I. INTRODUCTION
Because of their high energy and power densities, lithium ion batteries have been used extensively in many portable electronic devices. Lots of requirements demand extension of the battery application to electric vehicles and stationary power sources. Particularly, batteries in electric vehicles ought to have not only high energy and power densities; they also need extremely long life-time and remarkable safety. Generally speaking, the electrode material is a key material that strongly affects battery performance. Actually, LiMn1.5Ni0.5O4 spinel cathode is well known to show high operating voltage, which engenders development of batteries with high energy density. In this compound, the electrochemical reaction is two-electron reaction with Ni2+/Ni4+ redox, which results in large capacity comparable with commercialized α-NaFeO2-type layered oxide cathode. And the electrochemically inactive Mn4+ provides three-dimensional structural framework, which can yield higher working voltage than those of the α-NaFeO2-type cathodes. Nevertheless, it has been extremely difficult to synthesize the spinel compound with stoichiometric composition and high purity (Zhong et al., Reference Zhong, Bonakdarpour, Zhang, Gao and Dahn1997; Nakamura et al., Reference Nakamura, Tabuchi and Yamada2005; Yi et al., Reference Yi, Mei and Zhu2016). Recently, LiMn1.5Ni0.5O4 particles with high crystallinity and high purity were successfully synthesized with considering their low-temperature magnetic properties (Hanafusa et al., Reference Hanafusa, Kotani, Ishidzu, Oka and Nakamura2016; Nakamura et al., Reference Nakamura, Konya, Shiramata, Kobayashi and Tabuchi2018) which are very sensitive to the crystallinity and purity of the spinel compounds. Many more studies must be conducted to support the practical application of this high-purity spinel cathode to battery devices. In this study, the structural evolution of this spinel cathode during charge-discharge reaction was studied using operando X-ray diffraction, which provides valuable information about the cathode during operation. A battery cell with a specifically designed X-ray window and battery attachments for this operando X-ray diffraction study is explained briefly.
II. EXPERIMENTAL
LiMn1.5Ni0.5O4 particles were synthesized by two-step calcination process using the citrate complex precursors. Reagent grade LiNO3, (CH3COO)2Mn·4H2O and (CH3COO)2Ni·4H2O were dissolved in distilled water. The molar ratio was adjusted to the stoichiometry ratio. After a large amount of citric acid (molar ratio of citrate to acetate was adjusted to 3.0) was added, it was dissolved completely with stirring. The solution was heated to 70 °C until it became a viscous gel-like solution. It was calcined at 900 °C for 20 h in ambient atmosphere. Then it was annealed at 700 °C in O2 atmosphere for 40 h with slow cooling (cooling rate of 10 °C/h).
Using Li metal anode and 1.0 M LiPF6/EC-DMC (volume ratio of 3:7) electrolyte solution, the electrochemical performance of the specimens was evaluated. The cathode films were prepared using the doctor blade technique, where the sample powders (86 wt%) were blended with 7 wt% acetylene black and 7 wt% polyvinylidene difluoride in N-methyl-2-pyrrolidone solvent. The cathode films on Al current collector in this study had active material loading of approximately 10 mg cm−2 and approximately 50 µm average thickness. Galvano-static charge–discharge cycling was conducted at the upper-limit voltage of 4.9 V and lower-limit voltage of 3.5 V. The current rate was calculated assuming that 150 mA g−1 corresponds to 1 C.
Figure 1 shows the pictures of battery cell attachments with reflection and transmission geometries. X-ray diffraction measurements using transmission optics are used for pouch cell batteries that shows good cell operation. First, the cell is set as perpendicular to the incident X-ray beam; the diffraction patterns contain information about both cathode and anode along with that current collectors, separator and Al laminates. Therefore, a lot of care is necessary for the analysis. Additionally, short-wavelength as Mo radiation is required for transmission because of its availability of transmission for pouch cell batteries in the laboratory X-ray diffraction system. In this study, focus on the structural evolution of the high-voltage spinel cathode material during electrochemical cycling, the other geometry, reflection geometry, is useful. A home-made battery cell was developed with a specifically designed X-ray window. There, the current collector was utilized as X-ray window, and the electrode film was prepared directly on the window. Springs and spacers were introduced into the coin cell to adjust pressure onto the cathode film. They enabled us to attain good and stable cell operation with the operand X-ray diffraction measurement, where one-dimensional detector (D/teX ultra) was applied. The XRD measurements were performed with Rigaku SmartLab using CuK α radiation (45 kV and 200 mA). The scanning speed was 10° min−1 and the sampling step was 0.01°. The measurement time of one pattern for operando measurement was less than 2 min. Under these conditions, the Li content variation per X-ray scan was kept below 2%.
