Sepiolite is a fibrous clay mineral with an ideal chemical formula per half unit cell of Si12O30Mg8(OH)4(OH2)4.xH2O, where x is usually 8 and decreases depending on the drying temperature and applied vacuum (Brauner & Preisinger, Reference Brauner and Preisinger1959). The xH2O, (OH2)4 and (OH)4 groups are zeolitic water, hydroxyls along the edges of the octahedral strip and hydroxyls in the inner octahedral strips, respectively (Ahlrichs et al., Reference Ahlrichs, Serna and Serratosa1975; Prost, Reference Prost1975; Kiyohiro & Otsuka, Reference Kiyohiro and Otsuka1989; Frost & Ding, Reference Frost and Ding2003). Zeolitic water occupies the inter-channels via consecutive multi-molecular adsorption and then capillary condensation. Physical bonds between the magnesium cations and hydroxyls along the edges of the octahedral strip originate from the ion-dipole attractive forces. By contrast, covalent bonds exist between the structural hydroxyls and magnesium atoms. Synthetic sepiolite may be synthesized from SiO2 gel and magnesium chloride (Jeans, Reference Jeans1971; Aetahi, Reference Aetahi1985).
In the past, several studies have been carried out on the dehydration, dehydroxylation and decomposition of sepiolite (Perraki & Orfanoudaki, Reference Perraki and Orfanoudaki2008; Ogorodova et al., Reference Ogorodova, Kiseleva, Vigasina, Kabalov, Grishchenko and Mel'chakova2014; Wang et al., Reference Wang, Liang, Tang, Chen and Chen2014). The changes in the various physicochemical properties of sepiolite after heating in air and under vacuum have been examined extensively (McCarter et al., Reference McCarter, Krieger and Heinemann1950; Kulbicki, Reference Kulbicki1959; Dandy & Nadiye-Tabbiruka, Reference Dandy and Nadiye-Tabbiruka1975; Shuali et al., Reference Shuali, Yariv, Steinberg and Müller-Vanmoos1991). The effects of heating, acid leaching and various chemical treatments on the crystal structure and properties of sepiolite have been discussed qualitatively (Serna et al., Reference Serna, Ahlrichs and Serratosa1975; Yebra-Rodriquez et al., Reference Yebra-Rodriquez, Martin-Ramos, Del Rey, Viseras and Lopez-Galindo2003; Yılmaz et al., Reference Yılmaz, Kalpaklı and Pişkin2013, Fitaroni et al., Reference Fitaroni, Venâncio, Tanaka, Gimenez, Costa and Cruz2019). Sepiolite has been used widely in many processes, such as adsorption, catalysis, bleaching of edible oils, clarification of fruit juices, preparation of polymer nanocomposites and pharmaceutical applications (Galan, Reference Galan1996; Murray, Reference Murray1999; Saneeri et al., Reference Saneeri, Goli and Keramat2015, González-Santamaria, Reference González-Santamaria, Lopez, Ruiz, Fernández, Ortega and Cuevas2017; Mirzaaghaei et al., Reference Mirzaaghaei, Goli and Fathi2017; Al-Ani et al., Reference Al-Ani A. and & Zholobenko2018; Tian et al., Reference Tian, Han, Wang, Liang, Wang and Wang2019).
Major sepiolite deposits occur in the USA, Spain, France and Turkey. The geology and genesis of sepiolite deposits mined in the Eskişehir (Turkey) zone have been investigated extensively (Ece & Çoban, Reference Ece and Çoban1994). The Eskişehir sepiolite, known as pipestone, has a white colour. The physicochemical properties of this material, such as the chemical and mineralogical composition, thermal shrinkage, particle morphology and size distribution of nano- and macro-pores, have been determined (Yener et al., Reference Yener, Önal, Üstünışık and Sarıkaya2007; Önal et al., Reference Önal, Yılmaz and Sarıkaya2008). In addition, the modification of some of these properties depending on the applied acid treatment has been investigated (Çetişli & Gedikbey, Reference Çetişli and Gedikbey1990; Inukai et al., Reference Inukai, Miyawaki, Tomura, Shimosaka and Irkeç1994; Erdoğan et al., Reference Erdoğan, Demirci and Akay1996), and the influence of heating on the crystal structure, porosity, sintering surface structure and catalytic properties of sepiolite have been discussed (Göktaş et al., Reference Göktaş, Mısırlı and Baykara1997; Balcı, Reference Balcı1999; Yılmaz et al., Reference Yılmaz, Kalpaklı and Pişkin2013; Meşe et al., Reference Meşe, Figen, Filiz and Pişkin2018). Despite there being several experimental and theoretical interpretations, a numerical analysis of the thermal degradation of sepiolite crystals has not yet been performed extensively. Thus, the aim of this contribution is to use thermal analysis data to estimate some of the physicochemical parameters of the thermal degradation of sepiolite that accompany dehydration and dehydroxylation.
