Published online by Cambridge University Press: 13 May 2004
The host immune response has profound effects on parasitic nematode infections. Here we have investigated how a range of infection parameters are affected by host immune responses and by their suppression and enhancement. The infection parameters considered were the number of parasitic females, their size, per capita fecundity and intestinal position. We found that in immunosuppressive treatments worms persist in the gut, sometimes with a greater per capita fecundity, maintain their size and have a more anterior gut position, compared with worms from control animals. In immunization treatments there are fewer worms in the gut, sometimes with a lower per capita fecundity and they are shorter and have a more posterior gut position, compared with worms from control animals. Worms from animals immunosuppressed by corticosteroid treatment reverse their changes in size and gut position. This description of these phenomena pave the way for a molecular biological analysis of how these changes in infection parameters are brought about by the host immune response.
The host immune response has profound effects on parasitic nematodes. With the parasitic nematode Strongyloides ratti detailed ultrastructural observations of worms early and late in a primary infection found that later in an infection there were degenerate changes in a range of worm tissues, such as the accumulation of lipid droplets and dense granules in intestinal cells (Moqbel & McLaren, 1980). As infections progressed there was evidence of oral plugs which it was thought may inhibit worm feeding, and which may result in the degenerate ultrastructure changes (Moqbel & McLaren, 1980). As infections proceed worms were found to be substantially shorter, to have a reduced diameter, that their reproductive tract was reduced in size and that worms become more posteriorly positioned in the gut (Moqbel & McLaren, 1980; Kimura et al. 1999). Experiments in which S. ratti parasitic adults from a primary infection which had been damaged by the immune response were transplanted to naïve hosts showed that the worm damage could be reversed (Moqbel, McLaren & Wakelin, 1980). Similarly, with S. stercoralis in dogs, parasitic females become damaged and their fecundity decreases as a primary infection proceeds, but this is reversed by transplant to naïve dogs (Schad et al. 1997). The numbers of eggs in utero have been used as a measure of worm fecundity, with a maximum of 8·36 at day 9 (Kimura et al. 1999) and 8·60 at day 10 p.i. (Moqbel & McLaren, 1980). However, the relationship between this measure of fecundity and the per capita fecundity as measured by eggs passed in faeces (Paterson & Viney, 2002) is unclear. For example, a reduction in the number of eggs in utero could be due to an increase in the rate at which eggs are laid or due to a reduction in the rate at which eggs are produced (and hence deposited in the uterus), or a combination of both these effects. Analysis of the later (day 25 p.i.) phase of S. ratti infections have found worms present in the caecum and colon that continue to produce eggs, though this effect was dependent on the infective dose used (Kimura et al. 1999).
Effects of the host immune responses on worm fecundity have been observed with other nematodes. Variation between lambs in their faecal output of Teladorsagia circumcincta eggs is principally due to variation between hosts in worm length rather than variation in worm number (Stear, Park & Bishop, 1995a; Stear et al. 1997). In experimental infections there is a significant association between T. circumcincta-specific IgA responses, abomasal eosinophil and globule leucocyte density and worm length (with globule leucocytes also affecting worm number) (Stear et al. 1995a,b) which suggests that different features of the immune response affect different aspects of worm fitness. The intestinal position of Trichinella spiralis in mice becomes more posterior and there is a reduction in the per capita fecundity as an infection progresses and these effects are reversed upon transplant to naïve hosts (Kennedy, 1980; Kennedy & Bruce, 1981).
The purpose of this work was to investigate how a range of S. ratti infection parameters (namely the number of parasitic females in the gut, their size, their per capita fecundity and their intestinal position) are affected by different immune manipulations. These are a more extensive range of parameters of infection, compared with previous work. We have also used a greater range of immune manipulations, namely, immunosuppression by infection of nude (T cell deficient) rats or the administration of corticosteroids and ‘immune enhancement’ by immunization with the pre-intestinal, migratory phase or with the migratory and intestinal phase of S. ratti (Gemmill, Viney & Read, 1997; Harvey et al. 2000; Paterson & Viney, 2002). Understanding how different infection parameters are affected by manipulation of the host immune response may lead to a greater understanding of the functional relationship between these parameters. This is complimentary to other work which is generating S. ratti DNA microarrays which will be used to determine the effect of the host immune response on qualitative and quantitative changes in gene expression with the aim of elucidating the basis of the observed changes in infection parameters. This is important, because it is not clear how the host immune response changes worm morphology, size and fecundity (Viney, 2002b).
