikinite and we obtained a very high R int value of 0.44 for merging in point group 6mm. Trigonal and other hexagonal point groups also gave very poor reflection merging, e.g. 6/m, $\bar{3}\ {\rm and}\ \bar{3}$![]() m, had R int = 0.42. E-statistics, which should work well for sluzhenikinite as Pd, Sn and Sb have very similar atomic numbers, indicated that the structure is highly likely (79% probability) to be centrosymmetric. Clearly, the stibiopalladinite space group is not relevant for sluzhenikinite. The poor reflection merging for hexagonal/trigonal point groups also excludes hexagonal/trigonal symmetry. Consequently, after a thorough exploration of possible options, we consider the monoclinic (P21/m) model to be most appropriate for sluzhenikinite.
m, had R int = 0.42. E-statistics, which should work well for sluzhenikinite as Pd, Sn and Sb have very similar atomic numbers, indicated that the structure is highly likely (79% probability) to be centrosymmetric. Clearly, the stibiopalladinite space group is not relevant for sluzhenikinite. The poor reflection merging for hexagonal/trigonal point groups also excludes hexagonal/trigonal symmetry. Consequently, after a thorough exploration of possible options, we consider the monoclinic (P21/m) model to be most appropriate for sluzhenikinite.
All atoms of the monoclinic structure were found by structure solution using direct methods and were initially assigned to Pd. Examination of isotropic displacement factors indicated that some sites had much lower values than others, suggesting occupancy by a more electron-rich element, namely Sn/Sb. Residual electron-density maxima of 3–4 e–/Å3 in difference-Fourier maps were located within 0.4 Å of Pd atoms, confirming the presence of an element of higher atomic number. However, due to the almost identical atomic numbers of Sn (50) and Sb (51), it was not possible to distinguish between these two elements. Consequently, the non-Pd sites were modelled as Sb. Pd17 was modelled as either Pd or Sb. When Pd17 = Sb there is a corresponding electron density minimum of –2 e–/Å3. When Pd17 = Pd there is a corresponding maximum of 3 e–/Å3 at 0.36 Å away from Pd17. The site-scattering value obtained by refining Sb or Pd occupancy is 48 electrons (0.97Sb or 1.04Pd). However, consideration of interatomic distances implies that the occupant of Pd17 is Pd or Pd-dominant. It is conceivable that the minor amount of Pt (Z = 78) found by EMPA is ordered at this site, which would lead to a small increase in site-scattering value. In addition to the +3 e–/Å3 maximum at Pd17, the twenty highest residual electron-density maxima in the difference-Fourier map for the final refinement have values between +1.4 and +2.1 e–/Å3; the most negative electron density difference is –1.9 e–/Å3.
Lastly, given the close metric pseudosymmetry of orthorhombic and monoclinic cells, we anticipated that the crystal could be twinned. Searches for monoclinic/orthorhombic-C and monoclinic/hexagonal twinning for known twin laws using CrysalisPro® (based upon reflection intensities) did not reveal the presence of twins. Furthermore, none of the small residual unmodelled electron-density maxima could be mapped onto a possible twin. The close agreement between values of observed and calculated structure factors for the final refinement does not suggest twinning (F obs >> F calc if missed twinning is present, which is not the case here).
From a crystallographic viewpoint there are two plausible stoichiometries, depending upon how Pd17 is assigned: if Pd17 is Sb then the implied formula is Pd7(Sn,Sb)4; if Pd17 is Pd then the implied formula is Pd15(Sn,Sb)7. The well-defined composition determined by EMPA, Pd14.88(Sn3.20Sb3.92)Σ7.12, leads to the clear choice of Pd15(Sn,Sb)7 as the correct ideal formula, implying that that Pd17 is Pd. Further topological support for the identification of Pd17 as Pd is discussed below. Information summarising the details of the crystal, data collection and structure refinement are given in Table 3. Atom coordinates and equivalent-isotropic displacement parameters are given in Table 4. U ij and interatomic distances can be found in the accompanying crystallography information file (deposited with the Principal Editors of Mineralogical Magazine and available as Supplementary material – see below). Calculated d spacings and intensities for powder X-ray diffraction, calculated using PowderCell 2.3 (Krause and Nolze, Reference Massalski, Okamoto, Subramanian and Kacprzak1996; Nolze, Reference Nolze2017), are shown in Table 5.