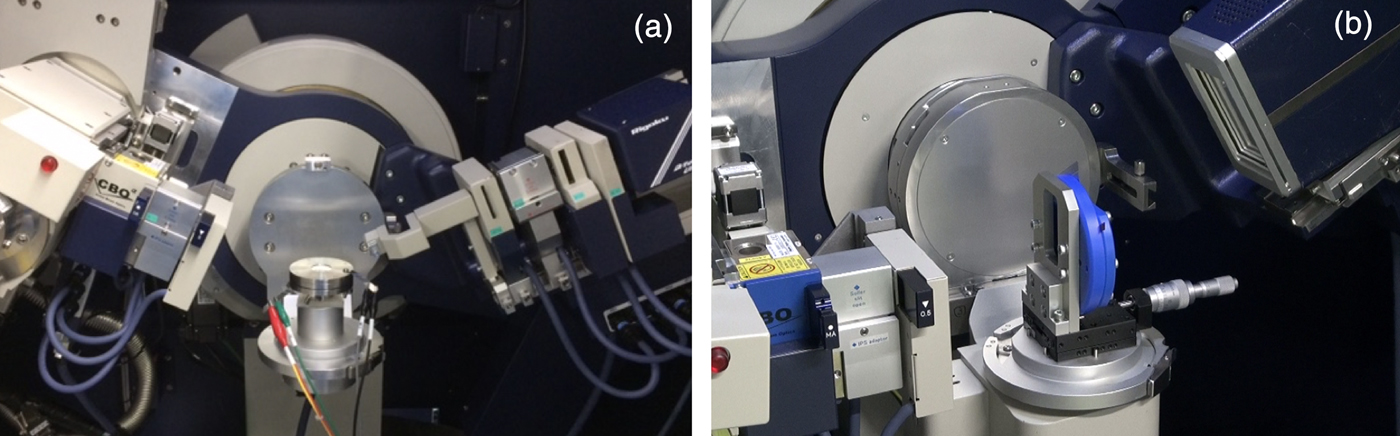
Figure 1. Battery cell attachments for SmartLab. (a) Coin cell attachment (reflection geometry) and (b) Pouch cell attachment (transmission geometry).
III. RESULTS AND DISCUSSION
Figure 2 shows the powder XRD profile of the synthesized cathode material with scanning electron micro-photograph. From the results, the synthesized LiMn1.5Ni0.5O4 cathode material is a single phase of cubic spinel with lattice parameters of a = 0.8165 nm, and the line-width is sharp enough, which indicates good crystallinity. Some weak peaks related to Ni-Mn ordering in the octahedral site were detected, and it indicated the space group of P4 332 rather than Fd ![]() $\bar{3}$m (Idemoto et al., Reference Idemoto, Narai and Koura2003). The obtained particles were isotropic; their primary particle size was approximately 2 µm with a narrow size distribution.
$\bar{3}$m (Idemoto et al., Reference Idemoto, Narai and Koura2003). The obtained particles were isotropic; their primary particle size was approximately 2 µm with a narrow size distribution.
