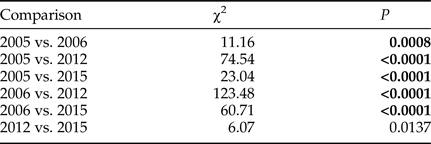Introduction
The timing of ecologically critical events like flowering, mating, migration, and appearance of different developmental stages has recently, and often dramatically, shifted in numerous taxa across diverse geographic regions (Bradley et al., Reference Bradley, Leopold, Ross and Huffaker1999; Hughes, Reference Hughes2000; Walther et al., Reference Walther, Post, Convey, Menzel, Parmesan, Beebee, Fromentin, Hoegh-Guldberg and Bairlein2002; Gordo & Sanz, Reference Gordo and Sanz2005; Menzel et al., Reference Menzel, Sparks, Estrella, Kochz, Aasa, Ahas, Alm-Kubler, Bissollik, Braslavska, Briede, Chmielewskizz, Crepinsek, Curnel, Dahl, Defila, Donnelly, Filella, Jatczak, Mage, Mestre, Nordli, Penuelas, Pirinen, Remisova, Scheifingerz, Striz, Susnik, Van Vliet, Wielgolaski, Zach and Zust2006; Schwartz et al., Reference Schwartz, Ahas and Aasa2006; Primack et al., Reference Primack, Ibáñez, Higuchi, Lee, Miller-Rushing, Wilson and Silander2009; Richardson et al., Reference Richardson, Keenan, Migliavacca, Ryu, Sonnentag and Toomey2013; Brown et al., Reference Brown, O'Connor, Poloczanska, Schoeman, Buckley, Burrows, Duarte, Halpern, Pandolfi, Parmesan and Richardson2016; Thackeray et al., Reference Thackeray, Henrys, Hemming, Bell, Botham, Burthe, Helaouet, Johns, Jones, Leech and Mackay2016). Examples include the earlier onset of avian migration (Cotton, Reference Cotton2003; Gill et al., Reference Gill, Alves, Sutherland, Appleton, Potts and Gunnarsson2014) and earlier (Moyes et al., Reference Moyes, Nussey, Clements, Guinness, Morris, Morris, Pemberton, Kruuk and Clutton-Brock2011) or prolonged (Qu et al., Reference Qu, Yang, Li, Chen, Mi, Xu and Zhang2016) mammalian breeding seasons than in previous decades. The direction and magnitude of these changes in phenology can vary among populations of the same species. This is seen in grasshoppers that exhibit delayed development in high altitude populations, but advanced development in low altitude populations (Buckley et al., Reference Buckley, Nufio, Kirk and Kingsolver2015). Such changes in biological timing are increasingly attributed to global climate change and human activity (Rosenzweig et al., Reference Rosenzweig, Karoly, Vicarelli, Neofotis, Wu, Casassa, Menzel, Root, Estrella, Seguin and Tryjanowski2008).
Shifts in phenology can impact ecological community structure, because different species may respond in different ways to environmental changes (Boggs, Reference Boggs2016). Such asynchrony can uncouple long-established ecological relationships among species. For example, Operophtera brumata caterpillars feed on young leaves of the oak Quercus robur. Egg hatch dates for the caterpillar have shifted 2 weeks earlier than the budburst dates of the oak, leading to starvation of caterpillars (Visser & Holleman, Reference Visser and Holleman2001). Phenological change in one species can also affect the timing of events in other species, for example, earlier availability of arthropod prey appears to have advanced the optimal breeding period for arctic birds (Tulp & Schekkerman, Reference Tulp and Schekkerman2008).
In this paper, we discuss a recent and apparently persistent shift in the reproductive phenology of a North American giant waterbug (Heteroptera: Belostomatidae), Belostoma flumineum Say. Belostomatids are generalist predators that inhabit freshwater ponds and small lakes, feeding on other aquatic insects, snails, small fish, and anuran larvae (Torre Bueno, Reference Torre Bueno1906). They are relatively K-selected insects, with large bodies, large eggs produced in relatively small broods, and extensive parental care of eggs by males (Lauck & Menke, Reference Lauck and Menke1961). Males of some species (subfamily Lethocerinae) guard eggs deposited on aquatic vegetation by females, but in other species, like B. flumineum (subfamily Belostomatinae), males carry eggs oviposited onto their dorsum by females (Smith, Reference Smith1980). Population growth in the Belostomatinae is therefore limited by, among other factors, the availability of male backspace during the breeding season (Kruse, Reference Kruse1990).