The isofemale line ED321 Heterogonic was used throughout; worm lines were maintained by serial passage in female Wistar rats (B & K Universal), with food and water provided ad libitum; all infections were initiated by the subcutaneous injection of 1000 infective 3rd-stage larvae (iL3s), unless otherwise stated. Faecal material was collected from rats overnight in grid-bottomed cages and was cultured and maintained at 19 °C. To determine the reproductive output of an infection (Paterson & Viney, 2002), the total number of worms that developed in such cultures was determined.
To recover parasitic females, rats were sacrificed at various times post-infection (as detailed in specific experiments, below), the small intestine removed, divided into 3 lengths, slit longitudinally, rinsed in pre-warmed RPMI and hung in 100 ml measuring cylinders filled with fresh, pre-warmed RPMI and maintained at 37 °C. After 1 h, the intestines were removed to fresh RPMI for a further hour. In both cases, the RPMI from which the guts had been removed was centrifuged to collect the worms. This pellet was placed on a 4[ratio ]1[ratio ]3, 60[ratio ]50[ratio ]40% (v/v) Percoll gradient in RPMI and centrifuged at 850 g, after which worms concentrated in the 50% fraction and debris in the remaining fractions. The worms were further washed by centrifugation and resuspension in two changes of fresh RPMI and were then fixed in steaming 10% (v/v) formal saline and prepared for measurement as described by Viney, Ashford & Barnish (1991).
To determine the intestinal location of worms, the small intestine was removed from sacrificed animals at various times post-infection (p.i.) or post-challenge (p.c.) (as detailed in specific experiments, below) and stored at −20 °C. Defrosted intestines were divided into 4 cm long sections from the pyloric sphincter to the start of the caecum and slit longitudinally, the digesta removed, the gut sections squashed between two glass plates and viewed with a dissecting microscope and the number and position of worms recorded.
Infections were established in immune manipulated hosts to determine the effect of the host immune response on S. ratti.
Infections were established in 15 nude (HsdHan:RNU-rnu) (Harlan, UK) and 15 control Wistar rats and sacrificed at 6, 15, 20, 32 and 43 days p.i. Three rats were sacrificed from each group at each time-point, of which 2 were used to determine the intestinal position of parasitic females and one was used to recover parasitic females. All rats were maintained on water containing 0·01% (v/v) Baytril (Bayer), a broad-spectrum antimicrobial agent.
Infections were established in 60 rats of which 36 were administered intraperitonealy daily with 10 mg Betamethasone (Betsolan, Schering-Plough Animal-Health, UK) per kg body weight from 15 to 19 days p.i., inclusive; 24 control animals were administered with PBS contemporaneously. Faecal material was collected at 14, 17, 20, 24, 28 and 31 days p.i. to determine the reproductive output of the infection. Animals were sacrificed at days 15, 18, 21, 25, 29 and 32 p.i. Six corticosteroid-treated rats and 4 control rats were sacrificed at each time-point, of which 4 corticosteroid-treated and 2 control animals were used to determine the intestinal position of parasitic females and the remaining 2 from each group were used to recover parasitic females. All rats were maintained on water supplemented with Baytril throughout, as above. The per capita fecundity of parasitic females was calculated as the reproductive output of an infection (at day×p.i.) divided by the number of parasitic females recovered (at day ×+1 p.i.).
Animals were immunized so that they were exposed to both the migratory larval and adult intestinal stages of S. ratti. To do this, 35 animals were infected with 100 iL3s; 21 control animals were given a sham inoculation. On days 11 and 12 p.i., all animals were treated with thiabendazole orally, as described previously (Paterson & Viney, 2002). All animals were given a challenge infection of 1000 iL3s on day 14 p.i. and rats were sacrificed on days 5, 6, 7, 8, 9, 11 and 14 p.c. Five immunized and 3 control animals were sacrificed at each time-point, of which 3 immunized and 2 controls were used to determine the intestinal position of parasitic females and the remaining 2 immunized and 1 control were used to recover parasitic females.
