Significant outcomes
-
∙ Exposure of Flinders sensitive line (FSL) rats to time-dependent sensitisation (TDS) stress reduces active coping, amplifies depressive-like behaviour and attenuates the antidepressant effects of imipramine (IMI) in FSL rats.
-
∙ The beneficial effects of IMI on limbic monoamine levels in FSL rats are compromised in combined FSL+TDS-exposed rats, especially its effects on the serotonergic system.
-
∙ Post-traumatic stress disorder (PTSD) is highly comorbid with depression and contributes to the development of treatment-resistant depression (TRD). Combining a genetic animal model of depression with a PTSD paradigm may represent a putative animal model of TRD.
Limitations
-
∙ The initial (severe) stress sequence and subsequent re-stresses may promote several adaptive changes in the animals that complicate interpretation of monoaminergic responses. Limiting the procedure to a single re-stress could be considered.
-
∙ Assessment of corticosterone levels immediately post severe stress as well as before and after re-stress may provide a more comprehensive picture of the bio-behavioural responses observed and their relevance to TRD.
-
∙ Behavioural assessment of anhedonia (sucrose preference test), which has been demonstrated to be an important symptom of TRD, would be a valuable addition.
-
∙ Challenging FSL+TDS animals with first-line antidepressants (SSRIs, NSRI’s) and/or ketamine would expand predictive validity, and is presented in a companion paper to this manuscript.
-
∙ Would this model present with altered biomarkers of TRD that contribute to construct validity?
Introduction
The occurrence of non- or partial response to antidepressant treatment in the depressed population creates a major problem in effectively treating and managing the disorder. Less than two-thirds of patients respond to drug-centred therapy (Reference Fava1) and up to half of patients fail to achieve a full response when treated with first-line antidepressant drugs (Reference Trivedi, Rush and Wisniewski2). These initial observations were confirmed by the Sequenced Treatment Alternatives to Relieve Depression (STAR D) study, designed to mimic clinical conditions by incorporating the most commonly used strategies in treating patients exhibiting drug resistance (Reference Rush, Fava and Wisniewski3). Even after applying several treatment strategies in this population, approximately 30% of these patients still did not respond to treatment (Reference Nierenberg and Amsterdam4).
On-going work has described the underlying biology of depression as being driven by the presence of chronic psychosocial stress and associated disturbances in monoaminergic, γ-amino-butyric acid (GABA)-glutamate, neuroendocrine (Reference Brand, Möller and Harvey5) and cardio-metabolic and immune-inflammatory disturbances (Reference Harvey6). However, the exact cause of TRD remains obscure. As with depression, TRD is believed to be heterogeneous in nature (Reference Krishnan and Nestler7) and, although most pathophysiological factors contributing to depression appear to be similar in TRD, many of these conditions are significantly exaggerated in the resistant form, resulting in more severe symptoms (Reference Wijeratne and Sachdev8).
The treatment of depression may be further complicated by the co-occurrence of other underlying psychiatrc disorders. The prevalence rate of a co-existing anxiety disorder is 50–60% (Reference Rush, Trivedi and Wisniewski9,Reference Zarate, Singh and Carlson10) – a figure that increases to 72% in TRD (Reference Rush, Trivedi and Wisniewski9). With a prevalence rate of 17.8%, PTSD is one of the more commonly co-occurring anxiety disorders in patients with depression, and increases to 22.4% in TRD (Reference Rush, Trivedi and Wisniewski9). Conversely, more than half of patients seeking treatment for PTSD are diagnosed with comorbid depression (Reference Ford, Elhai, Ruggiero and Frueh11). This high comorbidity stems largely from overlapping symptoms of anhedonia, sleep difficulty, irritability and poor concentration (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria) (Reference Elhai, De Francisco Carvalho, Miguel, Palmieri, Primi and Christopher Frueh12). Both depression and PTSD require exposure to stressful events for onset (Reference Elhai, De Francisco Carvalho, Miguel, Palmieri, Primi and Christopher Frueh12), whereas both illnesses exhibit hippocampal atrophy related to hypothalamic–pituitary–adrenal (HPA) axis abnormalities (Reference Manji, Drevets and Charney13).
In recent years, it has become widely accepted that genetic susceptibility plus adverse environmental situations are an important prodromal event to the development of depression (Reference Tennant14–Reference Sullivan, Neale and Kendler16). Animal models that are based on this construct have contributed significantly to our knowledge of mood and anxiety disorders (Reference Brand, Möller and Harvey5,Reference Harvey and Shahid17). However, a shortage of suitable and validated animal models of TRD is a major contributing factor to our current lack of understanding of the pathophysiology of TRD. Recent studies have therefore set out to explore the processes that underlie treatment resistance in animal models (Reference Levinstein and Samuels18). In their review, Willner and Belzung (Reference Willner and Belzung19) emphasise models that incorporate predisposing factors leading to heightened stress responsiveness. Chronic mild stress (CMS), a paradigm primarily identified as a depression model (Reference Katz20), has been demonstrated to successfully reproduce antidepressant treatment response rates resembling those observed in clinical studies, with chronic escitalopram treatment found to induce response rates of only 50% (Reference Jayatissa, Bisgaard, Tingström, Papp and Wiborg21). However, it being labour intensive and exhibiting poor cross-laboratory reproducibility is a concern (Reference Willner22,Reference Samuels, Leonardo and Gadient23).