B. flumineum has usually exhibited two annual reproductive peaks in North America, first among overwintered adults in spring (April–May) followed by a second reproductive peak among young adults of a new generation (or generations) in the fall (August–October, Kruse, Reference Kruse1990; Kight et al., Reference Kight, Tanner and Coffey2011). We are not certain if the second (fall) reproductive peak is contained to a single generation of young adults. Flosi (Reference Flosi1980) describes populations of B. flumineum in central Iowa with two to three annual generations: a first generation produced in the spring by overwintered adults, a second generation produced in the fall by young first-generation adults, and a possible third generation also produced in the fall by the earliest second-generation adults. Flosi indicates that late second and third generation adults enter winter diapause before reproducing. It seems likely that adults reproducing in the fall would not survive to reproduce again in the spring, just as overwintered adults that breed in the spring do not appear to survive to reproduce again in the fall. This is consistent with a laboratory study by Gilg & Kruse (Reference Gilg and Kruse2003) in which reproduction was negatively associated with longevity in both male and female B. flumineum.
We have intermittently studied several local populations of B. flumineum in northern New Jersey, USA, over the last two decades (Kight et al., Reference Kight, Batino and Zhang2000, Reference Kight, Steelman, Coffey, Lucente and Castillo2008, Reference Kight, Tanner and Coffey2011). We typically collected specimens during the fall breeding peak and housed them in the laboratory for behavioural studies. However, in the fall of 2012, we encountered a dramatic change in field reproduction: despite normal sampling efforts, we collected no egg-bearing males between August and December. Here we report our observations of reproduction in these populations, under both field and laboratory conditions, in four separate years: 2005 and 2006, when the populations exhibited two reproductive peaks, and 2012 and 2015, when the populations did not exhibit a fall reproductive peak. We then discuss the potential significance of this change in reproduction for the population ecology of this K-selected insect predator.
Materials and methods
Field observations
We collected male and female B. flumineum between early August and December in 2005, 2006, 2012, and 2015. Collections typically occurred twice each week. Sampling locations included several ponds and small lakes in northeastern New Jersey, USA. The most frequently sampled site was Silver Lake (41°07′17.7″N, 74°31′58.3″W), but we also sampled other ponds within 10 km of this site. Because waterbugs tend to perch on underwater shoots of aquatic vegetation, we collected all specimens in shallow water (<1.5 m depth) near the shoreline by sweeping dip nets through patches of submerged vegetation. We collected approximately 50 specimens per sampling trip.
Laboratory observations
We transported collected waterbugs to the laboratory in plastic coolers containing pond water, aquatic vegetation, and odonate naiads as a temporary food source. In the laboratory, we housed waterbugs in controlled environmental chambers (15L:9D at 25 °C in 2005, 2006, 2015, and 14L:10D at 26–28 °C in 2012) in 40L glass aquaria (61 × 23 × 33 cm) containing dechlorinated tap water and plastic substrate for perching. Each aquarium held mixed-sex groups of approximately 20 males and 20 females, fed commercially available crickets (Acheta domesticus Linnaeus) and cockroaches (Blatta lateralis Walker) daily ad libitum.
We inspected aquaria each day for the appearance of eggs on the backs of males and recorded the date for each reproductive event. Brooding males were thereafter removed from the aquaria and studied in other experiments (Kight et al., Reference Kight, Steelman, Coffey, Lucente and Castillo2008, Reference Kight, Tanner and Coffey2011). To compare patterns of laboratory reproduction between the 4 years of study, we used the earliest date that an egg pad was observed on any captive male in any year as the Day 1 frame of reference for all years.
Statistical analysis
We analyzed data with JMP PRO (v. 11.0) statistical software (SAS Institute, Cary, North Carolina, USA). We used Product-Limit Survival Fit procedures to analyze cumulative egg-pad deposition in the laboratory, and compared data from different years with Wilcoxon tests between groups with α = 0.05. We used a more conservative Bonferroni correction to alpha (α = 0.0083) for six pairwise post-hoc comparisons between specific years.
Results
Field observations
At the beginning of each collection season (early August), there were several indications that waterbugs in our samples recently emerged and nulliparous adults. For example, young adults exhibit light green-brown exoskeletons that continue to darken over the lifespan. In all four study years, adults collected in August almost exclusively had the characteristic light pigmentation of recent eclosion. Furthermore, adult males captured in August rarely had egg pads in any study year. In 2005 and 2006, the earliest samples in August rarely included males with egg pads, but the frequency of egg brooding males increased in samples taken from September to October. Males collected in late October rarely had egg pads.
In contrast, we captured no egg-bearing young adult males in any sample taken between August and December in 2012 and 2015. Only on the very first sampling trip of 2012 (8 August 2012) did we collect a single egg-encumbered male, but its dark melanistic exoskeleton indicated it was an older male from the previous overwintered cohort. No other samples collected in August–December 2012 or 2015 included egg brooding males of any age.