Animals were immunized so that they were exposed to the migratory larval phase of S. ratti only. To do this, 35 animals were infected with 100 iL3s; 21 control animals were given a sham inoculation. On days 2 and 3 p.i., all animals were treated with thiabendazole orally, as above, and the efficacy of this against migrating larvae was confirmed by faecal examination at day 6 p.i. All animals were given a challenge infection of 1000 iL3s on day 14 p.i. Faecal material was collected at days 4, 5, 6, 7, 8, 11 and 14 p.c. to determine the reproductive output of the infection. Animals were sacrificed at days 5, 6, 7, 8, 9, 12 and 15 p.c. The numbers of animals used to determine the intestinal position and to recover parasitic females at each time-point was the same as the long immunization experiment, above. The per capita fecundity of parasitic females was calculated as described for the corticosteroid treatment (above).
Worm length, the number of parasitic females and their per capita fecundity with respect to TREATMENT (immune manipulation vs. control) and TIME (days p.i.) were analysed by ANOVA. Means are ±S.E.M. throughout.
To investigate the effect of immune manipulation on the intestinal position of worms, for each rat the cumulative number of worms along the gut was calculated and expressed as a percentage of the total number of worms present and plotted against the cumulative percentage of gut which contained all those worms. Inspection of plots of these data showed that there is an essentially linear relationship between these measures for the approximate anterior-most 90% of worms, after which there is an asymptotic relationship (Fig. 1). A regression analysis of the non-asymptotic part of this relationship (i.e. for the cumulative distribution of the anterior-most 90% of worms) for rats within a treatment group was performed. To test whether the slopes of regression lines were different we used a two-tail t-test, in which t was calculated as the difference in the slopes divided by the standard error of the difference in the slopes with (n1−2)+(n2−2) degrees of freedom, where n1 and n2 are the sample sizes (Zar, 1999). To determine the proportion of worms to use to calculate the slope of the non-asymptotic part of the distribution in all analyses, the slopes of regression lines of 60, 70, 80, 90 and 100% of worms against gut length were compared for infections in naïve Wistar rats at 6 days p.i. This showed that there was no difference between slopes calculated for 60 and 70%, 70 and 80% and 80 and 90% (t22=0·422, P=0·60; t25=0·778, P>0·40; t25=0·239, P>0·80, respectively) of worms, but that there was in comparisons of 90 and 100% and 80 and 100% (t54=4·52, P<0·001; t50=3·087, P<0·01, respectively) of worms. Animals from whom less than 50 parasitic females were recovered were excluded from this analysis. All analyses were performed using the statistical package JMP (SAS Institute Inc.). A significance level of P<0·01 was used throughout.

Fig. 1. The relationship between the cumulative percentage of parasitic females along the cumulative percentage gut length of 2 Wistar rats at 6 days p.i. (solid lines) and a regression line of the anterior-most 90% of worms (dotted line).
Data for the length of parasitic females and their intestinal position are presented for all 4 immune manipulations. Data for the number of parasitic females present in the gut and their per capita fecundity are presented for the corticosteroid and short immunization treatments only. Data for these parameters in nude rats and in the long immunization regime are presented by Paterson & Viney (2002).
The mean lengths of parasitic females recovered from nude and normal, control rats at 6 days p.i. were not significantly different (2·42±0·024 and 2·43±0·015 mm, respectively) (F1,47=0·270, P=0·605) (Fig. 2A). As the infection progressed the worms recovered from nude rats maintained their size (there was no significant difference in the length of worms from 6 and 43 days p.i., F1,71=0·004, P=0·9498), whereas those from normal, control rats became progressively shorter, such that at day 20 p.i. worms were approximately 60% of their original length (1·48±0·014 mm), which is significantly different from their length at day 6 p.i. (F1,71=1631·2, P<0·0001). At day 32 p.i. too few worms, and at day 43 p.i. no worms, were recovered, from the normal, control rats to determine worm length.

Fig. 2. The mean (±S.E.M.) length (mm) of worms from animals immune manipulated by (A) use of nude rats, (B) corticosteroid administration (arrow-heads show time-range of corticosteroid administration), (C) long immunization and (D) short immunization (solid lines) and respective controls (dotted lines) at days p.i. (A and B) and p.c. (C and D).