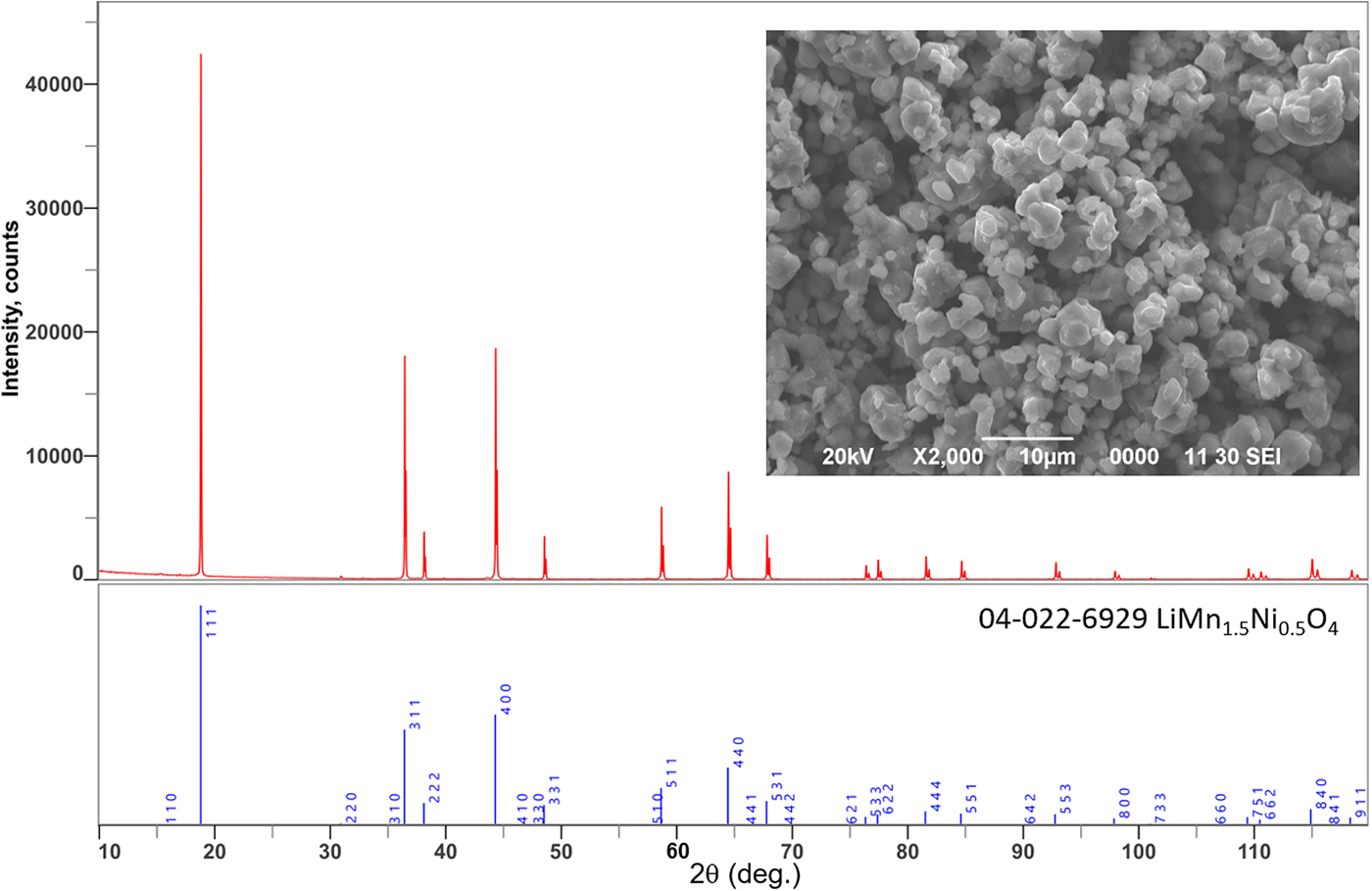
Figure 2. Powder X-ray diffraction pattern and SEM photograph of the synthesized LiMn1.5Ni0.5O4 specimen.
The charge–discharge profile of the high-voltage spinel cathode was depicted in Figure 3, where the upper/lower voltage limit was set to 4.9/3.5 V and the constant current was adjusted to be 15 mA g−1 (0.1 C). The redox reactions were detected only in the 4.7 V region, which is attributed to the Li+ extraction-insertion accompanying the Ni2+/Ni4+ redox reaction. No redox signal around 4.0 V related to Mn3+/Mn4+ redox was found. They mean that the spinel compound has the stoichiometric composition (Ni:Mn = 1:3) (Nakamura et al., Reference Nakamura, Tabuchi and Yamada2005). The redox capacity was estimated as 140 mAh g−1, which was close to 95% of the theoretical capacity of the stoichiometric spinel compound. It means high purity of the obtained spinel compound. Other properties of the obtained cathode, such as rate capability and cycle performance, were referred from the previous work (Hanafusa et al., Reference Hanafusa, Kotani, Ishidzu, Oka and Nakamura2016; Nakamura et al., Reference Nakamura, Konya, Shiramata, Kobayashi and Tabuchi2018). For more detailed study on the high-voltage redox reaction around 4.7 V, the cyclic voltammetry curve was obtained and shown in Figure 4, where the voltage sweeping rate was adjusted to 1.67 µV sec−1 and the scanning range was 4.6–4.9 V. The redox reaction around 4.7 V actually comprised two pairs of the redox reaction: the oxidation current peaks were located at 4.73 and 4.76 V, and the reduction current peaked at 4.71 and 4.72 V. These peaks, which correspond to the plateaus in the charge-discharge potential profile, imply that the redox reaction proceeds via two consecutive two-phase reactions (Idemoto et al., Reference Idemoto, Sekine and Koura2005), which are certified in operando X-ray diffraction.