The lack of field-collected egg-bearing young males in 2012 and 2015 suggests that fall reproduction at the study sites did not occur (or was so rare that it was not detected by the same sampling effort employed in 2005 and 2006). Nonetheless, each fall we collected large numbers of young adults, indicating that a spring reproductive season had previously occurred. To confirm spring reproduction, we sampled the collection sites from March 2016 until we began capturing egg-encumbered males on 12 May 2016.
Laboratory observations
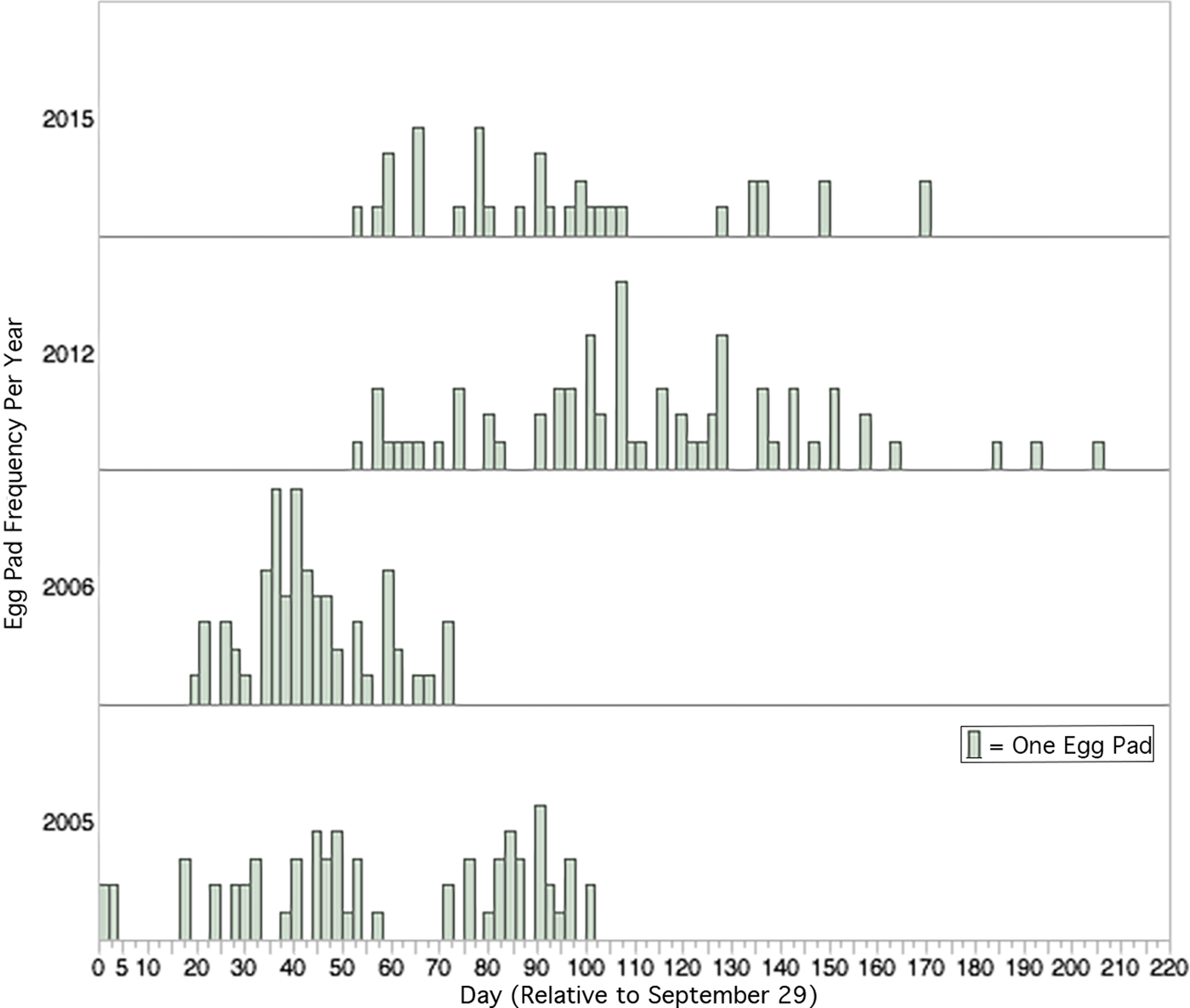
The earliest date that an egg pad was observed on any captive male was 29 September 2005, which we considered the Day 1 frame of reference. We dated all other reproductive events by the number of calendar days that had elapsed since 29 September of each respective year (fig. 1).