The FSL rat, a genetic animal model of depression, is a robust and well-studied preclinical model of depression with good construct, predictive and face validity (Reference Yadid, Nakash and Deri24–Reference Overstreet and Wegener27). Furthermore, FSL rats only display anhedonic responses after exposure to CMS (Reference Matthews, Forbes and Reid28,Reference Pucilowski, Overstreet, Rezvani and Janowsky29), thus tagging the strain as a good candidate for gene-X-environment studies. Indeed, FSL and Flinders Resistant Line (FRL) rats display differential sensitivity to rearing conditions (early-life stress) and rat strain (genes) that in turn modify treatment response by altering serotonin transporters (SERT) (Reference Shrestha, Pine and Luckenbaugh30). This is a valuable quality, seeing that abnormal SERT function has been implicated in the pathology of depression (Reference Shrestha, Hirvonen and Hines31,Reference Ruf and Bhagwagar32). Interestingly, by exposing FSL rats to maternal separation, Carboni et al. (Reference Carboni, Becchi and Piubelli33) demonstrated the induction of biological correlates reminiscent of those observed in human TRD, prompting them to propose that the gene-environment paradigm offers important construct validity in modelling TRD. However, the model lacked predictive validity due to the inability of antidepressant treatment to alter immobility time in maternally separated FSL rats either before or after treatment when compared with control animals (Reference Carboni, Becchi and Piubelli33).
Considering the strong comorbidity between depression and PTSD, and that depression in patients with PTSD is more treatment resistant (Reference Green, Krupnick and Chung34,Reference Thase and Rush35), we have developed an animal model of TRD based on the premise that exposing animals genetically predisposed to depressive-like behaviour to a PTSD-related paradigm would yield animals displaying more pronounced depressive-like behaviour. Moreover, such behaviour would be resistant to antidepressant treatment. To this end we have considered the TDS or stress re-stress model of PTSD. TDS is based on a trauma plus contextual reminder principle of PTSD (Reference Oosthuizen, Wegener and Harvey36), and has shown good predictive, construct and face validity for PTSD (Reference Harvey, Naciti, Brand and Stein37–Reference Liberzon, Krstov and Young40). In this study, face, construct and predictive validity were assessed in the forced swim test (FST) using a behavioural sampling method to study serotonergic and noradrenergic-driven behaviours, assessment of limbic noradrenaline (NA) and 5-hydroxyindoleacetic acid (5HIAA) levels, and response to chronic treatment with the tricyclic antidepressant, IMI.
Materials and methods
Subjects
Animals were bred and housed at the Vivarium (SAVC reg. number FR15/13458; SANAS GLP compliance number G0019) of the Pre-Clinical Drug Development Platform of the North-West University. Ambient temperature was maintained at 22±2°C with a relative humidity of 40–60% and full spectrum of light in a 12-hour light/dark cycle, with lights switched on at 06:00 a.m. and off at 06:00 p.m. Food and water were provided ad libitum. All experiments were approved by the AnimCare animal research ethics committee (NHREC reg. number AREC-130913-015) of the North-West University. Animals were maintained and procedures performed in accordance with the code of ethics in research, training and testing of drugs in South Africa and complied with national legislation (ethics approval number: NWU-00111-12-A5).
The original colonies of FSL and FRL rats were obtained from Dr. David H Overstreet, University of North Carolina, USA. Subjects were male adult FSL (n=48 for behavioural assessment and n=32 for monoamine analysis each) and FRL (n=12 for behavioural assessment and n=8 for monoamine analysis) rats. Table 1 describes the layout of the experimental groups. Half of the FSL animals in each of the above groups were subjected to TDS (see below) at the start of the protocol with behaviour in the open field test (OFT) and FST assessed at the end of the protocol (3 weeks following single prolonged stress (SPS)). Monoamine analysis was performed in animals naive to behavioural assessment. The animals were housed four per cage, with the TDS paradigm initiated at an age of 40 (±1) days in order to conclude the experiments while the rats were still of an appropriate weight for the behavioural assessments. Handling of the animals was initiated 1 week before starting the experimental procedure by taking bodyweight measurements daily until the last day of the study to monitor weight gain and calculate drug dosages.
Table 1 Layout of experimental groups

FRL, Flinders resistant line; FSL, Flinders sensitive line; IMI, imipramine; n/s, non-stressed; TDS, time-dependent sensitisation; VEH, vehicle.
Time-dependent sensitisation (TDS)
TDS is an animal model of PTSD. Animals exposed to a severely traumatic situation, and followed by subsequent but less stressful contextual reminders, exhibit significant physiological and behavioural alterations that show a time-dependent sustaining or worsening in the absence of the initiating stressor (Reference Yehuda and Antelman41,Reference Harvey, Brand, Jeeva and Stein42).
The TDS paradigm used in this study incorporated an acute SPS sequence comprising a somatosensory stressor (restraint), a psychological stressor (forced swimming with brief submersion) and a complex stress-stimuli (exposure to ether vapours) followed by re-exposure to restraint stress 7 and 14 days later (Reference Harvey, Brand, Jeeva and Stein42).
Restraint stress
Rats were placed in Perspex® restrainers for 2 h with the tail-gates adjusted to keep each animal well-contained without impairing circulation to the limbs. The same procedure was followed on days 7 and 14 during the re-stress phase of the TDS protocol.
Forced swim stress
Rats were placed individually in cylindrical Perspex® swim tanks containing 40 cm of ambient water (25°C) and allowed to swim for 15 min while being forcefully submerged for the last 20 s. Thereafter animals were removed from the cylinders, dried and returned to their home cages to recover for 15 min. Forced swimming was performed 21 days before behavioural testing (only as part of the SPS procedure and not during re-stress) in the FST so that any possible conditioned response to swim stress in the FST is unlikely.
Exposure to ether vapours
15 min after swim stress, rats were exposed to 5 ml of 100% ether vapours in a 5 l sealed plastic container until loss of consciousness (±2 min). Ether was poured onto a paper towel at the bottom of the container with the animal placed on a raised metal platform to avoid direct contact with the substance. After loss of consciousness, the animals were immediately removed from the container, returned to their home cage for observation until regaining full consciousness and then returned to their holding room. Animals were left mostly undisturbed, only subjecting them to routine handling until re-exposure to restraint stress during the re-stress phase of the TDS protocol.
