Introduction
Tourette syndrome (TS) is phenotypically and etiologically heterogeneous, characterized by motor and phonic tics (American Psychiatric Association, 2013), often with symptoms of obsessive-compulsive disorder (OCD) and attention-deficit/hyperactivity disorder (ADHD) (Hirschtritt et al. Reference Hirschtritt, Lee, Pauls, Dion, Grados, Illmann, King, Sandor, McMahon, Lyon, Cath, Kurlan, Robertson, Osiecki, Scharf and Mathews2015), both of which consist of multiple symptom subgroups. Between 3 and 5 OCD symptom groups have been identified, including contamination/cleaning, taboo/forbidden thoughts, and hoarding, and less consistently symmetry, superstitions/repeating rituals, doubts, fear-of-harm, and checking (Bloch et al. Reference Bloch, Landeros-Weisenberger, Rosario, Pittenger and Leckman2008; Leckman et al. Reference Leckman, Denys, Simpson, Mataix-Cols, Hollander, Saxena, Miguel, Rauch, Goodman, Phillips and Stein2010; Delucchi et al. Reference Delucchi, Katerberg, Stewart, Denys, Lochner, Stack, den Boer, van Balkom, Jenike, Stein, Cath and Mathews2011). Between 2 and 3 symptom groups have been identified for ADHD: inattentive, impulsive, and hyperactive symptoms (the latter two are often combined) (Pillow et al. Reference Pillow, Pelham, Hoza, Molina and Stultz1998; Collett et al. Reference Collett, Crowley, Gimpel and Greenson2000; Dumenci et al. Reference Dumenci, McConaughy and Achenbach2004; Toplak et al. Reference Toplak, Pitch, Flora, Iwenofu, Ghelani, Jain and Tannock2009). For both OCD and ADHD, parsing these symptom subtypes has led to improved understanding of the pathophysiology of these disorders (Freitag et al. Reference Freitag, Rohde, Lempp and Romanos2010; Katerberg et al. Reference Katerberg, Delucchi, Stewart, Lochner, Denys, Stack, Andresen, Grant, Kim, Williams, den Boer, van Balkom, Smit, van Oppen, Polman, Jenike, Stein, Mathews and Cath2010).
Subgroups of OCD and ADHD may also exist in TS samples; however, they may also (1) differ from those seen in OCD or ADHD populations, and (2) be associated with distinct pathophysiology or treatment outcomes. Early studies in TS samples primarily examined these dimensions at the diagnostic level (e.g. combinations of TS, OCD, and ADHD diagnoses) (Pauls et al. Reference Pauls, Leckman and Cohen1993; Robertson et al. Reference Robertson, Althoff, Hafez and Pauls2008; Mathews & Grados, Reference Mathews and Grados2011). Only a few have conducted symptom-level analyses across ⩾3 disorders (e.g. TS, OCD, and ADHD, ±autism) (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017; Huisman-van Dijk et al. Reference Huisman-van Dijk, Schoot, Rijkeboer, Mathews and Cath2016). These studies are useful in identifying cross-disorder phenotypes (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017); however, subtle but important disorder-specific patterns may not be discernable in cross-disorder studies because the strong internal cohesion of any single group of symptoms (e.g. tics) can lead to a somewhat over-simplified cross-disorder model, thus prohibiting further investigation of potentially relevant, but less dominant, symptom subgroups. Thus, there is utility in examining specific symptom types (e.g. tics, OCD symptoms, and ADHD symptoms) separately, providing a nuanced characterization that complements the cross-disorder approach. For example, we conducted both cross-disorder analyses (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017) and a separate tic-only analysis in the same TS family sample (Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016). The cross-disorder analysis yielded a tic symptom group, an OCD symptom group, and symmetry and disinhibition symptom groups that included both tics and OC symptoms. In contrast, the tic-only analysis identified six tic symptom subgroups that paralleled the somatotopic representation of the somatosensory cortex, a finding that was not identified in the larger cross-disorder analysis, but which has potential relevance for future etiological studies.
As with the tic-only analysis, separate examinations of the patterns of OCD and ADHD symptoms in TS families may also be useful, given the substantial comorbidity and likely genetic pleiotropy between TS, OCD, and ADHD. Thus, in this report, to clearly elucidate the complex relationships of OCD and ADHD symptoms in TS, we used factor and latent class analyses (LCA) to identify OCD and ADHD symptom patterns and subsequently examined the heritability and clinical associations of these symptom subgroups in a well-phenotyped sample of subjects with TS and their family members.