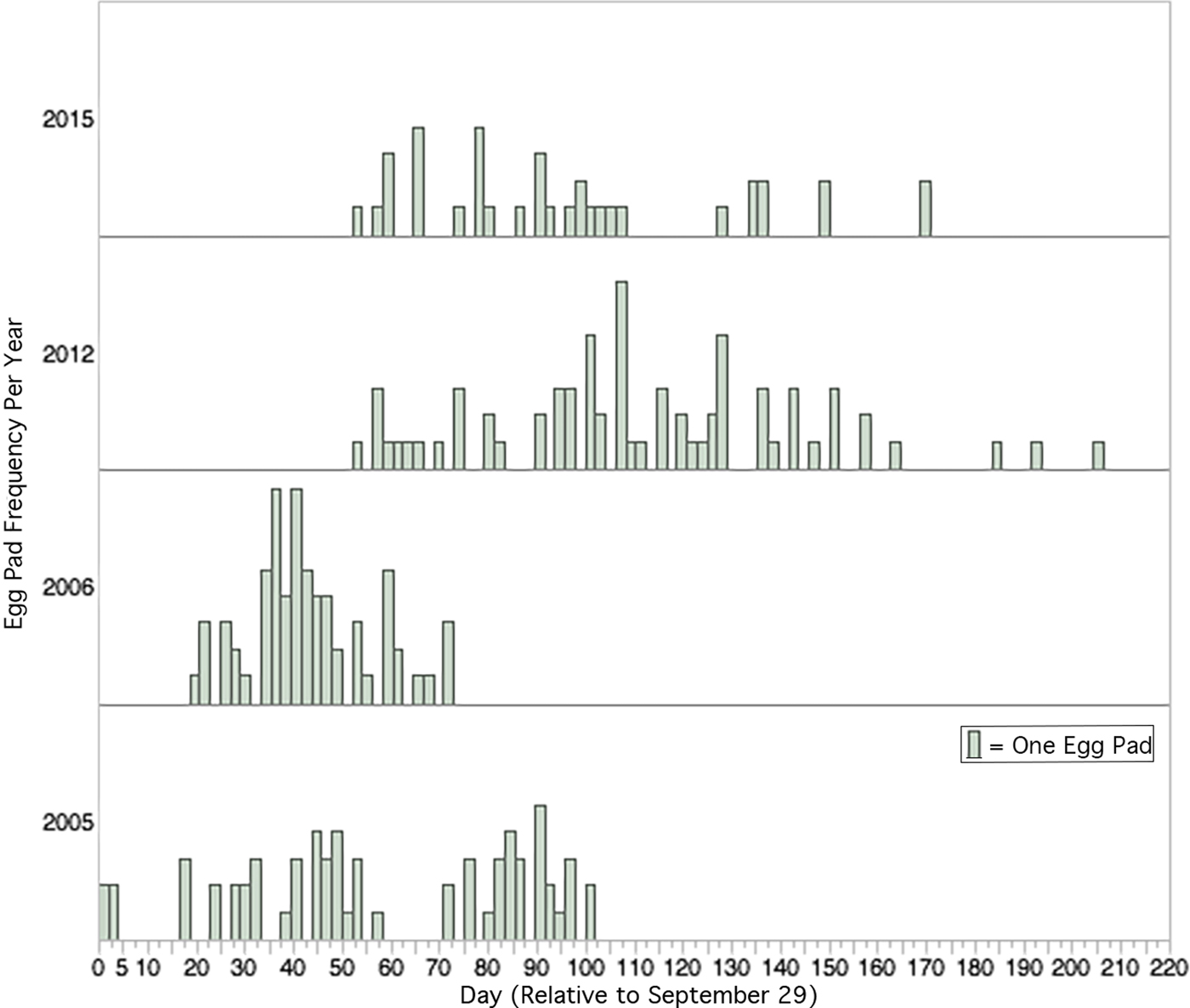
Fig. 1. Relative frequency of deposition of new egg pads in the laboratory (15L:9D at 25 °C in 2005, 2006, 2015, and 14L:10D at 26–28 °C in 2012). Day of egg deposition is relative to 29 September, the earliest date that laboratory egg deposition occurred in any year. Total number of egg pads each year was 2005, N = 65, 2006, N = 66, 2012, N = 70, 2015, N = 36.