Open field test (OFT)
This test is generally performed before the FST to control for locomotor activity. The OFT was performed half an hour before subjecting animals to the FST. Rats were individually placed in a square arena (100×100×50 cm) facing the centre of the arena. Behaviour was recorded for 5 min using a ceiling-mounted digital camera. The video files were subsequently analysed using EthoVision® XT software (Noldus® Information Technology, Wageningen, The Netherlands). Total distance moved was used as a measure of locomotor activity.
Forced swim test (FST)
The FST can reliably predict antidepressant-like effects after drug treatment and is considered a model of behavioural despair that is typically manifest in human depression, and expressed in rodents as a decrease in escape-driven behaviour, i.e. increased immobility (Reference Porsolt, Anton, Blavet and Jalfre43). During behavioural analysis, rats were placed individually in cylindrical Perspex® swim tanks containing 30 cm of ambient water (25°C) for 7 min and their behaviour recorded. The first and last minute of the video files were discarded and the remaining 5 minutes scored for characteristic escape-directed behaviours, including swimming, climbing (struggling) and immobility. These sub-scores of the FST provide useful information relating to serotonergic (swimming) and noradrenergic (climbing) directed behaviours that may extend whole brain monoamine analyses (Reference Harvey, Duvenhage and Viljoen44).
Drug administration
After weighing all animals daily (between 09:00 a.m. and 11:00 a.m.), IMI (Sigma-Aldrich, Kempton Park, South Africa) was dissolved in physiological saline (0.9% NaCl) and administered subcutaneously at a dose of 10 mg/kg (Reference Wróbel, Serefko, Wlaź and Poleszak45,Reference Wainwright, Workman and Tehrani46) to unstressed animals (FSL−TDS+IMI) and animals exposed to TDS (FSL+TDS+IMI) (Table 1). Treatment started on day 15 (after completing the TDS protocol on day 14) and persisted for 7 days before behavioural testing commenced on the evening of day 21. This duration of treatment is adequate for establishing an antidepressant response in rats (Reference Harvey, Duvenhage and Viljoen44,Reference Breuer, Groenink, Oosting, Westenberg and Olivier47,Reference Breuer, Chan and Oosting48). Stressed and unstressed control animals (FSL and FRL) were injected with saline vehicle according to the same procedure as in IMI-treated animals.
Quantitative analysis of brain NA and 5HIAA
Several valid indices of central serotonergic activity may be applied, including serotonin (5-hydroxytryptamine; 5HT) and 5HIAA levels and the 5HIAA/5HT ratio (Reference Shannon, Gunnet and Moore49). In this regard, in vivo microdialysis is a more reliable method to directly measure extracellular levels of 5HT, whereas whole- or regional brain monoamine analysis provides total levels of 5HT – both extracellular and unreleased from nerve terminals (Reference Duncan50). 5HT is metabolised primarily to 5HIAA, hence 5HIAA has been demonstrated to reflect reliable insights into time-dependent alterations in 5HT response (Reference Mehlman, Westergaard and Hoos51). 5HIAA has previously been correlated with 5HT function (Reference Shannon, Gunnet and Moore49) and was therefore applied as an indicator of serotonergic function in the current study. Following sacrifice of the rats by decapitation, total hippocampus and frontal cortices were dissected out on an ice-cooled dissection slab, weighed, snap frozen in liquid nitrogen and stored at −80°C until the day of analysis, as described previously (Reference Yehuda and Antelman41). Quantification of NA and 5HIAA was performed by high-performance liquid chromatography (HPLC) coupled with electrochemical detection (HPLC-EC), as previously described (Reference Harvey, Brand, Jeeva and Stein42). An Agilent 1200 series HPLC (Agilent Technologies, California, USA), equipped with an isocratic pump, auto sampler and coupled to an ESA Coulochem Electrochemical detector (Dionex, California, USA), and Chromeleon® Chromatography Management System software (version 6.8), was used. NA and 5HIAA concentrations in the tissue samples were determined by comparing the area under the peak of each monoamine with that of the internal standard, isoprenaline (range 5–50 ng/ml). Linear standard curves (regression coefficient >0.99) were found in this particular range. Monoamine concentrations were expressed as ng/g wet weight of tissue (mean±SEM).
Bodyweight analysis
Decreased bodyweight and loss of appetite have been observed in both depressed individuals (20) and FSL rats (26). Sustained decreases in weight gain have been reported in rats following chronic stress (Reference Harris, Zhou, Youngblood, Rybkin, Smagin and Ryan52,Reference Harris, Palmondon, Leshin, Flatt and Richard53) which may be initiated by increased energy metabolism during stress coupled with acute increases in stress-related peptides (Reference Harris, Palmondon, Leshin, Flatt and Richard53). In order to establish the impact of the applied stressors on the well-being of the animals, bodyweight was measured daily from 7 days prior to SPS and continued until the final day of the experiment.
Statistical analysis
Statistical analyses were performed using Graphpad Prism® 6 and IBM® SPSS® 22 software under the guidance of the Statistical Consultation Service of the North-West University. In pairwise comparisons of the behaviour and neurochemistry between unstressed FRL and FSL animals, unpaired student’s t-tests with Welch’s correction (normally distributed data as indicated by Shapiro–Wilk test for normality p>0.05) or Mann-Whitney U-tests (data not distributed normally) were performed. Two-way repeated measures analysis of variance (RM-ANOVA) followed by Bonferroni post-hoc analysis was applied to comparisons of the treatment naive cumulative weight gain of FRL and stressed and unstressed FSL animals. Time and cohort was set as within-subject factors, whereas weight was set as between-subject factor. Ordinary two-way ANOVA was applied in between-group comparisons of behaviour and neurochemistry in treatment-naive and IMI-treated unstressed and stressed FSL animals. In this case, exposure to TDS and treatment was set as within-subject factors, whereas the respective behavioural and neurochemical parameters were set as between-subject factors. Significance was set at p<0.05 for all comparisons. Where Cohen’s d effect sizes were calculated, large effect sizes are indicated by d>0.8 and very large effect sizes by d>1.3.