We hypothesized that, in addition to finding empirically-based OCD- and ADHD-symptom dimensions similar to those found in non-TS samples, we would identify additional, unique heritable symptom patterns with specific relevance to TS. We also hypothesized that individual OCD and ADHD symptom subgroups would be differentially associated with other clinically relevant characteristics, such as comorbid psychiatric disorders and/or symptom severity. For example, based on our previous work we hypothesized that we would identify specific symmetry and disinhibition dimensions from within the OCD and ADHD symptoms that would be more closely associated with TS than with OCD or ADHD.
Methods
Sample
Sample characteristics and assessments used in this study are described in detail elsewhere (Hirschtritt et al. Reference Hirschtritt, Lee, Pauls, Dion, Grados, Illmann, King, Sandor, McMahon, Lyon, Cath, Kurlan, Robertson, Osiecki, Scharf and Mathews2015, Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016; Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017). Subjects included 3494 individuals from 1365 families collected by the multi-site Tourette Syndrome Association International Consortium for Genetics (TSAICG) for genetic studies; all participants provided written informed consent (parental consent and written assent was obtained for individuals <18 years). This study was approved by the Institutional Review Boards of all participating sites. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
The sample included 283 sib-pair families (two or more TS-affected siblings plus parents) and 1082 trio families (TS-affected individuals plus both parents). In addition to parents and TS-affected sibling pairs, there were 91 TS-unaffected siblings; 26 families had extended family members (grandparents, uncles or aunts, and cousins). Sib-pair families were excluded if both parents had TS, chronic tics, or OCD; no such exclusions were made for trio families. Inclusion criteria for probands (the first identified TS-affected individual in a given family) were: age ⩾6 years, established TS diagnosis, and availability of living parents for family-based analyses. Exclusion criteria included: intellectual disability, and tics caused by neurologic disorders other than TS. All analyses except for the exploratory factor analysis (EFA) used all family members with sufficient data, independent of TS diagnosis.
Procedure
Research staff assessed demographic data, tic severity, OCD and ADHD symptoms using the TSAICG Tic and Comorbid Symptom (TICS) Inventory (Tourette Syndrome Association International Consortium for Genetics, 1999, 2007), which also includes detailed checklists of lifetime-encountered OCD and ADHD symptoms, age-of-onset, and (for OCD symptoms only) global severity (online Supplementary Methods).
Other psychiatric diagnoses were assessed using the Structured Clinical Interview for the DSM (First et al. Reference First, Spitzer, Gibbon and Williams1995) or the Schedule for Affective Disorders and Schizophrenia-Lifetime Version, Modified for the Study of Anxiety Disorders (Fyer et al. Reference Fyer, Endicott, Mannuzza and Klein1985) for adults and the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (Kaufman et al. Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997) and Epidemiologic Version (Polanczyk et al. Reference Polanczyk, Eizirik, Aranovich, Denardin, da Silva, da Conceicao, Pianca and Rohde2003) for children. These data were only collected during the first wave of recruitment and were available for ~19% of participants. We established all psychiatric diagnoses using a best-estimate approach (Leckman et al. Reference Leckman, Sholomskas, Thompson, Belanger and Weissman1982; Hirschtritt et al. Reference Hirschtritt, Lee, Pauls, Dion, Grados, Illmann, King, Sandor, McMahon, Lyon, Cath, Kurlan, Robertson, Osiecki, Scharf and Mathews2015). Psychiatric diagnoses other than TS, OCD, and ADHD were combined into categories; mood (depression and bipolar disorder), anxiety (panic, generalized anxiety, social phobia, and separation anxiety), and disruptive behavior disorders (conduct disorder and oppositional defiant disorder).