Figure 3. Charge-discharge profile of the LiMn1.5Ni0.5O4 cathode under constant current at 15 mA g−1 (0.1C), the upper limit voltage of 4.9 V and the lower limit voltage of 3.5 V.

Figure 4. Cyclic voltammetry curve of the LiMn1.5Ni0.5O4 cathode with the scanning rate of 1.67 μV sec−1 in the voltage range from 4.6 to 4.9 V.
Next, structural evolution of the spinel compound during high-voltage redox reaction was studied using operando X-ray diffraction. First, the charge-discharge current was adjusted to 0.1 C, where the redox reaction was slow enough to be close to the equilibrium state. In the diffraction patterns, three cubic spinel phases having different lattice parameters were identified, and the appearance of the intermediate phase was reported in the previous studies (Idemoto et al., Reference Idemoto, Narai and Koura2003; Idemoto et al., Reference Idemoto, Sekine and Koura2005; Kunduraci and Amatucci, Reference Kunduraci and Amatucci2006; Arai et al., Reference Arai, Sato, Orikasa, Murayama, Takahashi, Koyama, Uchimoto and Ogumi2013; Takahashi et al., Reference Takahashi, Mori, Yoshinari, Orikasa, Koyama, Murayama, Fukuda, Hatano, Arai, Uchimoto and Terai2016). The cubic spinel phases had narrow peak widths, which meant that each crystal phase had high crystallinity. Both the lattice parameters and phase fractions of these three phases were evaluated numerically and plotted as a function of the state of charge or depth of discharge in Figure 5. From these figures, the lattice parameters of three cubic spinel phases remained almost unchanged through the charge-discharge reaction: the values were 0.8165, 0.8087, and 0.800 nm, which almost coincided to those reported in the previous work (Kunduraci and Amatucci, Reference Kunduraci and Amatucci2006). Hereinafter, cubic spinel phases having lattice parameter of 0.8165, 0.8087, and 0.800 nm denote phase I, phase II, and phase III, respectively. It was found that their phase fractions changed linearly with the state of charge and also with depth of discharge. Initial phase I was replaced by intermediate phase II in the first half of the charging process. The phase fraction of final-charged phase III was grown at the expense of intermediate phase II in the second half. Reverse evolution of the accompanying phases was observed in the discharged reaction. In other words, the electrochemical reaction proceeded reversibly as two consecutive two-phase reactions, where one comprised phase I and phase II and the other comprised phase II, and phase III. The unit cell volume change in the whole reaction was estimated as 6.0%, and the presence of the intermediate phase may relax the large volume change with separating the whole volume change into 2.8% in the first half of the charge and 3.2% in the second half of the charge.

Figure 5. Operand X-ray diffraction results. (a) lattice parameters during the charge reaction, (b) phase fractions during the charge reaction, (c) lattice parameters during the discharge reaction, and (d) phase fractions during the discharge reaction under the constant current charge-discharge at 15 mA g−1 (0.1C) and 3.5 ~ 4.9V (□: phase I, ○: phase II, △: phase III).
The experimental results of the operando X-ray diffraction at low current demonstrated symmetric and reversible phase evolution in the high-voltage spinel cathode compound. Actually, the appearance of the intermediate phase might be a key factor for the electrochemical reaction. Considering them, operando X-ray diffraction at various currents were conducted. Figure 6 presents the experimentally-obtained results, where the charge-discharge currents were 15 mA g−1 (0.1C), 75 mA g−1 (0.5C) and 150 mA g−1 (1C). The contour maps of X-ray diffraction patterns were generated using Rigaku SmartLab Studio II software. At the lower current rate, the three phases had high crystallinity. It is possible to detect the two-consecutive two-phase reaction. However, as the reaction current was raised, it was found that the crystallinity of phase II was reduced, especially for the discharge process. Furthermore, it was also shown that the separation of the two two-phase reactions became obscure and that they were overlapped in the intermediate region. Moreover, this feature was more significant in the discharge process than that in the charge process. These phenomena indicate asymmetry of the charge-discharge reaction at a high reaction rate, which can be originated to the deviation from the equilibrium state. Similar phenomena were reported in an earlier paper (Arai et al., Reference Arai, Sato, Orikasa, Murayama, Takahashi, Koyama, Uchimoto and Ogumi2013; Takahashi et al., Reference Takahashi, Mori, Yoshinari, Orikasa, Koyama, Murayama, Fukuda, Hatano, Arai, Uchimoto and Terai2016). The charge-discharge reaction asymmetry, which might be attributed to the phase transition rate, is explainable under the following assumption: (i) the transition rate from phase II to phase I is faster than that from phase III to phase II, and (ii) the transition rate from phase III to phase II is lower than that from phase II to phase III. That is, the phase transition from phase II to phase III may be a rate-determining step on the charge reaction, although the phase transition from phase III to phase II may be a rate determining process in the discharge process. As described earlier, the presence of the intermediate phase II might be a key for the electrochemical reaction kinetics of the high-voltage spinel cathode, which also has practical importance for high power density battery fabrication, especially for rapidly chargeable battery devices.