The mean length of parasitic females from rats at day 15 p.i. prior to administration of corticosteroids was 1·62±0·022 mm (Fig. 2B). After administration of corticosteroids the mean length increased to a maximum of 2·55±0·030 mm at day 25 p.i., which was significantly greater than the length at day 15 p.i. (F1,38=607·38, P<0·0001), and that of worms from control rats at day 25 p.i., which were 1·37±0·0369 mm long (F1,32=610·46, P<0·0001). The length of worms from the corticosteroid-treated animals after the end of the treatment subsequently decreased so that at day 32 p.i. their mean length was 1·47±0·033 mm. This shows that the host immune response brings about a reduction in the length of parasitic females, and that this reduction in length is reversible following the administration of corticosteroids.
There was a significant difference in the length of worms from immunized animals compared with non-immunized, control animals (TREATMENTF1,263=1179·6, P<0·0001), which was also affected by the duration of infection (TIMEF6,263=25·4, P<0·0001; TREATMENT*TIMEF6,236=13·98, P<0·0001) (Fig. 2C). The mean length of worms from immunized rats at 5 days p.c. was 1·75±0·046 mm, which is significantly shorter than worms from non-immunized, control rats (2·35±0·024 mm, F1,36=133·07, P<0·0001). At 15 days p.c. the mean length of worms from immunized animals is (1·52±0·032 mm) which was significantly shorter than those from non-immunized, control animals (1·87±0·0422 mm, F1,38=42·84, P<0·0001). Thus, the effect of this immunization regime is that when worms established in the gut they were substantially shorter than worms in non-immunized, control rats and that as the infection progresses they got shorter still, as did worms in non-immunized, control rats.
There was a significant difference in the length of worms from immunized animals compared with non-immunized, control animals (TREATMENTF1,266=99·4, P<0·0001), which was also affected by the duration of infection (TIMEF6,266=25·6, P<0·0001; TREATMENT*TIMEF6,266=3·55, P<0·0021) (Fig. 2D). The mean length of worms from immunized rats at 5 days p.c. was 2·00±0·042 mm, which decreased significantly to 1·82±0·032 mm at day 15 p.c. (F1,38=9·817, P=0·0033). For days 5 to15 p.c. combined, worms from immunized rats (2·01±0·017 mm) were significantly shorter than those from non-immunized, control rats (2·20±0·017 mm) (F1,278=62·67, P<0·0001). Thus, the effect of this immunization regime was that when worms established in the gut they were substantially shorter than worms in non-immunized, control rats and that as the infection progresses they got shorter still, as did worms in non-immunized, control rats.
In infections in normal, control rats there was a significant difference in the distribution of worms at 6 and 15 days p.i. (t52=5·78, P<0·001) with a more posterior distribution at 15 days p.i. In infections in nude rats there was no significant difference in the distribution of worms between 6 and 15, 15 and 20, 20 and 32, 32 and 43 days p.i. (t42=0·722, P>0·05; t44=1·90, P>0·05; t47=0·138, P>0·05; t46=0·152, P>0·05, respectively) nor between worms at 6 and 43 days p.i. (t43=2·83, P>0·01). There was a significantly different distribution of worms between nude and normal, control rats both at 15 days, p.i. with a more anterior distribution in nude rats (t57=4·71, P<0·0001). Therefore in infections in nude rats there was no change in the position of worms during the infection from 6 to 43 days p.i., which is different to infections in normal immunocompetent rats in which the worms became more posteriorly positioned as the infection progressed from days 6 to 15 p.i.
In non-corticosteroid-treated, control rats there was a significantly different distribution of worms between 15 and 18 days p.i. (t52=4·89, P<0·001), with a more posterior distribution at 18 days p.i. In corticosteroid-treated rats there was a significant difference in the distribution between days 18 and 22 p.i. (t94=5·36, P<0·001) with a more anterior distribution at 22 days p.i., but there was no significant difference in distribution between days 22 and 25 p.i. (t74=0·77, P>0·05). After this the distribution of worms in corticosteroid-treated rats became more posterior, such that there was a significant difference in the distribution between days 25 and 29 p.i. (t84=4·32, P<0·001), but there was no significant difference between days 29 and 32 p.i. (t74=1·73, P>0·05). The distribution of worms at 22 days p.i. was not different from that at 15 days p.i. in non-corticosteroid-treated, control rats (t59=0·948, P>0·05). These data are consistent with worms in non-corticosteroid-treated, control rats moving to a more posterior position as the infection progressed. However, with corticosteroid treatment, assuming a delay in the action of the corticosteroid treatment, the worms moved to a more anterior position (22 and 25 days p.i.), after which (29 and 32 days p.i.), the worms resumed a more posterior distribution as the effect of the corticosteroid treatment waned.