Table 3. Information relating to the data collection and structure refinement of sluzhenikinite.
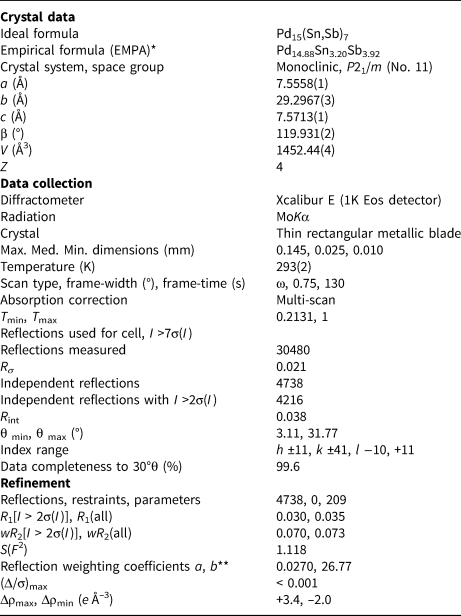
* Average of 13 analyses by electron microprobe (WDS) calculated to 22 atoms per formula.
** SHELX
Table 4. Atom coordinates and equivalent-isotropic displacement parameters (Å2) for sluzhenikinite (in reality Sb positions represent Sb/Sn mixed positions).
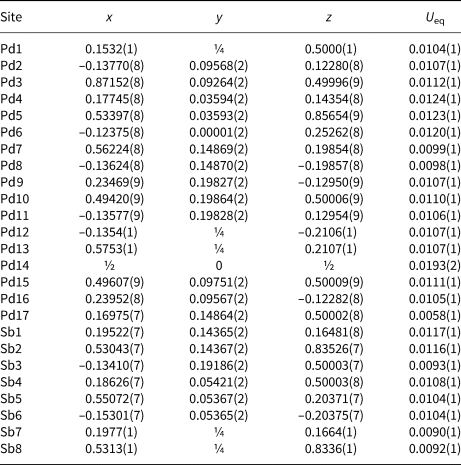
Table 5. Calculated powder X-ray diffraction values for sluzhenikinite (d, Å, I, %)*.
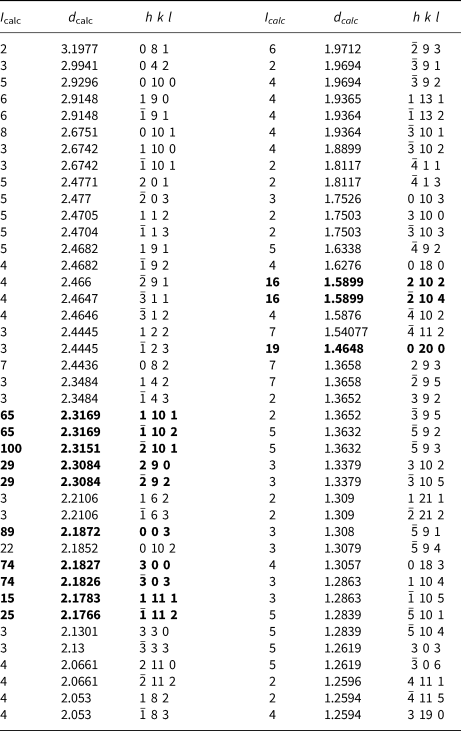
* Calculated using PowderCell 2.3 (Krause and Nolze, Reference Kraus and Nolze1996; Nolze, Reference Nolze2017). The strongest bands are given in bold.
Structure description
There are seventeen non-equivalent Pd sites and eight non-equivalent (Sb,Sn) sites in the crystal structure. Nineteen of these sites are on general positions and have a multiplicity of four; six sites (Pd1, Pd12, Pd13, Pd14, Sb7 and Sb8) lie within mirror planes and have a multiplicity of two.
The structure of sluzhenikinite is shown in Figs 5, 6 and 7. It is composed of three distinct lamellar components: slab A = Pd7(Sn,Sb)6; slab B = Pd10(Sn,Sb)3; and sheet C = Pd3(Sn,Sb)2.