Results
Bodyweight
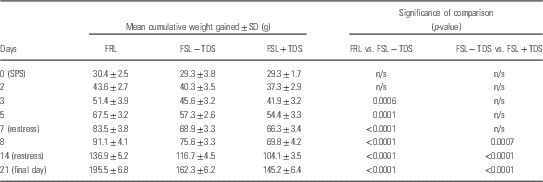
Data are represented in Fig 1. Two-way RM-ANOVA revealed a significant interaction between time and cohort [F(81,1188)=61.98] with respect to the mean cumulative weight gain of animals, whereas both time [F(27,1188)=13 940, p<0.0001] and cohort [F(3,44)=102.0, p<0.0001], respectively, also had significant main effects on weight gain. Although the mean cumulative daily weight gain of rats between the respective cohorts demonstrated no significant differences before SPS (day 0), significant age and stress-related differences between FSL and FRL animals, both within (FSL) and between strain, became apparent post-SPS. From day 3 post-SPS, unstressed FSL animals lagged behind the FRL controls (day 3, 45.6±3.2 vs. 51.4±3.9 g, p=0.004). Moreover weight gain in stressed FSL rats soon lagged behind that of unstressed control animals (Table 2). On day 8 post-SPS the difference in weight gain between stressed and unstressed FSL rats began to reveal significance (69.8±4.2 vs. 75.6±3.3; p=0.005). The observed disparities in the rates of weight gain in the various groups persisted until the last day of observation. In addition to the curbed weight gain of TDS-exposed FSL rats, they were also observed to present with a general decrease in fur quality and porphyrin staining around the eyes (visual inspection; data not shown).

Fig. 1 Mean cumulative weight gain in FRL and TDS naive and TDS exposed FSL animals. Data are represented as the mean of 12 animals. Descriptive statistics are provided in Table 2. FRL, Flinders resistant line; FSL, Flinders sensitive line; n/s, non-stressed; SPS, single prolonged stress; TDS, time-dependent sensitisation.
Table 2 Cumulative weight gain over time
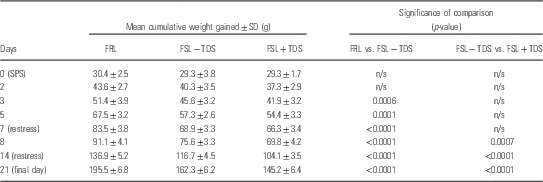
FRL, Flinders resistant line; FSL, Flinders sensitive line; IMI, imipramine; n/s, non-stressed; SPS, single prolonged stress; TDS, time-dependent sensitisation.
Mean cumulative weight gain of rats measured daily from 1 week before commencement of the TDS protocol until the final day of behavioural testing. Data are provided as mean of matched daily values in each group.
Two-way repeated measures ANOVA with Bonferroni post-hoc.
Behaviour
In order to establish the translational relevance of the FSL rat for depression, data and statistics relating to the behavioural comparisons made between stress and treatment-naive FRL and FSL animals are provided in Table 3. The behavioural differences between FSL versus FRL rats are henceforth described separately under the OFT and FST sections below.
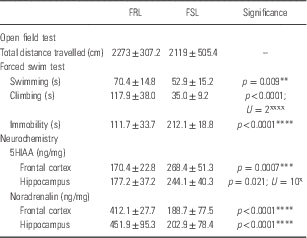
Table 3 Open field test, forced swim test and frontal-hippocampal monoamine data in unstressed Flinders resistant line (FRL) vs. Flinders sensitive line (FSL) animals
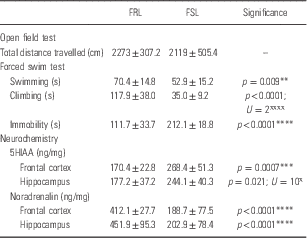
*Unpaired Student’s t-test; ˣMann–Whitney U-test.
Open field test (OFT)
Locomotor activity
FSL and FRL rats were similar with respect to distance travelled in the OFT (2119±505.4 vs. 2273±307 cm, Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 2. Considering the locomotor activity of FSL animals, two-way ANOVA did not reveal a significant interaction between TDS-exposure and treatment [F(1,11)=0.95, p=0.35]. However, a main effect of treatment was observed [F(1,11)=5.67, p=0.04] in the locomotor activity of the TDS-exposed group with post-hoc analysis revealing a trend towards decreased locomotor activity in IMI-treated animals that narrowly missed statistical significance (1640±422.8 vs. 2296±971.7 cm, p=0.07, d=0.94).

Fig. 2 Comparison between locomotor activity of unstressed and TDS exposed FSL rats before (white) and after (black) sub-chronic IMI treatment. TDS VEH versus TDS IMI; d=0.94. All data analysed by two-way analysis of variance followed by Bonferroni’s post-hoc tests and Cohen’s d analysis. Data are represented as mean±SEM. IMI, imipramine; n/s, non-stressed; TDS, time-dependent sensitisation; VEH, vehicle.
Forced swim test (FST)
Swimming
FSL rats presented with significantly reduced swimming behaviour compared with FRL rats (59.9±15.2 vs. 70.4±14.8 s; p<0.05, Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 3a. Although no significant interaction between TDS and treatment was displayed in the behaviour of FSL animals [F(1,44)=1.54, p=0.2], both factors had statistically significant main effects on swimming behaviour [TDS, F(1,44)=11.8, p=0.001; treatment, F(1,44)=5.32, p=0.03]. As such, post-hoc analysis demonstrated that treatment-naive FSL animals exposed to TDS showed an even greater reduction in the average swimming time compared with unstressed treatment-naive rats (52.9±15.2 vs. 24.4±9.8 s, p=0.004). Finally, IMI treatment significantly reversed the reduced swimming time in TDS-exposed FSL animals (46.0±36.7 vs. 24.4±9.8 s, p=0.03), whereas failing to affect the behaviour of animals in the unstressed group.