There was a significant difference in the distribution of worms between immunized and non-immunized, control rats at 5, 6, 7, 8 and 9 days p.c. (t61=5·87, P<0·001; t60=5·57, P<0·001; t59=4·92, P<0·001; t48=5·90, P<0·001; t54=7·79, P<0·001, respectively) with a more anterior distribution in the non-immunized, control rats. There was no significant difference between the distribution of worms at 5 and 9 days p.c. in the immunized (t72=0·317, P>0·05) and non-immunized, control rats (t43=0·624, P>0·05). Therefore, the result of the long immunization is that worms established in the gut in a more posterior position compared to worms in non-immunized, control rats, but during the infection in immunized and non-immunized, control rats there was no change in the position of worms.
There was no significant difference in the distribution of worms between immunized and non-immunized, control rats at 5, 6, 7 and 8 days p.c. (t46=2·31, P>0·01; t49=1·64, P>0·05; t46=1·66, P>0·05; t53=0·4812, P>0·05, respectively) but there was a significantly different distribution at 9 and 12 days p.c. (t57=3·024, P<0·01; t68=4·2604, P<0·001, respectively) with a more anterior distribution in the non-immunized, control rats. Therefore, the short-immunization had no effect on the gut position of worms early in an infection, but latterly worms in immunized rats were in a more posterior position than those in control rats.
There was a significant difference in the number of parasitic females present in corticosteroid-treated and non-corticosteroid-treated, control rats (313±38·0 and 15·7±53·7, respectively, across days 18–32 p.i., inclusive) (TREATMENTF1,20=33·4, P<0·0001) but this did not change during infection (TIMEF4,20=2·39, P=0·085) (Fig. 3). This difference was due to loss of worms from non-corticosteroid-treated, control rats but their persistence in corticosteroid-treated rats. In treated rats there was no significant difference in the number of parasitic females present in corticosteroid-treated animals at days 18 and 22, 22 and 25, 25 and 29, (F1,6=0·705, P=0·433; F1,6=2·89, P=0·14; F1,6=0·63, P=0·457, respectively).

Fig. 3. The mean (−S.E.M.) number of parasitic females (solid lines) and their per capita fecundity (+S.E.M.) (dotted lines) in corticosteroid-treated ([bull ]) and non-corticosteroid-treated (×), control rats at days p.i. Arrow-heads show time-range of corticosteroid administration.
There was no significant difference in the per capita fecundity of worms between days 15 and 18 p.i. in corticosteroid-treated rats nor in non-corticosteroid-treated, control rats (F1,4=0·0347, P=0·861; F1,2=1·63, P=0·329, respectively) (Fig. 3). There was no significant difference between fecundity of worms from corticosteroid-treated and non-corticosteroid treated, control rats at day 18 p.i. (F1,4=0·564, P=0·49) and by day 22 p.i. the fecundity of worms from non-corticosteroid-treated, control rats was zero. In the corticosteroid-treated rats, there was no significant difference in the per capita fecundity of worms between days 18 and 22, 22 and 25, 25 and 29, 29 and 32 (F1,4=0·0347, P=0·861; F1,6=2·18, P=0·189; F1,6=1·00, P=0·35; F1,6=1·46, P=0·271; F1,6=0·804, P=0·402, respectively). Thus, the administration of corticosteroids allowed worms to persist and to maintain their fecundity when they would otherwise be removed and become less fecund.
There was a significant difference in the number of parasitic females present in immunized and non-immunized, control rats (128±18·6 and 189±26·6, respectively, across all time-points) (TREATMENTF1,21=13·77, P=0·0013) and this changes during the infection (TIMEF6,21=13·40, P<0·0001) (Fig. 4).

Fig. 4. The mean (+S.E.M.) number of parasitic females (solid lines) and their per capita fecundity (−S.E.M.) (dotted lines) in immunized (short regime) ([bull ]) and non-immunized (×), control rats at days p.c.
There was a significant difference in the per capita fecundity of worms from immunized and non-immunized, control rats (26±4·0 and 66±16·3, respectively, across all time-points) (TREATMENTF1,21=19·90, P=0·0002) and this changed during the infection (TIMEF6,21=8·03, P=0·0001). Thus, this immunization regime reduced the number of parasitic females present in the gut and their per capita fecundity.