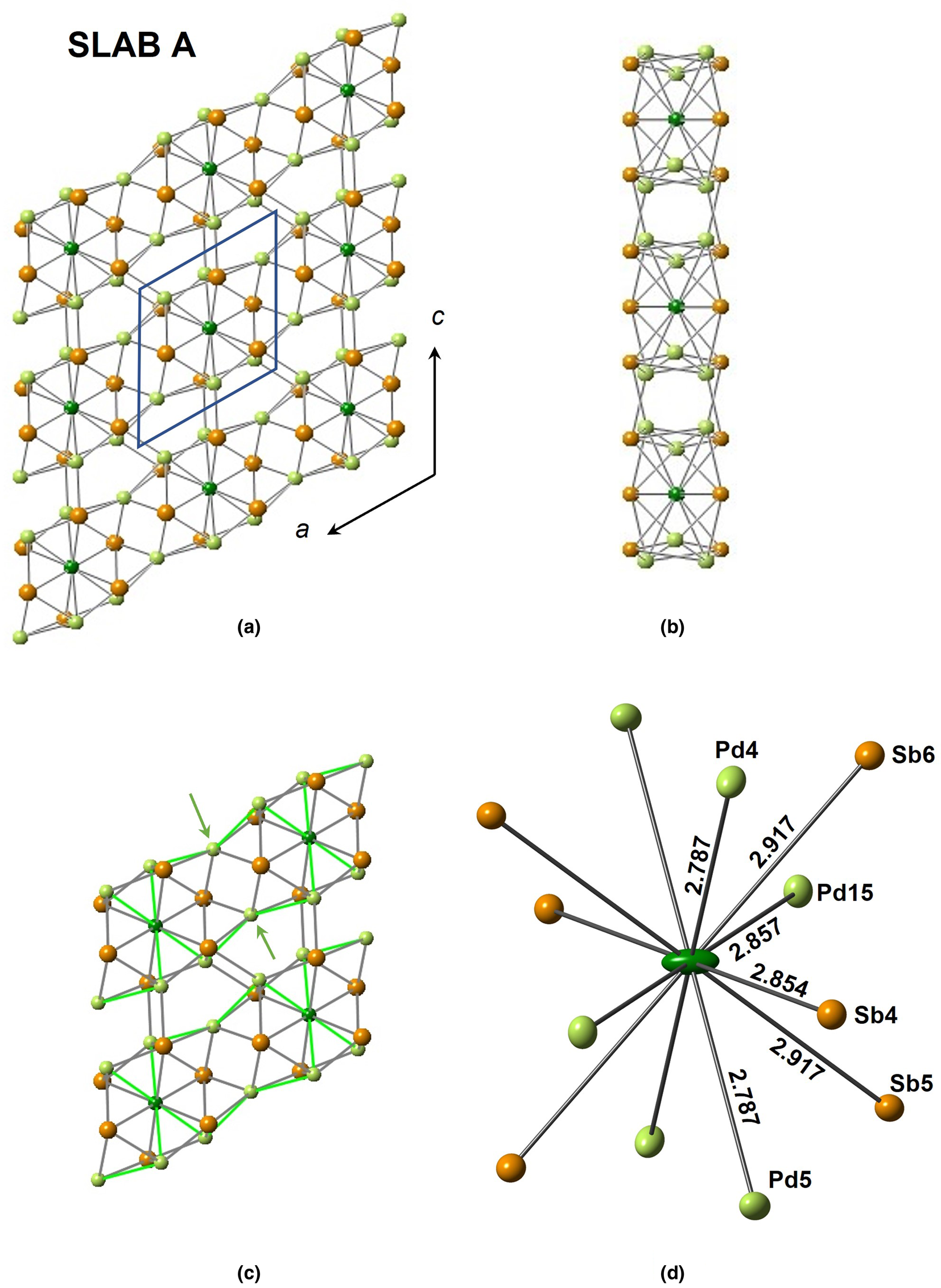
Fig. 5. The three different structural modules of sluzhenikinite. (a) Slab A projected onto (010). Rows of Pd14[Pd4Sb6] polyhedra extend parallel to [100]. (b) A view of the slab looking along the a-axis and showing tunnels extending parallel to [100]. (c) Close-up of the linkages between polyhedra involving pairs of Pd atoms (arrowed) within a row and Pd–(Sn,Sb) bonds between rows. (d) A Pd14[Pd6Sb6] polyhedron. Atom displacement ellipsoids are shown at the 68% level.