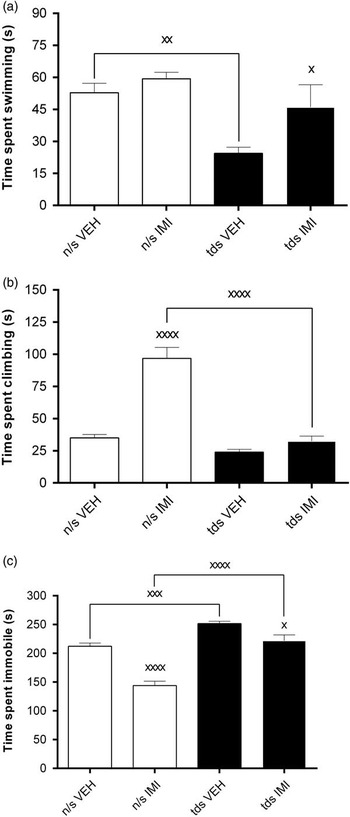
Fig. 3 Comparisons between behavioural parameters measured in the forced swim test [panel (a)=time swimming, panel (b)=time climbing, panel (c)=immobility time] in unstressed and TDS-exposed FSL rats before and after sub-chronic IMI treatment. (a) Time spent swimming (s). n/s VEH versus TDS VEH, xx p<0.01; TDS VEH versus TDS IMI, x p<0.05. (b) Time spent climbing (s). n/s VEH versus n/s IMI, xxxx p<0.0001; n/s IMI versus TDS IMI, xxxx p<0.0001. (c) Time spent immobile (s). n/s VEH versus n/s IMI, xxxx p<0.0001; n/s IMI versus TDS IMI, xxxx p<0.0001; n/s VEH versus TDS VEH, xxx p<0.001; TDS VEH versus TDS IMI, x p<0.05. All the data were analysed by two-way analysis of variance followed by Bonferroni’s post-hoc tests and Cohen’s d analysis. Data are represented as mean±SEM. IMI, imipramine; n/s, non-stressed; TDS, time-dependent sensitisation; VEH, vehicle.
Climbing
FSL rats presented with significantly reduced climbing behaviour compared with FRL rats (35.0±9.2 vs. 117.9±38.0 s; p<0.0001, Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 3b. A significant interaction was displayed between TDS and treatment [F(1,44)=28.5, p<0.0001], whereas both treatment [F(1,44)=49.6, p<0.0001] and stress [F(1,44)=57.1, p<0.0001] had significant main effects on climbing behaviour. Although no difference between the climbing behaviour of treatment naive stressed and unstressed FSL animals was demonstrated, post-hoc analysis revealed that IMI treatment significantly increased climbing in unstressed FSL animals (96.8±29.2 vs. 35.0±9.3 s, p<0.0001), but was without effect in FSL+TDS animals (32.4±13.9 vs. 24.0±7.9 s, n/s). In fact, in comparing FSL+TDS with non-stressed FSL rats receiving IMI, exposure to TDS significantly negated the response to IMI in FSL animals (32.4±13.9 vs. 96.8±29.2 s, p<0.0001).
Immobility
FSL rats presented with significantly increased immobility compared to FRL rats (212.1±18.8 vs. 111.7±33.70 s; p<0.0001, Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 3c. As a significant two-way interaction was revealed between TDS and treatment with respect to the immobility scores of FSL animals [F(1,44)=6.8, p=0.01], simple main effects of both factors were run. Exposure to TDS [F(1,44)=64.4, p<0.0001] significantly increased the average immobility score of treatment naive FSL animals (251.7±14.7 vs. 212.1±18.8 s, p=0.0008). Although IMI treatment [F(1,44)=45.4, p<0.0001] resulted in significant reductions in the immobility scores of both unstressed (143.8±27.1 vs. 212.1±18.8, p<0001) and stressed (221.5±35.7 vs. 251.7±14.2, p=0.01) FSL rats, immobility in IMI-treated FSL+TDS animals remained significantly greater than that in unstressed FSL animals receiving IMI (221.5±35.7 vs. 143.8±27.1, p<0.0001).
Monoamine analysis
In order to establish the translational relevance of the FSL rat for depression, data and statistics relating to the neurochemical comparisons made between stress and treatment-naive FRL and FSL animals are provided in Table 3. The neurochemical differences between FSL versus FRL rats are henceforth described separately below.
5HIAA
FSL rats presented with significantly increased 5HIAA levels in the frontal cortex (268.4±51.3 vs. 170.4±22.8 ng/mg; p<0.005) and hippocampus (244.1±40.3 vs. 177.2±37.2 ng/mg; p<0.05) versus FRL rats (Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 4a. Two-way ANOVA revealed significant interactions between TDS and treatment in both brain areas [frontal cortex, F(1,27)=7.6, p=0.01; hippocampus, F(1,27)=4.45, p=0.04] and simple main effects were run. As such, TDS and treatment significantly influenced the concentrations of 5HIAA measured in both the frontal cortex [TDS, F(1,27)=30.8, p<0.0001; treatment, F(1,27)=6.1, p=0.02] and hippocampus [TDS, F(1,27)=15.72, p=0.0005; treatment, F(1,27)=11.1, p=0.002]. As such, post-hoc analyses revealed that 5HIAA levels in treatment naive stressed FSL animals tended to be lower compared with the unstressed FSL animals (Fig 4ai, 216.4±45.6 vs. 268.4±51.3 ng/mg, d=1.07; Fig 4aii, 201.5±59.1 vs. 244.1±40.4 ng/mg, d=0.85). Although IMI treatment significantly increased both frontocortical (Fig 4ai, 366.0±69.0 vs. 268.4±51.3 ng/mg, p=0.007) and hippocampal (Fig 4aii, 369.1±87.3 vs. 244.1±40.4 ng/mg, p=0.004) 5HIAA levels in unstressed FSL rats, exposure to TDS negated this effect (Fig 4ai, frontal cortex, 210.8±32.4 vs. 216.4±45.6 ng/mg, n/s; Fig 4aii, hippocampus, 229.7±58.7 vs. 201.5±59.0 ng/mg, n/s) and resulted in significantly lower levels of 5HIAA levels measured in IMI-treated animals after TDS-exposure relative to stress-naive animals (Fig 4ai, frontal cortex, 210.8±32.4 vs. 366.0±69.0 ng/mg, p<0.0001; Fig 4aii, hippocampus, 229.7±58.7 vs. 369.1±87.3 ng/mg, p=0.0005).