Material and methods
A sample of Eskişehir sepiolite was ground gently in a mortar and air dried at room temperature for 1 week. The air-dried samples, each having a mass of 10 g, were heated at pre-set temperatures within the range of 25–1000°C for periods of 4 h.
The chemical analysis of the sample after heating at 100°C for 2 h was carried out using a Hitachi Z-8200 Atomic Absorption Spectrophotometer. The X-ray diffraction (XRD) trace of the natural sepiolite was obtained from random mounts using a Rigaku D-Max 2200 Powder Diffractometer with Cu-Kα radiation and a Ni filter. The morphology of the sample was examined by scanning electron microscopy (SEM) using a JEOL JSM-490LV instrument operating at an accelerating voltage of 15 kV. Thermogravimetric and differential thermal analysis (TG/DTA) curves were recorded for the natural and heated samples between 25°C and 1000°C under atmospheric pressure with a heating rate of 10°C min–1 using a Setaram Instrument (Labsys SETSYS-1780).
Results and discussion
Mineralogy, morphology and chemical composition of the sepiolite
A SEM image and XRD trace of the sepiolite are shown in Fig. 1. The SEM image shows the fibrous morphology and porous texture of the sepiolite. The XRD trace indicates that the sepiolite contains minor dolomite (CaMg(CO3)2) impurities. The chemical analysis of the sepiolite (mass%) was: SiO2 (57.60), MgO (25.06), Al2O3 (1.85), Fe2O3 (0.55), TiO2 (0.13), CaO (0.86), Na2O (0.08), K2O (0.15) and loss on ignition (13.20). The abundance of dolomite was estimated to be 3% from the CaO content. The Al2O3 and Fe2O3 are due to the partial replacement of Mg2+ by Al3+, Fe2+ and Fe3+ in sepiolite.
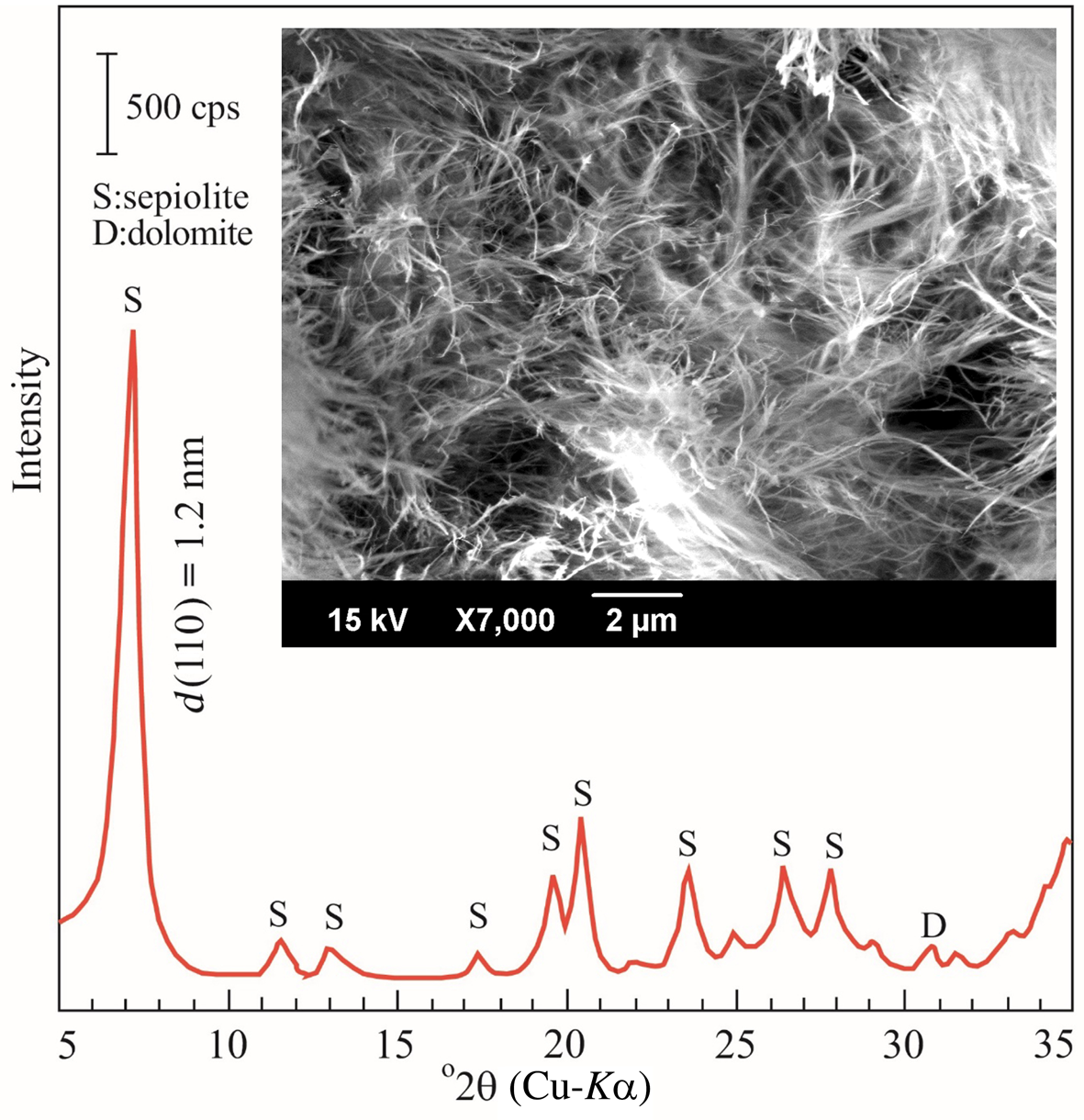