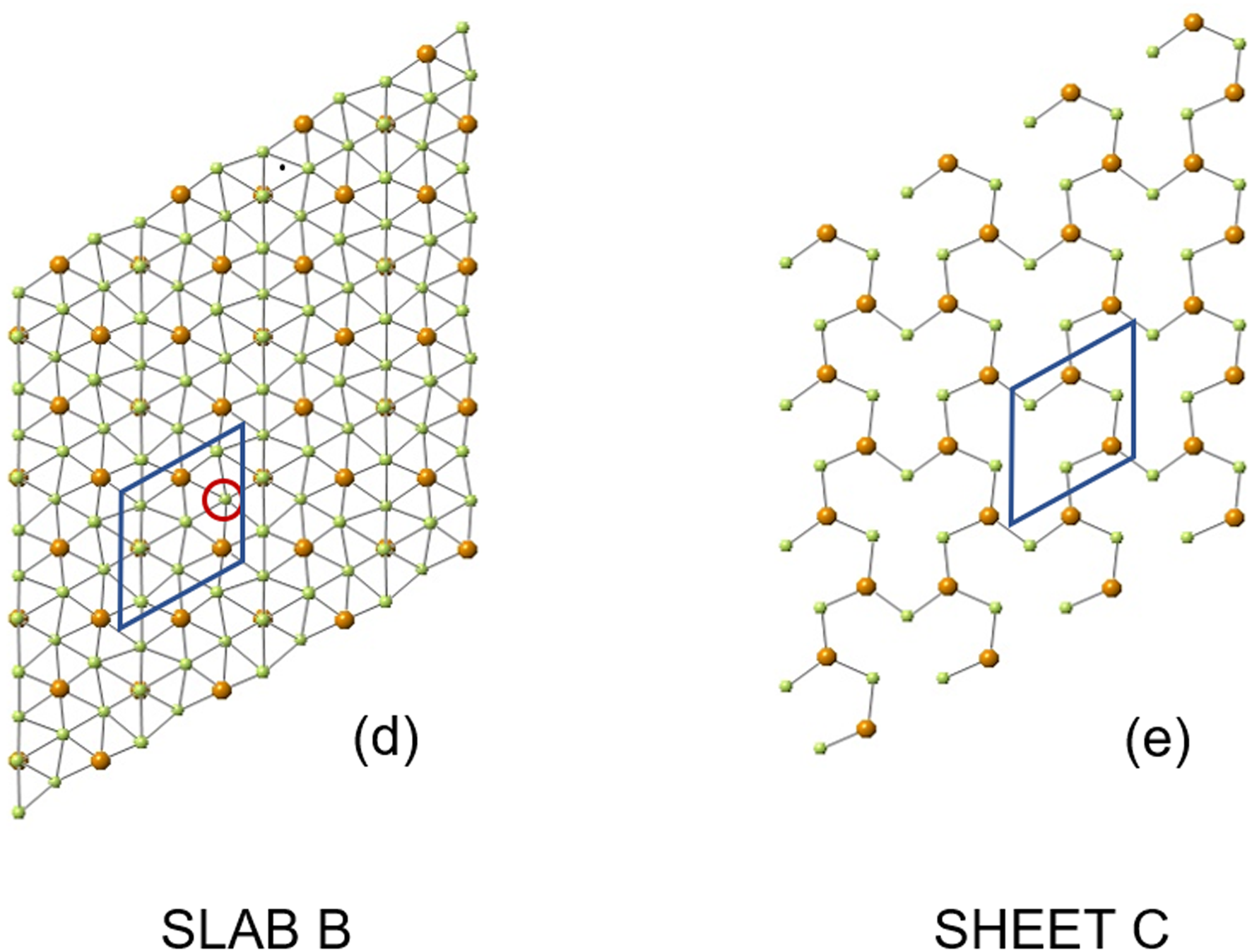
Fig. 6. (a) Slab B composed of a puckered sheet of (Sn,Sb) atoms defining a centred-pseudohexagonal motif and bonded to Pd atoms, (Sn,Sb) atoms are bonded to nine Pd atoms; Pd atoms are bonded to six other Pd atoms and three (Sn,Sb) atoms. (b) Sheet C comprising chains of alternating Pd and (Sn,Sb) atoms extending parallel to [001] and linked by rows of Pd atoms. See text for further details.