Fig. 4 Comparisons between frontocortical and hippocampal 5HIAA [panel (a)] and NA [panel (b)] in unstressed and TDS-exposed FSL rats before and after sub-chronic IMI treatment. Panel (ai) Frontal-cortical 5HIAA concentrations. n/s VEH versus n/s IMI, xx p<0.01; n/s IMI versus TDS IMI, xxxx p<0.0001; n/s VEH versus TDS VEH, d=1.07. Panel (aii) Hippocampal 5HIAA concentrations. n/s VEH versus n/s IMI, xx p<0.01; n/s IMI versus TDS IMI, xxx p<0.001, n/s VEH versus TDS VEH, d=0.87. Panel (bi) Frontal-cortical NA concentrations. n/s VEH versus n/s IMI, d=0.98; TDS VEH versus TDS IMI, d=1.22. Panel (bii) Hippocampal NA concentrations. TDS VEH versus TDS IMI, xx p<0.01; n/s VEH versus n/s IMI, d=1.03. All data analysed by two-way analysis of variance followed by Bonferroni’s post-hoc tests and Cohen’s d analysis. Data are represented as mean±SEM. FC, frontal cortex; HC, hippocampus; 5HIAA, 5-hydroxyindoleacetic acid; IMI, imipramine; NA, noradrenaline; n/s, non-stressed; TDS, time-dependent sensitisation; VEH, vehicle.
NA
FSL rats presented with significantly reduced NA levels in the frontal cortex (188.7±77.5 vs. 412.1±27.7 ng/mg; p<0.0001) and hippocampus (202.9±78.4 vs. 451.9±95.3 ng/mg; p<0.0001) versus FRL rats (Table 3).
Data describing the effects of stress and IMI treatment in FSL animals are presented in Fig 4b. No significant two-way interactions between TDS and treatment were observed in either brain area [frontal cortex, F(1,27)=0.01, p=0.9; hippocampus, F(1,26)=1.3, p=0.3]. However, treatment demonstrated a main effect on NA concentrations in the frontal cortex [F(1,27)=8.4, p=0.007] and hippocampus [F(1,26)=11.29, p=0.002]. Although IMI resulted in trends toward increased NA in both brain areas of unstressed FSL animals (frontal cortex, 285.2±119.1 vs. 188.7±77.5 ng/mg, d=0.98; hippocampus, 308.5±126.1 vs. 202.9±78.4 ng/mg, d=1.03), it significantly increased the hippocampal NA levels in stressed FSL animals to levels comparable with that observed in unstressed animals (Fig 4bii, 364.9±212.8 vs. 151.9±41.4 ng/mg, p=0.04). Furthermore, although narrowly missing statistical significance, IMI also tended to increase NA in the frontal cortex of stressed FSL animals (Fig 4bi, 243.8±96.2 vs. 154.0±51.1 ng/mg, p=0.054, d=1.2).
Discussion
As expected, FSL rats presented with significant depressive-like manifestations versus their FRL controls at both the behavioural and neurochemical level (Table 3), with IMI for the most part reversing these changes (Figs 3 and 4). Exposure of FSL rats to TDS profoundly inhibited growth (Fig 1), with behavioural and neurochemical sequelae (Figs 3 and 4). TDS further reduced active coping (swimming) behaviour and amplified depressive-like behaviour (immobility) in FSL rats (Fig 3a, c). Importantly, the above-noted antidepressant-like effects of IMI in FSL rats were significantly attenuated after TDS exposure. Although IMI altered brain monoamine levels in unstressed FSL rats, it failed to do so in combined FSL+TDS rats – especially effects on 5HIAA (Fig 4). As such, combining FSL+TDS stress may represent a novel animal model of TRD, a schematic outline of which is depicted in Fig 5.

Fig. 5 Procedural outline of the treatment-resistant depression (TRD) model. The TRD model is a coming together of two translational animal models, namely exposing a genetic animal model of depression (FSL rats) with the time-dependent sensitisation (TDS) model of post-traumatic stress disorder (PTSD). Rats are exposed to single prolonged stress (SPS; day 0) – a triple stressor sequence comprising a somatosensory stressor (restraint), a psychological stressor (forced swimming with brief submersion), and a complex stress-stimuli (exposure to ether vapours), followed by a less stressful but situational reminder of the original stressor (restraint stress on days 7 and 14). The latter is to enable consolidation of contextual fear memory to promote the progression from an acute stress disorder to PTSD. Thereafter, the animals are left undisturbed for another 7 days before being subjected to behavioural and neurochemical analysis (day 21; two separate cohorts of animals). Drug treatment takes place during the latter 7-day period immediately prior to bio-behavioural testing. FST, forced swim test; 5HIAA, 5-hydroxyindoleacetic acid; FSL, Flinders sensitive line; NA, noradrenalin; OFT, open field test.