Figure 6. Contour maps of the operand X-ray diffraction pattern: the charge-discharge current of (a) 15 mA g−1 (0.1C), (b) 75 mA g−1 (0.5C) and (c) 150 mA g−1 (1C).
In addition to high-voltage operation, crystal structure evolution in low voltage range using operando X-ray diffraction was also examined briefly. In general, the low-voltage operation of the Mn-spinel cathode compounds includes the Jahn-Teller active Mn3+ formation at the octahedral site. It causes remarkable structural deformation, mechanical degradation of the cathode materials, and capacity fading with electrochemical cycling. The charge-discharge potential profile in low voltage region 2.1–3.5 V and current of 15 mA g−1 was shown in Figure 7, where a long plateau was observed at around 2.7 V, and the reversibility was relatively good for this low current reaction. Numerical analysis of the operando X-ray diffraction yielded the results presented in Figure 8. The 2.7 V plateau redox reaction proceeded via two-phase reaction of cubic phase (phase I) and tetragonal phase. This tetragonal phase formation is related to the Jahn-Teller active Mn3+ formation at the octahedral site. Lattice parameters of the tetragonal phase were a = 0.5742 nm and c = 0.8661 nm (a tetra ~ a cub/![]() $\sqrt 2 $ and c tetra ~ a cub), and the lattice volume difference from the cubic phase was about 4.9%, which was not so large as ordinary spinel compounds. The tetragonal phase fraction reached 72% at the end of the discharge reaction, and the phase transition was completely reversible during the charge process. These analyses suggest the possible low-voltage operation of the spinel cathode, which remains as a subject of future work.
$\sqrt 2 $ and c tetra ~ a cub), and the lattice volume difference from the cubic phase was about 4.9%, which was not so large as ordinary spinel compounds. The tetragonal phase fraction reached 72% at the end of the discharge reaction, and the phase transition was completely reversible during the charge process. These analyses suggest the possible low-voltage operation of the spinel cathode, which remains as a subject of future work.

Figure 7. Charge-discharge profile of the LiMn1.5Ni0.5O4 cathode under constant current at 15 mA g−1 in the voltage range from 2.1 to 3.5 V.

Figure 8. Variation of the phase fractions (○: cubic phase and □: tetragonal phase) during discharge reaction (a) and charge reaction (b) under the constant current of 15 mA g−1 at 2.1 ~ 3.5 V.
IV. CONCLUSIONS
Structural evolution of LiMn1.5Ni0.5O4 high-voltage cathode material during the charge-discharge reaction in a half cell was studied using operando X-ray diffraction measurement with a laboratory X-ray diffractometry system and a home-made cell. The electrochemical Li+ extraction/insertion reaction in the range of 3.5–4.9 V proceeded with two consecutive two-phase reactions having high crystallinity, high symmetry, and reversibility under low current. Then, the unit cell volume change was divided into two stages because of the presence of the intermediate phase. On the other hand, under high current reaction, the crystallinity of the intermediate phase was reduced, which caused the asymmetry in the charge-discharge reaction. These results suggest that the properties of the intermediate phase have an important role in cathode performance. Furthermore, operando X-ray diffraction study was conducted for low-voltage cycling at 2.1–3.5 V. Results obtained for a two-phase reaction between cubic and tetragonal phases show that the lattice volume change was not so large and that the reaction proceeded reversibly. It implied that there was a possibility of the electrochemical cycling at low voltages.
ACKNOWLEDGEMENT
The authors gratefully appreciate Dr. Y. Kobayashi (CRIEPI) and Dr. M. Tabuchi (AIST) for their fruitful discussion and encouragement of this work.