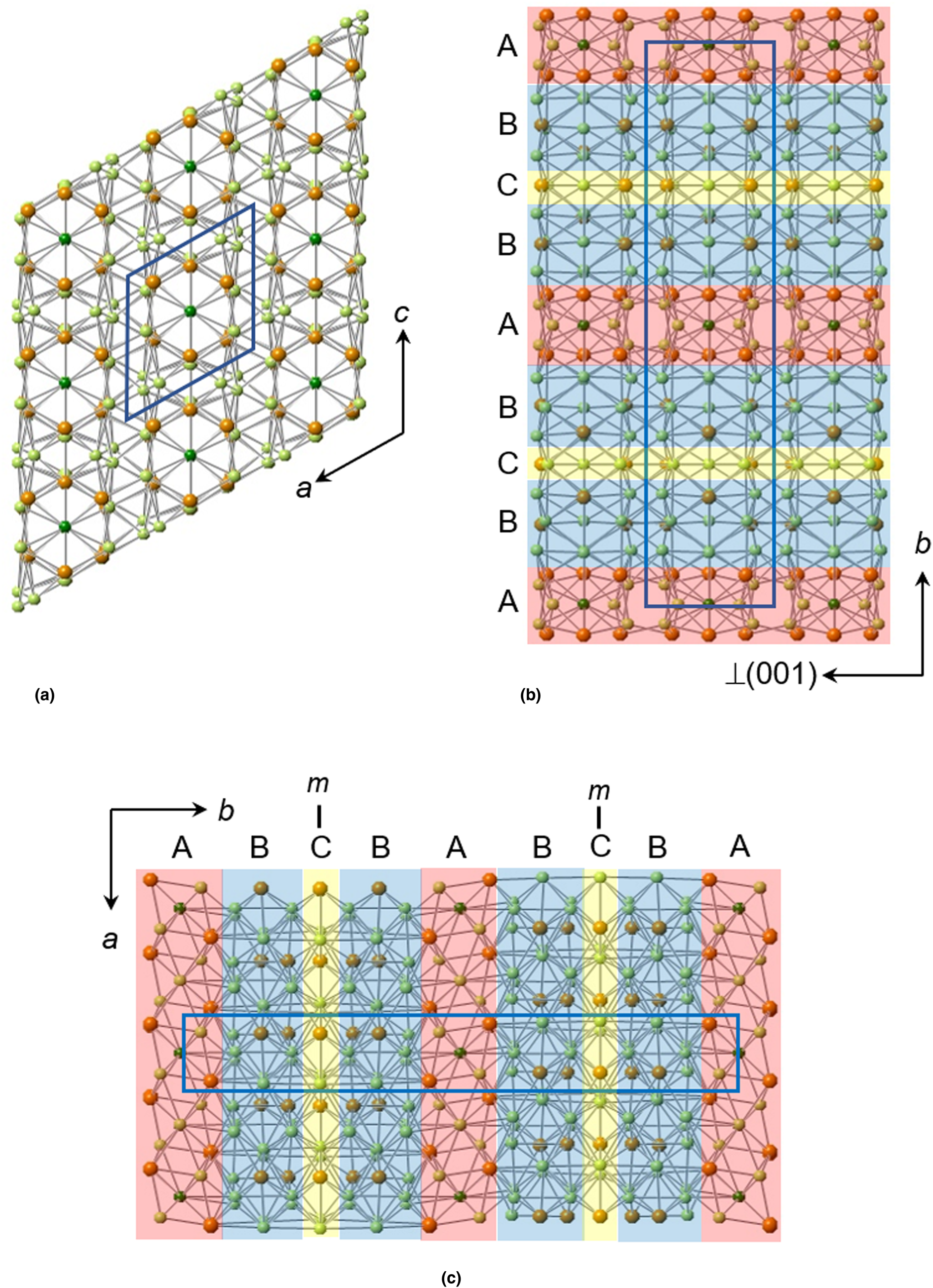
Fig. 7. The modular structure of sluzhenikinite showing the sequence of A, B and C modules stacked along [010] in ratio AB2C. (a) Structure projected onto (010) and showing pseudohexagonal ‘channels’ running parallel to the b-axis and occupied by Pd14 and (Sn,Sb) atoms. (b) Structure viewed along the x axis and showing the module stacking sequence. Vacant tunnels extend parallel to [100]. (c) Structure projected onto (001) and showing the module stacking sequence.
These three components are stacked regularly along the y axis ….ABCBA…. in the ratio AB2C, giving a corresponding overall composition Pd7(Sn,Sb)6 + 2[Pd10(Sn,Sb)3] + Pd3(Sn,Sb)2 = Pd30(Sn,Sb)14. The three components are structurally very different as we now describe.