Depression is a multifactorial disorder (Reference Krishnan and Nestler7) with both genetics and environmental stress contributing to its development (Reference Tennant14,Reference Caspi, Sugden and Moffitt15). The FSL rat is a well-validated genetic animal model of depression (Reference Overstreet and Wegener27). Considering the high comorbidity of depression in PTSD and as a contributing factor in treatment resistance (Reference Green, Krupnick and Chung34,Reference Thase and Rush35), introducing these animals to conditions conducive to PTSD may serve as a suitable gene-X-environment model of TRD. The aim of this study was therefore to explore this notion by studying behaviour and neurochemistry in such a model and, in so doing, to aid preclinical research into TRD and developing novel drug options for the disorder. By using FRL rats as a control, we demonstrated the depressive phenotype of the FSL rat, thereafter subjecting this stress-sensitive animal to a TDS paradigm and assessing its response to standard antidepressant treatment. The negative impact of TDS on physical development, as illustrated by its detrimental effects on growth during a 4-week period (Fig 1), is indicative of the degree to which the physical and, no doubt, “psychological” well-being of these animals were affected by these interventions. The results of the comparison between cumulative weight gain in FRL rats and stressed and unstressed FSL rats provide an accurate portrayal of the character and resilience of the two strains. At baseline, FSL rats already displayed decreased ability to gain weight even before exposure to environmental stressors. Bearing this in mind, TDS expectedly proved to further worsen the overall well-being of these animals.
Rats exposed to CMS have previously been observed to exhibit impaired locomotor activity (Reference Willner22), although TDS did not negatively affect locomotor activity in the current study (Fig 2). Although IMI treatment resulted in a trend toward decreased locomotor activity in TDS exposed animals, this failed to reach statistical significance. As such, this finding provides a robust departure point for interpreting treatment effects in the FST without having to consider any confounding effects on locomotor activity.
Immobility time is a characteristic depressive-like behaviour measured in the FST, whereas the assessment of swimming and climbing behaviour allows for generating a more holistic account of coping behaviour and also aids in understanding the behavioural effects of drug treatment (Reference Espejo and Miñano54). Results obtained from the FST showed that FSL rats displayed significantly less active coping (swimming and climbing) behaviour as well as being significantly more immobile than their FRL counterparts (Table 3). Important to note is that both decreased swimming behaviour and increased immobility observed in FSL control animals were augmented to a significant degree following exposure to TDS (Fig 3a and c). Of even greater importance is that the antidepressant-like effect exhibited by IMI treatment in unstressed FSL animals was negated in TDS-exposed FSL rats in respect to climbing and immobility (Fig 3b and c) although not swimming (Fig 3a). Further, the anti-immobility effects of IMI in FSL animals were also significantly compromised by TDS compared with that in unstressed FSL animals (Fig 3c). Thus TDS stress exaggerates depressive-like (immobility) behaviour evident in a genetic animal model of depression and abrogates the antidepressant-like effects of IMI in these animals. This not only supports the validity of combining FSL rats with a PTSD paradigm as a gene-X-environment model of TRD, but reinforces the clinical presentation of TRD in depressed patients with a history of severe psychological trauma.
In spite of diffuse distribution of 5HT throughout the central nervous system, uncertainty still surrounds the exact function of 5HT and its relationship to other neurochemicals (Reference Hayes and Greenshaw55,Reference Andrews, Bharwani, Lee, Fox and Thomson56). TDS represents a severely traumatic series of events which prompts the activation of various bio-behavioural responses geared to maximise the animal’s survival. Serotonin is crucial in survival behaviour and has been suggested to play a critical role in adapting to aversive events (Reference Deakin57,Reference Daw, Kakade and Dayan58). In fact, stress re-stress has been demonstrated to alter 5HT receptors in limbic structures that in turn adversely affect memory and other cognitive processes (Reference Harvey, Naciti, Brand and Stein37). Further, the changes in 5HT concentration in response to stress vary between brain regions and also according to the duration of stress applied (Reference Kirby, Allen and Lucki59). FSL rats have previously been characterised by increased levels of 5HT and 5HIAA in limbic regions that are altered in response to antidepressant treatment (Reference Zangen, Overstreet and Yadid60). Increased cortical and hippocampal 5HIAA levels in FSL rats compared with FRL controls in the current study concur with this observation (Table 3) and would suggest a compromised serotonergic system. 5HIAA levels were decreased in FSL rats 1 week after TDS (Fig 4a) – this decrease correlating with significantly reduced swimming activity measured in the FST and concurs with decreased 5HIAA levels measured in the frontal cortex of Sprague–Dawley rats subjected to CMS (Reference Ahmad, Rasheed, Banu and Palit61). Although TDS may be viewed as a series of aversive and traumatic events (Reference Oosthuizen, Wegener and Harvey36), it should be kept in mind that the current data reflects NA and 5HIAA changes 1 week subsequent to completion of the TDS procedure, and thus represents a late emerging event that may be pathological. Previous data demonstrated that an initial increase in 5HT levels after SPS was followed by decreased levels after re-stress (Reference Harvey, Brand, Jeeva and Stein42) which may be suggestive of an adaptive response to stress.