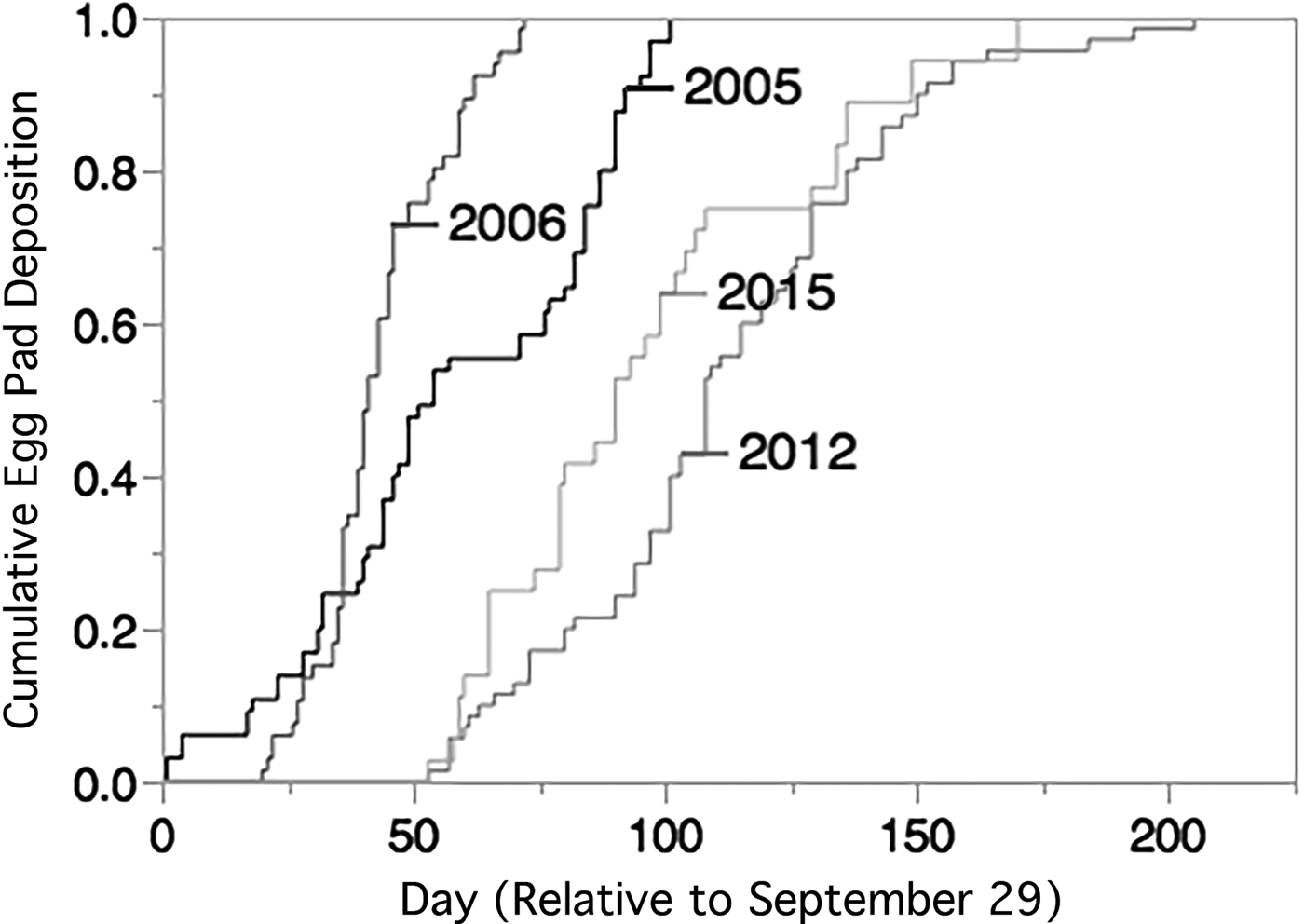
Cumulative egg pad deposition between the four sampled years was statistically significantly different (Wilcoxon Test, χ2 = 164.6612, DF = 3 P < 0.0001, fig. 2). Post hoc pairwise comparisons (with Bonferroni corrected α = 0.0083) between years indicated that cumulative egg pad deposition occurred significantly earlier in 2005 and 2006 than in 2012 and 2015 (table 1). There was also a significant difference in cumulative egg pad deposition between 2005 and 2006, but no significant difference between 2012 and 2016.