Fig. 1. XRD trace and SEM image of the sepiolite.
Thermal analysis
The TG/DTA curve of the sepiolite is given in Fig. 2. The temperature intervals and mass-loss percentages for the dehydration, dehydroxylation, thermal degradation and thermal decomposition of the sepiolite and decomposition dolomite are also shown in Fig. 2. The removal of hydroxyls along the edges of the octahedral strip occurs in two steps and the removal of hydroxyls from the inner octahedral strips of the sepiolite are endothermic, whereas its thermal decomposition is strongly exothermic (Fig. 2).

Fig. 2. Thermal degradation steps of the sepiolite as shown in the TG/DTA curves.
The TG/DTA curves of the heated samples showed that the exothermic heat flow for the thermal decomposition decreases at preheating temperatures >250°C (Fig. 3). The heights (h) of the heat-flow peaks for various preheating temperatures are listed in Table 1. The plot of h against temperature showed that the structure of the sepiolite remained unchanged at <250°C and then degraded linearly (Fig. 4). The equation and slope for the straight line obtained were determined from Eqs (1) and (2), respectively:
 $$h ={-}0.051\hskip-1pt T + 51.867$$
$$h ={-}0.051\hskip-1pt T + 51.867$$ $$\lpar \partial h/\partial T\rpar _p ={-}0.051\;{\rm mW}\;{\rm K^{-1}}$$
$$\lpar \partial h/\partial T\rpar _p ={-}0.051\;{\rm mW}\;{\rm K^{-1}}$$where the subscript p represents the atmospheric pressure, which is constant in the thermal treatments.

Fig. 3. Heat flow (h) vs increasing preheating temperature for the exothermic thermal decomposition of the sepiolite.

Fig. 4. Heat flow (h) vs absolute preheating temperature (T) for the thermal decomposition of the sepiolite.