All infection parameters investigated were affected by the host immune response. In normal (control) animals mounting an immune response parasitic females become progressively shorter as the infection (and hence the immune response) progressed. However, in nude rats, this process does not occur and worms maintain the same size during an infection. Parasitic females in animals that are being treated with corticosteroids reverse their reduction of length and regain their maximum length, which is equivalent to that seen in nude rats. ‘Immune enhancement’ by both immunization regimes resulted in worms that established in the gut at 5 days p.c. that were shorter than those in control animals and that worms got progressively shorter as the infections proceeded. The long immunization treatment had a greater effect on worm length than the short immunization regime. These effects of the immunization treatments suggest that immune responses against migrating iL3s and L4s negatively affects worm growth such that parasitic females at 5 days p.c. are significantly shorter compared with controls. In nematodes, pre-maturation tissue migration is associated with an increased size of adult worms and there is therefore a relationship between the optimal time of maturation and the mortality rate during this phase (Read & Skorping, 1995; Gemmill, Skorping & Read, 1999). This therefore predicts that when pre-maturation mortality is low, there will then be a longer maturation, which will result in greater adult size and fecundity and, alternatively, when pre-maturation mortality is high (e.g. due to host immunity) then shorter maturation time and smaller adults worms will occur (Guinnee et al. 2003). Therefore, an alternative explanation for the observation that parasitic females from immunized hosts are shorter than those from controls is that in immunized animals, migrating larvae facultatively shorten their pre-intestinal migration time, which has the consequence that they are smaller when they establish in the gut. This explanation also requires that these smaller worms matured more early, but this cannot be known because in these experiments observations only commenced at 5 days p.c. An unresolved question arising from these experiments is whether worm growth during the migration phase determines the maximum size that intestinal worms can ever achieve.
The effects of the host immune response on the intestinal position of parasitic females are broadly analogous to the effect on worm size. Thus, in normal (control) hosts mounting an immune response parasitic females become more posteriorly positioned in the gut as the infection (and hence the immune response) progresses. However, in nude rats, this process does not occur and worms maintain their intestinal position. Parasitic females in animals that are being treated with corticosteroids reverse their posterior movement. Both immunization regimes result in a more posterior position of worms, compared with controls, but the different regimes result in subtle differences. Thus, the long immunization treatment results in a more posterior position during all of the infection, whereas the short immunization treatment only results in a more posterior (than the control) position of worms later in the infection. The result from the immunization regimes implies that the, presumably deleterious, effects of the immune response during the migratory (and worm growth) phase of worms has consequences for the biology of adult parasitic females from their establishment in the gut (5 days p.c.).
The more posterior position of worms in the presence of the immune response is a population effect and as such reflects the distribution of worms over a greater extent of the gut, i.e. the anterior limit of worms is maintained. The reasons for this in response to the host immune response are unclear. It may be an adaptive behaviour by which movement along the gut positions worms in more profitable habitats due to, for example, better physiological conditions or food availability and, or reduced immune pressure (Sukhdeo & Bansemir, 1996). The manipulation of the immune response, and the administration of corticosteroids, may also have significant effects on the physiology, histology etc of the gut which may affect worm position. T. spiralis has been artificially selected over seven generations for ‘anterior’ or ‘posterior’ gut position which resulted in a change in the average gut position of the two populations (Sukhdeo & Bansemir, 1996). Given that the host conditions have remained constant during this selection, these results suggest that these parasites have changed their habitat preference. The intestinal position of Nippostrongylus brasiliensis becomes more anterior as an infection progresses (Brambell, 1965).
In S. ratti infections, the movement along the gut as the infection progresses also reduces the local, average S. ratti density, which may go someway to moderating the per capita effects of the immune response (Paterson & Viney, 2002). Alternatively, this posterior movement may be a reflection of the consequence of the host immune response. Thus, a proportion of worms that are under severe pressure and which will die shortly may then be unable to maintain their gut position and thus become more posteriorly positioned. However, the results of the corticosteroid treatments show that this situation, whatever its cause, is reversible. Kimura et al. (1999) observed fecund parasitic adults in the caecum and colon of rats late (>25 days p.i.) in a primary infection. We did not observe egg/larval production in infections of normal, control animals beyond day 25 p.i. All infections used in these experiments were initiated with 1000 infective larvae. This is a large dose compared with natural infections, but is less than used in other studies, which ranged from 4000 (Moqbel & McLaren, 1980) to 3000 or 8000 iL3s (Kimura et al. 1999). These different infective doses produced different results, with a greater temporal persistence of a fecund infection at a dose of 8000 iL3s compared with a dose of 3000 (Kimura et al. 1999). It is likely that these and other differences between our observations presented here and those of other studies are, at least in part, dose dependent.