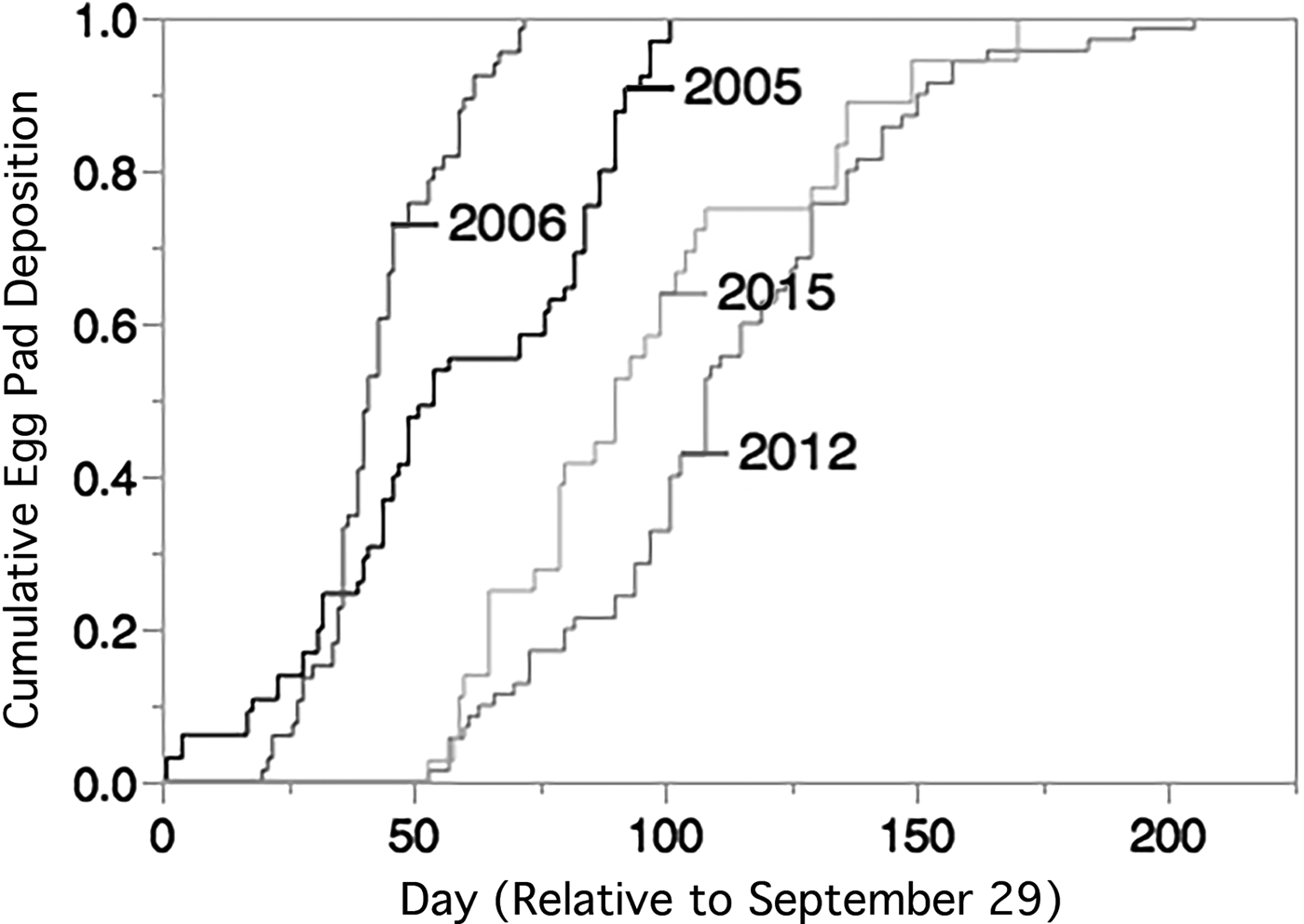
Fig. 2. Cumulative deposition of new egg pads in the laboratory (15L:9D at 25 °C in 2005, 2006, 2015, and 14L:10D at 26–28 °C in 2012). Day of egg deposition is relative to 29 September, the earliest date that laboratory egg deposition occurred in any year. Total number of egg pads each year was 2005, N = 65, 2006, N = 66, 2012, N = 70, 2015, N = 36.
Table 1. Pairwise statistical comparisons (Wilcoxon Test, DF = 1, Bonferroni corrected α = 0.0083) of cumulative egg pad deposition in 2005, 2006, 2012, and 2015.
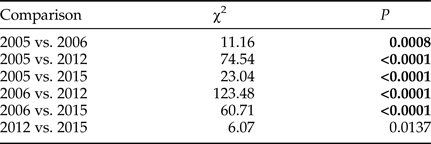
Bold font indicates Bonferroni corrected statistical significance.
Day of egg deposition is relative to 29 September, the earliest date that laboratory egg deposition occurred in any year. Total number of egg pads each year was 2005, N = 65, 2006, N = 66, 2012, N = 70, 2015, N = 36.
Discussion
There appear to be recent and potentially critical changes in the reproductive biology of B. flumineum populations in northern New Jersey, USA. There were previously two reproductive peaks, in which overwintered adults bred in the spring and young adults bred in the fall. It is uncertain whether the second peak was distributed over one vs. two fall generations, but late-maturing new adults likely entered winter diapause before breeding and reproduced for the first time in the spring (Flosi, Reference Flosi1980; Gilg & Kruse, Reference Gilg and Kruse2003). Although we observed spring breeding by overwintered adults, extensive sampling did not detect fall breeding by young adults in 2012 or 2015, the two recent years we sampled. This change in reproductive phenology was associated with delayed reproduction in the captive laboratory, presumably nulliparous, adults collected throughout the fall of 2012 and 2015. We considered the possibility that a slightly warmer and shorter laboratory photophase in 2012 might have caused the effect, but delayed reproduction persisted in 2015 when we replicated laboratory conditions from 2005 and 2006. We speculate that these changes are recent, probably beginning in the last decade, and persistent, occurring in more than 1 year and in adults collected in different water bodies.