Table 1. Kinetic and thermodynamic parameters obtained from the thermal analysis of the sepiolite (k = −(∂h/∂T)/h, h 0 = 24 mW and (∂h/∂T)p = −0.051 mW/K).

Kinetic considerations
A thermal degradation coefficient is proposed from Eq. (3):
 $$k ={-}\displaystyle{1 \over h}\;\left({\displaystyle{{\partial h} \over {\partial T}}} \right)_p$$
$$k ={-}\displaystyle{1 \over h}\;\left({\displaystyle{{\partial h} \over {\partial T}}} \right)_p$$by analogy from the thermal expansion and isothermal compression coefficients. As (∂h/∂T)p < 0, the negative sign is incorporated into the definition so that k is positive. This is not required in the case of this derivative being positive.
The k and lnk values for each preheating temperature calculated from Eq. (3) are listed in Table 1. The projection of lnk against 1/T shows three consecutive intersecting straight lines (Fig. 5). The presence of straight lines suggests that the k coefficient might be considered to be similar to the reaction rate constant. Thus, each straight line should fit to the Arrhenius equation in Eq. (4):
 $${\rm ln}k = \ln \hskip-1.5pt A-E^\#/{\rm R}T$$
$${\rm ln}k = \ln \hskip-1.5pt A-E^\#/{\rm R}T$$where A is the pre-exponential frequency factor, E # is the activation energy (J mol–1), T is the absolute temperature (K) and R = 8314 J mol–1 K–1 is the universal gas constant.
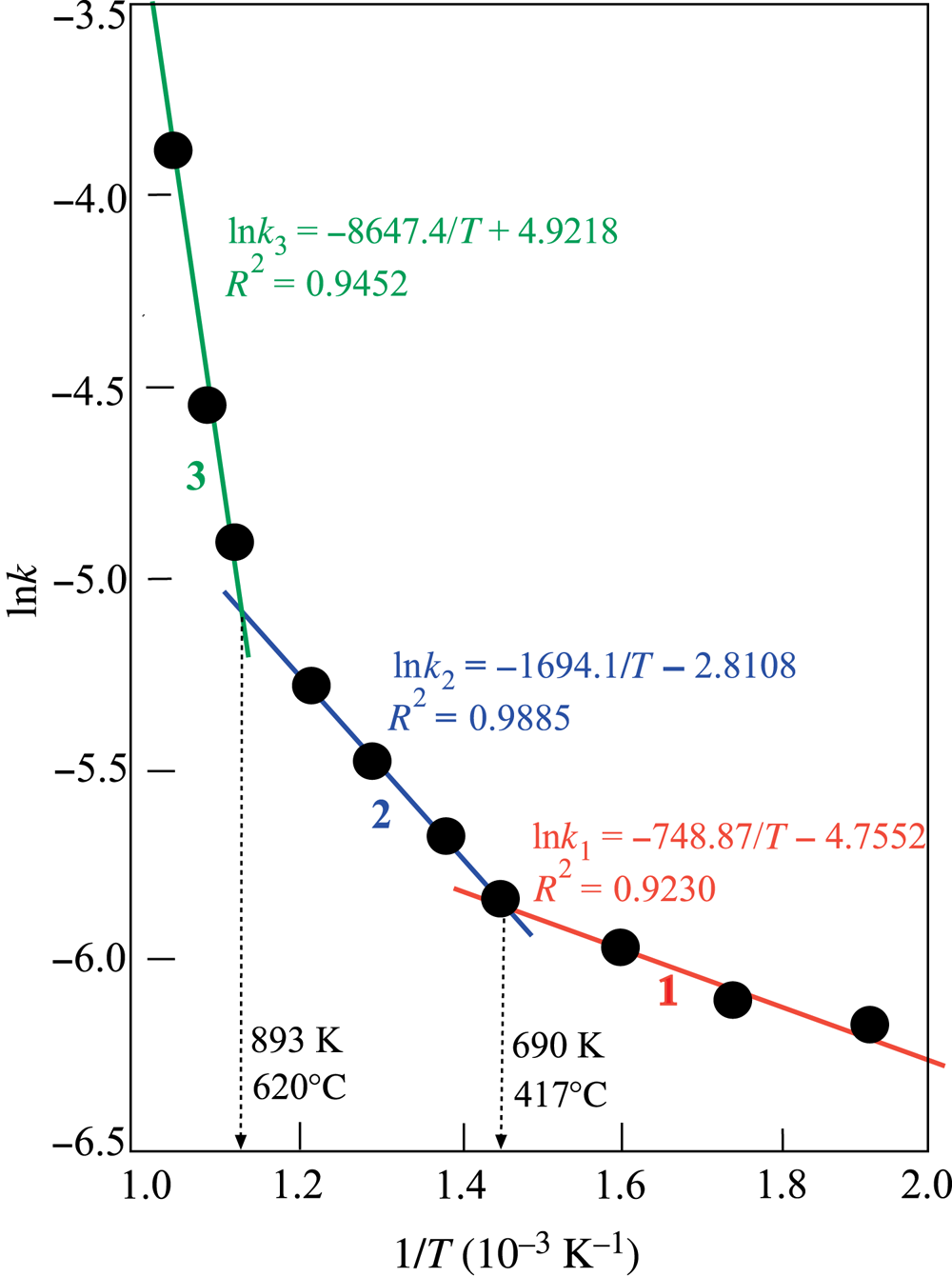
Fig. 5. Arrhenius graphs showing three intersecting straight lines obtained from lnk vs 1/T.
The intersect temperatures and the equations of the straight lines are shown in Fig. 5. The first term of each equation should be equal to the lnA value. The corresponding E # values were calculated from the slopes, being equal to –E #/R. Using the calculated lnA and E # values, three Arrhenius equations (Eqs 5–7) and corresponding temperature intervals were obtained:
 $${\rm ln}k_1 ={-}4.7552-6226/{\rm R}T\comma \;\;\;\;\;250\ndash 417^\circ {\rm C}$$
$${\rm ln}k_1 ={-}4.7552-6226/{\rm R}T\comma \;\;\;\;\;250\ndash 417^\circ {\rm C}$$ $${\rm ln}k_2 ={-}2.8108-16\;941/{\rm R}T\comma \;\;\;\;417\ndash 620^\circ {\rm C}$$