The above-mentioned coping strategies employed in the FST have been found to present with significant correlations with altered monoamines and to be of relevance for the neurochemical basis of depression (Reference Detke, Rickels and Lucki62). Thus, noradrenergic processes have been demonstrated to be altered in depression, but also in anxiety and PTSD, such as adrenergic receptor dysregulation in depression (Reference Cottingham and Wang63), increased NA precursors accompanied by a decrease in adrenergic receptor affinity in patients suffering from PTSD with comorbid depression (Reference Maes, Lin and Verkerk64), the association between catechol-O-methyl transferase single nucleotide polymorphisms and suicide risk in TRD patients (Reference Schosser, Calati and Serretti65), and increased 3-methoxy-4-hydroxyphenylglycol levels measured in patients suffering from anxiety disorders (Reference Yamada, Yamauchi and Yajima66). Furthermore, uncontrollable stress in animal models is associated with decreased central levels of NA (Reference Leonard67,Reference Weiss, Goodman, Losito, Corrigan, Charry and Bailey68) and may be the result of insufficient synthesis of the neurotransmitter relative to its utilisation (Reference Leonard67). A general decrease in NA levels were measured in the frontal cortex and hippocampus of treatment-naive FSL animals (Table 3). Owing to the premise that increased climbing and swimming behaviour in the FST may be a result of enhanced noradrenergic and serotonergic neurotransmission, respectively (Reference Detke, Rickels and Lucki62), the decreased frontal–hippocampal NA levels in both stressed and unstressed FSL rats (Fig 4bi and 4bii; Table 3) as well as the trend to raise NA levels and significantly elevate NA levels in the cortex and hippocampus, respectively, by IMI in TDS+FSL rats (Fig 4b) were expected and congruent with the current thinking on the role of NA in depression (Reference Brand, Möller and Harvey5). However, it is apparent that TDS-exposure abrogated the climbing-enhancing effect of IMI in FSL animals (Fig 3b) as well as sustained lowered NA in the cortex and hippocampus of untreated animals (Fig 4bi and bii). Indeed, TDS has been found to significantly increase NA after SPS, eventually falling to levels significantly lower than baseline 1 week after re-stress (Reference Harvey, Brand, Jeeva and Stein42). The inability of IMI to increase climbing behaviour in stressed FSL rats (Fig 3b), despite its tendency to elevate NA in the cortex as well as significantly increase NA in the hippocampus, is of interest but may be a result of a decrease in adrenergic receptor density and/or affinity as previously reported in both humans (Reference Maes, Lin and Verkerk64,Reference Dimsdale, Mills, Patterson, Ziegler and Dillon69) and animals (Reference Flügge70,Reference Tejani-Butt, Paré and Yang71) exposed to stress.
Given that limbic brain structures are involved in the stress response, changes in 5HT-related responses may be linked to changes in hippocampal and cortical 5HT neurotransmission (Reference Harvey, Naciti, Brand and Stein37,Reference Harvey, Naciti, Brand and Stein39). A general decrease in 5HIAA levels were measured in the frontal cortex and hippocampus of treatment-naive FSL animals (Table 3). TDS worsened swimming deficits as well as duration of immobility (Fig 3a and c) and sustained reduced cortical and hippocampal 5HIAA levels (Fig 4a). Although IMI significantly reduced immobility in unstressed and stressed FSL rats, immobility in the latter group remained significantly higher than that of unstressed IMI-treated rats (Fig 3c) and failed to reverse lowered 5HIAA in FSL+TDS animals (Fig 4a).
Tricyclic antidepressants such as IMI act by increasing the extracellular levels of NA and 5HT (Reference Richelson72). IMI significantly reversed deficits in swimming in FSL+TDS animals (Fig 3a), but failed to reverse lowered limbic 5HIAA levels in these animals (Fig 4a). On the other hand, IMI failed to reverse suppressed climbing in FSL+TDS animals (Fig 3b) despite provoking a tendency (in the FC) and to significantly (in the hippocampus) reverse lowered NA in these animals (Fig 4b). This paradox with respect to limbic monoamine levels and coping strategies may indicate other adaptive changes that underlie coping responses following sustained exposure to stress. Furthermore, it cannot be assumed that the effects of antidepressant drugs are simply to reverse and/or normalise dysfunctions in the brain (Reference Willner, Scheel-Krüger and Belzung73), including those of animals. This has been exemplified by CMS-induced behavioural effects in mice, demonstrating that while aberrant behavior was reversed by fluoxetine, the drug failed to alter most of the underlying stress-induced biological effects (Reference Surget, Wang and Leman74).
In conclusion, exposing FSL rats to TDS resulted in either bolstered or sustained reduction in coping and an increase in depressive-like behaviours, combined with altered monoaminergic profiles in hippocampal and frontocortical brain regions. Furthermore, the addition of TDS to FSL rats significantly abrogated the antidepressant-like effects of IMI at most behavioural levels (climbing and immobility) and with respect to limbic 5HT. Data presented here therefore supports the proposed hypothesis that exposure of a genetic animal model of depression to a PTSD-like paradigm results in a more severe depressive-like profile that is resistant to traditionally effective antidepressant treatment. The results of the current study have potential value in the search for a suitable animal model of TRD and warrants further investigation. Challenging FSL+TDS animals with first-line antidepressants (serotonin selective reuptake inhibitor or SSRI, or noradrenaline serotonin reuptake inhibitor or NSRI) and/or ketamine would expand predictive validity, and is presented in a companion paper to this manuscript (Reference Brand and Harvey75).
Acknowledgements
The authors wish to thank Antoinette Fick (GLP Manager, Vivarium, North-West University), De Wet Wolmarans and Francois Viljoen (faculty members) for their support and guidance. Authors’ contributions: S.J.B. performed all behavioural procedures, including treatment of the animals, performed all neurochemical analyses, undertook the statistical analysis, and prepared the first draft as well as the final version of the manuscript. B.H.H. devised the concept of the study as well as the layout of the manuscript, and finalised the pre-submission version of the manuscript.
Financial Support
The authors declare that this work has been funded by the South African Medical Research Council (MRC) (BHH) and the National Research Foundation (NRF) (BHH; grant number 77323). The grant holder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by NRF supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard. These funders have no other role in this study.
Conflicts of Interest
The authors declare that over the past 3 years, Brian Harvey has participated in advisory boards and received honoraria from Servier®, and has received research funding from Servier® and Lundbeck®. The authors declare that, except for income from the primary employer and research funding to BHH from the MRC, NRF, or the above-mentioned exceptions, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional services, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.