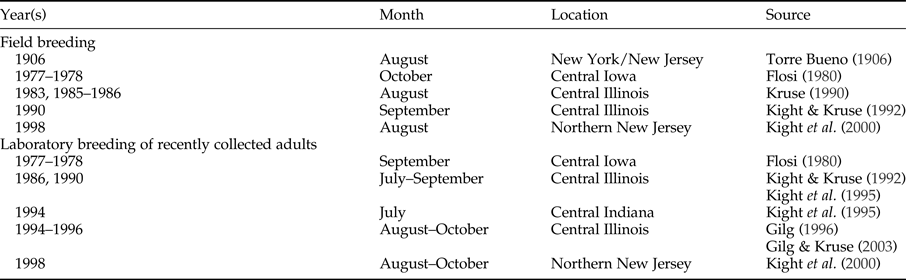
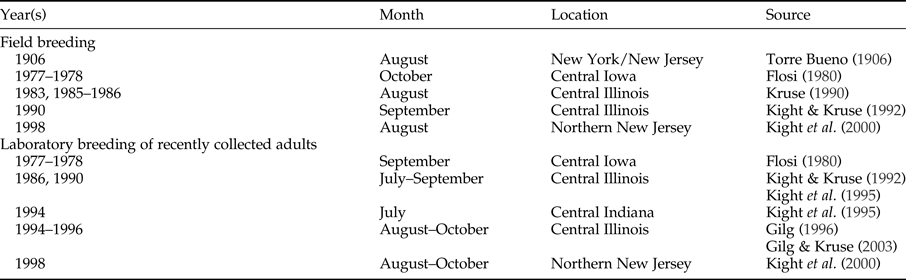
The absence of fall reproduction in the field in recent years is a considerable deviation from previously typical breeding patterns in North American B. flumineum populations (table 2). There are records of fall reproduction more than a century ago by Torre Bueno (Reference Torre Bueno1906), who observed egg-encumbered males in New York and New Jersey at the end of August. This pattern continued through the 20th century in central Iowa (Flosi, Reference Flosi1980), central Illinois (Kruse, Reference Kruse1990; Kight & Kruse, Reference Kight and Kruse1992), and northern New Jersey (Kight et al., Reference Kight, Batino and Zhang2000). As reported here, fall reproduction was still observed in northern New Jersey as recently as 2005 and 2006.
Table 2. Records of fall field and laboratory breeding in North American populations of Belostoma flumineum Say. Month is when egg pads were observed on males.
We did not investigate the mechanism(s) responsible for the absence of fall breeding, though there may be several mutually inclusive explanations. For example, in 2012 and 2015, we noticed an apparent reduction in abundance of odonate naiads collected alongside B. flumineum. This was especially true for immature dragonflies (Anisoptera), which we retained as temporary prey items for waterbugs during transport. Although these observations were anecdotal, they may be indicative of changes in lentic community structure, and are consistent with shifts in species composition and reduced odonate species richness at other locations (e.g., Flenner & Sahlén, Reference Flenner and Sahlén2008). Prey availability can influence the population ecology of belostomatids: Ohba et al. (Reference Ohba, Miyasaka and Nakasuji2008) found an association between tadpole abundance and emergence of Kirkaldyia deyrolli (Belostomatidae, Lethocerinae).
In the present study, however, delayed laboratory breeding in 2012 and 2015 suggests that changes in prey availability are insufficient to explain the lack of fall breeding among B. flumineum in the field. Captive waterbugs were fed ad libitum, but laboratory reproduction did not begin until late November in 2012 and 2015, representing a remarkable deviation from numerous North American laboratory studies over the last 40 years (table 2). Flosi (Reference Flosi1980) observed laboratory breeding among young adults during September in central Iowa, with similar patterns of laboratory breeding between July and September in central Illinois (Kight & Kruse, Reference Kight and Kruse1992; Kight et al., Reference Kight, Sprague, Kruse and Johnson1995; Gilg & Kruse, Reference Gilg and Kruse2003) and northern New Jersey (Kight et al., Reference Kight, Batino and Zhang2000). Indeed, Gilg (Reference Gilg1996) observed males in the fall under laboratory conditions with egg pads as early as 8 days after adult moulting.