$${\rm ln}k_2 ={-}2.8108-16\;941/{\rm R}T\comma \;\;\;\;417\ndash 620^\circ {\rm C}$$ $${\rm ln}k_3 = 4.9218-71\;894/{\rm R}T\comma \;\;\;\;\,\;620\ndash 730^\circ {\rm C}$$
$${\rm ln}k_3 = 4.9218-71\;894/{\rm R}T\comma \;\;\;\;\,\;620\ndash 730^\circ {\rm C}$$The three different activation energies and the corresponding Arrhenius equations indicated that the change proceeds via three consecutive mechanisms that result in the thermal degradation of sepiolite.
The first and second mechanisms result from the two consecutive stages of removal of hydroxyls along the edges of the octahedral strip. The various activation energies are attributed to the various positions and orientations of the H2O molecules physically bonded to magnesium cations in the intra-fibre channels of the sepiolite crystal. The greater value of E 3# compared to E 1# and E 2# is due to the chemical nature of the dehydroxylation, whereas dehydration is a physical process. Similar results were obtained using the intensity of the most characteristic XRD reflection of sepiolite (110) as a kinetic variable (Kiyohiro & Otsuka, Reference Kiyohiro and Otsuka1989; Perraki & Orfanoudaki, Reference Perraki and Orfanoudaki2008; Sarıkaya et al., Reference Sarıkaya, Önal and Pekdemir2019; Tian et al., Reference Tian, Han, Wang, Liang, Wang and Wang2019).
Conclusions
The temperature-dependent h of any exothermic or endothermic heat-flow peak on the DTA curve may be used to calculate the kinetic parameters of the thermal degradation of minerals. The volume, density, porosity, surface area, micro-hardness, intensity of any XRD peak and other mechanical properties that change linearly with temperature may also be used as system variables instead of the peak h of the heat flow. The possible apparent activation energies as well as the path of degradation with one or more step(s) could be evaluated from a thermal coefficient that is comparable to a reaction rate constant.
Financial support
This research was supported by Ankara University Scientific Research Projects Coordination Unit (Project No.: 12B4240016).