The effects of the immune response, its suppression and enhancement, on worm length and gut position occur together, which suggests that their causation is the same. Intestinal mastocytosis is the prominent feature of Strongyloides infections (Miller, 1984; Abe & Nawa, 1988) and there is evidence for a role of interleukin-5 and eosinophils (Ovington et al. 1998; Korenga et al. 1991) in worm clearance. These effector processes are also likely to have very significant effects on the gut physiology, which may affect the position of worms (above). The effect on worm length could be brought about by the direct action of these effectors on the worms. However, the view we favour is that these effects are indirect and, thus, rather that the differences in worm size are, for example, a consequence of their reduced ability to feed (Moqbel & McLaren, 1980) or their expenditure of energy protecting themselves and recovering from immune attack (Viney, 2002a).
In hosts that are mounting an immune response (control animals and Paterson & Viney, 2002), the number of worms present in the gut declines. This process is halted in animals treated with corticosteroids. Immunization (short regime) reduces the number of worms present in the gut during an infection, which is similar to previous observations using a long immunization regime (Paterson & Viney, 2002). The per capita fecundity of worms also declines in hosts that are mounting an immune response (control animals and Paterson & Viney, 2002). This decline is not altered in animals treated with corticosteroids. However, immunization (short regime) does reduce the per capita fecundity. In several lines of S. ratti the survivorship of parasitic females and their fecundity at day 23 p.i. have been found to vary, which may reflect differences between these lines in their response to host immunity (Paterson & Viney, 2003). It is also possible to speculate that lines of S. ratti will vary in other infection parameters, such as changes in gut position due to the host immune response.
An alternative way to consider the relationship between worm length and fecundity is to calculate the per capita fecundity per mm of worm. For worms from corticosteroid-treated rats at 22 days p.i. (when length is maximum and equivalent to that from nude rats) and from non-corticosteroid-treated, control rats at 18 days p.i., when worm length is at a minimum shows that fecundity per length of worm is 56·4 and 22·09 eggs per mm worm, respectively. This suggests that the reduction in per capita fecundity that occurs as the immune response develops is not solely due to the reduction in worm length. Rather, it appears that as worms become shorter, apparently due to the host immune response, the per length fecundity of worms also reduces. Indeed, worms from non-corticosteroid-treated, control rats at day 22 p.i. have a mean length of 1·58 mm, but are no longer fecund.
This work has provided a detailed understanding of the behaviour of a range of infection parameters due to the host immune response and how these change in response to manipulations of the host immune status. These analyses do not explain how or why these changes occur which, therefore, remains to be investigated.
We would like to thank Sadie Iles-Ryan and Louise Phelon for their excellent technical support and Simon Harvey and Matt Crook for discussions. This work was supported by the Medical Research Council.
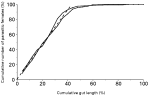
Fig. 1. The relationship between the cumulative percentage of parasitic females along the cumulative percentage gut length of 2 Wistar rats at 6 days p.i. (solid lines) and a regression line of the anterior-most 90% of worms (dotted line).

Fig. 2. The mean (±S.E.M.) length (mm) of worms from animals immune manipulated by (A) use of nude rats, (B) corticosteroid administration (arrow-heads show time-range of corticosteroid administration), (C) long immunization and (D) short immunization (solid lines) and respective controls (dotted lines) at days p.i. (A and B) and p.c. (C and D).
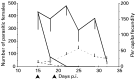
Fig. 3. The mean (−S.E.M.) number of parasitic females (solid lines) and their per capita fecundity (+S.E.M.) (dotted lines) in corticosteroid-treated ([bull ]) and non-corticosteroid-treated (×), control rats at days p.i. Arrow-heads show time-range of corticosteroid administration.

Fig. 4. The mean (+S.E.M.) number of parasitic females (solid lines) and their per capita fecundity (−S.E.M.) (dotted lines) in immunized (short regime) ([bull ]) and non-immunized (×), control rats at days p.c.