It is also possible that B. flumineum populations have responded to environmental factors associated with climate change, such as the statistically significant rise in average annual temperatures in the study region between 1895 and 2015 (New Jersey Department of Environmental Protection, 2017). The Intergovernmental Panel on Climate Change (IPCC) has concluded that regional climate changes are occurring across the planet, with significant anthropogenic input (Canziani et al., Reference Canziani, Palutikof, van der Linden, Hanson, Parry, Canziani, Palutikof, van der Linden and Hanson2007). Rapid changes in climate variables, such as annual temperature and precipitation, are associated with diverse perturbations to physical and biological systems (Rosenzweig et al., Reference Rosenzweig, Karoly, Vicarelli, Neofotis, Wu, Casassa, Menzel, Root, Estrella, Seguin and Tryjanowski2008), including phenological shifts in many taxa (Ovaskainen et al., Reference Ovaskainen, Skorokhodova, Yakovleva, Sukhov, Kutenkov, Kutenkova, Shcherbakov, Meyke and del Mar Delgado2013). Higher annual temperatures can increase winter survivorship and accelerate developmental rates such that reproduction and appearance of stadia are occurring earlier in many insect groups, including Lepidoptera (Roy & Sparks, Reference Roy and Sparks2000), Hymenoptera, Diptera (Gordo & Sanz, Reference Gordo and Sanz2005), Coleoptera (Faccoli, Reference Faccoli2009), Odonata (Hassall et al., Reference Hassall, Thompson, French and Harvey2007), Homoptera (Harrington et al., Reference Harrington, Clark, Weltham, Virrier, Denhol, Hullé, Maurice, Rounsevell and Cocu2007), and Heteroptera (Musolin, Reference Musolin2007). Such changes to the timing of life-cycles can potentially decouple ecological species relationships (Visser & Holleman, Reference Visser and Holleman2001), including those between predators and prey (Schmitz & Barton, Reference Schmitz and Barton2014), thereby influencing prey availability, as discussed above. It seems unlikely that warmer regional temperatures directly caused a change in reproductive patterns of B. flumineum, as we have observed breeding in water temperatures ranging from 15 to 35 °C (Kight et al., Reference Kight, Batino and Zhang2000). However, species at higher trophic levels, like B. flumineum, are indirectly sensitive to climate change (Voigt et al., Reference Voigt, Perner, Davis, Eggers, Schumacher, Bahrmann, Fabian, Heinrich, Kohler, Lichter, Marstaller and Sander2003) if trophic relationships are disrupted by differential responses among species to climate variables (Both et al., Reference Both, Van Asch, Bijlsma, Van Den Burg and Visser2009).
Although laboratory conditions were basically the same in 2005, 2006, and 2015 (slightly warmer in 2012), reproductive outcomes were not, indicating developmental plasticity and/or genetic differences between current and past waterbugs collected from the same study area. A developmental or acclimation effect could result from different environmental conditions experienced by immatures prior to collection. For example, differences in nutrition (e.g. difficulty finding prey) or abiotic factors (e.g. temperature) experienced by immatures can influence adult behaviour and physiology (Sgrò et al., Reference Sgrò, Terblanche and Hoffmann2016). Also, nutritionally restricted immatures are expected to require more and longer feeding after adult emergence to reach reproductive condition. In B. flumineum, this should apply to both sexes: females incur energetic demands for egg production, and males for egg brooding (Gilg & Kruse, Reference Gilg and Kruse2003). Alternatively, population genetic changes between generations may have evolved by natural selection acting on heritable variation for delayed reproduction, if adults in recent years that overwintered before reproducing had high fitness. However, we cannot distinguish between the hypotheses of phenotypic plasticity, evolutionary change to population genetics, or an interaction between the two processes, as all waterbugs in the present study developed from egg to adult in uncontrolled natural environments.
Regardless of causal mechanism(s), the reproductive changes we observed may have profound impacts on populations of B. flumineum in the study area. It appears that new adults are currently recruited into populations during only a single reproductive peak instead of two peaks, which seems likely to result in fewer overwintered adults to reproduce each spring. A shift from two peaks (distributed over two to three annual generations) to a single annual reproductive peak (only overwintered adults that did not breed prior to spring) in this relatively K-selected insect (e.g. the species exhibits parental care, large eggs, relatively small brood size), if sustained, could lead to decreased population abundance over time. We are uncertain whether the results of this study represent a long-term change to the reproductive ecology of B. flumineum or merely are a response to shorter-term environmental factors. It is also presently unclear whether this is a geographically limited phenomenon or if similar effects are occurring beyond the study region, and in other belostomatid species. Recent reports of phenology changes in many insect species across widespread geographic locations suggest at least the possibility that our observations of reproductive change in B. flumineum are associated with global climate change.
Acknowledgements
The authors are grateful to the following individuals for assistance with collection of study animals: Vladimir Bonhomme, Daniel Carrino, MaryAnn Castillo, Eric Chapman, Kaitlin Garvey, Michelle Kight, Julie Lucente, and Laura Steelman. The authors are also grateful to Joshua Galster for advice on the manuscript.