Slab A (Fig. 5) comprises rows of Pd6[Sn,Sb]6 polyhedra with a central Pd14 atom shown in dark green in this figure. These polyhedra are connected via pairs of additional Pd atoms to form a row, leading to a composition Pd7(Sn,Sb)6 for the row (and slab). Adjacent rows are linked via Pd–(Sn,Sb) bonds. This slab has tunnels running parallel to [100] (Fig. 5b).
Slab B (Fig. 6) is composed of Pd atoms coordinated by six Pd and three (Sn,Sb) atoms; (Sn,Sb) atoms are coordinated by nine Pd atoms. The (Sn,Sb) atoms form a puckered centred-pseudohexagonal motif. In this figure the central Pd and Sb,Sn atoms are superimposed. The red circle shows the Pd17 site. The assignment of this site to Pd is discussed in the text and is consistent with the simple motif of this slab. There are two B slabs per unit cell.
Sheet C (Fig. 6e) is a single layer of atoms thick and lies wholly within the mirror plane. All three non-equivalent Pd atoms (Pd1, Pd12, Pd13) are coordinated to six Pd and four Sb/Sn atoms. Atoms Sb/Sn 7 and 8 are coordinated to nine Pd atoms.
The modular structure of sluzhenikinite (Fig. 7) suggests the possibility of a polysomatic series of Pd–Sn–Sb phases in which different proportions of these modules occur.
It is conceivable that Sn and Sb are partitioned differently between these three structural components. If this were the case, then a range of structures (polysomes) with different Sn/Sb ratios could occur.
Relation to other minerals
There are several Pd-dominant Pd–Sn–Sb minerals and synthetic phases (Table 6): Mertieite Pd8Sb3, naldrettite Pd2Sb, stibiopalladinite Pd5Sb2, synthetic Pd2Sn and synthetic Pd20Sb7. The latter is the Sb-analogue of keithconnite Pd20Te7. The Pd–Pd and Pd–Sn/Sb interatomic distances are in the ranges 2.8−3.1 Å and 2.5−3.0 Å, respectively. The corresponding distances in sluzhenikinite fall within these ranges (see supplementary crystallography information file). The narrowest range of interatomic distances is in Pd2Sn for which d(Pd−Pd) is 2.8−3.0 Å and d(Pd−Sn) is 2.7−3.0 Å. The shortest Sb(Sn)–Sb(Sn) distance in sluzhenikinite is 3.115 Å and as such is not considered to constitute a bond. Hence, there are no Sb(Sn)–Sb(Sn) bonds in sluzhenikinite, which is also true of other Pd–Sb(Sn) structures. Topologically, sluzhenikinite is unlike other Pd–Sn–Sb structures, while sharing basic features such as comparable Pd–Pd and Pd–(Sn,Sb) interatomic distances. We suggest that sluzhenikinite should belong to the 01.AG PGE-metal alloys group of the Nickel and Strunz classification.
Table 6. Unit-cell parameters (Å,°) of sluzhenikinite and related Pd–Sb–Sn minerals.
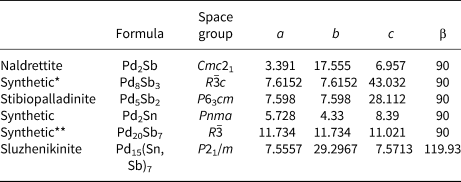
* Isostructural with mertieite-II, Pd8Sb2.5As0.5.
** Isostructural with keithconnite, Pd20Te7.
Acknowledgments
We would like to take this opportunity to acknowledge the major contribution of Pete Williams to the international mineralogical community and the many fruitful shared projects, discussions and IMA-related interactions with him over the past 15 years. We acknowledge Ritsuro Miyawaki, Chairman of the CNMNC of IMA and its members for helpful comments on the original proposal. We thank Luca Bindi, Peter Leverett and an anonymous reviewer for their very helpful comments and suggestions. This research was supported by the Grant Agency of the Czech Republic (project No. 22-26485S to A.V.), by the Czech Geological Survey (DKRVO/ČGS 2018–2022). Chris Stanley acknowledges Natural Environment Research Council grant NE/M010848/1 Tellurium and selenium cycling and supply. J.P. acknowledges CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110) for financial support for the collection of diffraction data.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.96