Statistical analyses
Exploratory factor analyses
We performed separate EFA on OCD and ADHD symptom data in probands using robust weighted least squares estimation for dichotomous variables (Muthén et al. Reference Muthén, du Toit and Spisic1997) and oblique rotation (geomin), which allows for correlation among factors, in MPlus version 7.1 (Muthén & Muthén, Reference Muthén and Muthén1998–2012). We subsequently used orthogonal (varimax) rotation as a secondary sensitivity analysis. As the orthogonal rotation yielded similar results, they are not presented here. We limited data to probands to examine independent cases. The best-factor solution was chosen using a stepwise approach based on established criteria (Preacher et al. Reference Preacher, Zhang, Kim and Mels2013). First, we only considered models containing eigenvalues ⩾1. Second, we examined the root mean square error of approximation (RMSEA) (Loehlin, Reference Loehlin2004; Raykov & Marcoulides, Reference Raykov and Marcoulides2006) and chi-square difference test (Floyd & Widaman, Reference Floyd and Widaman1995) values among models to provide quantitative measures of fit. Third, we prioritized models with minimal ‘cross-loading’ (i.e. had fewer variables that loaded on ⩾1 factor at ⩾40), and finally, we assessed the clinical applicability and interpretability of the models. Within each model, we retained items if factor loadings were ⩾40; items that loaded on ⩾2 latent factors at ⩾0.40 were assigned to the factor with the higher loading. Items with loadings <0.40 were excluded from the final model. We assessed the internal consistency of each factor using Cronbach's alpha and calculated mean factor sum scores for each factor in each participant by dividing the number of items the individual endorsed by the total number of items answered for each factor (Katerberg et al. Reference Katerberg, Delucchi, Stewart, Lochner, Denys, Stack, Andresen, Grant, Kim, Williams, den Boer, van Balkom, Smit, van Oppen, Polman, Jenike, Stein, Mathews and Cath2010). These factor sum scores were used in the LCA and in all clinical and heritability analyses.
Using generalized estimating equation models clustering on family and controlling for age at interview, we tested the association between OCD and ADHD factor sum scores and lifetime diagnoses of TS, OCD, ADHD, anxiety, mood, and disruptive behavior disorders; sex; TS, OCD, and ADHD age-of-onset; and tic and OCD severity. To account for multiple testing, we set our selection threshold to p < 0.005 (0.05/10, the total number of OCD and ADHD factors).
Latent class analyses
We conducted LCA in MPlus version 7.1, and fit latent classes to EFA-derived ADHD and OCD factor sum scores among all participants. We chose the best-fit models based on those with the lowest Bayesian Information Criterion (Schwarz, Reference Schwarz1978) and a significant Lo, Mendel, and Rubin likelihood ratio test (p < 0.05) (Lo et al. Reference Lo, Mendell and Rubin2001). If these criteria left the model choice unclear, we examined the clinical interpretability of the solutions. We performed an additional step by fitting latent classes to EFA-derived ADHD and OCD factor sum scores among probands only to examine the robustness of the LCA solution. For each latent class model, we added classes until the model failed to converge. In all LCA models, the probability distributions for class membership [ranging from 0 (no probability) to 1 (100% probability)] approximated a binary distribution; therefore, we assigned each individual to his/her most likely class. Class membership was categorical and mutually exclusive. We next examined the rates of psychiatric comorbidity in each class using the auxiliary variable function of MPlus, which accounts for uncertainty in class membership.
Heritability
We calculated heritability estimates for factor sum scores using the Sequential Oligogenic Linkage Analysis Routine statistical package (Almasy & Blangero, Reference Almasy and Blangero1998), covarying for age, sex, and sex × age. We inverse-normalized all factor sum scores to account for any skewing in the distributions of the raw data. We first examined the heritability of the OCD and ADHD factors individually. To estimate the genetic relationships between these symptom-based phenotypes and the core diagnoses (TS, OCD, and ADHD), we then conducted heritability analyses for TS, OCD, and ADHD, covarying for all OCD and ADHD factors (separately for both sets of factors). Because TS, OCD, and ADHD are heritable in TS-affected families (Hirschtritt et al. Reference Hirschtritt, Lee, Pauls, Dion, Grados, Illmann, King, Sandor, McMahon, Lyon, Cath, Kurlan, Robertson, Osiecki, Scharf and Mathews2015), we included the factors as covariates in the heritability analysis for each diagnosis to partition out any heritability in the model that was due to the symptom factor. We then used log-likelihood and associated p values (representing the model with and without the given factor) to determine which factors significantly changed the estimated heritability of each diagnosis, thus indicating a significant genetic relationship between the factor and the diagnosis.
Results
Sample characteristics
The final sample included 1191 probands (254 from sib-pair families, 937 from trios) and 3494 total participants (1147 from sib-pair families and 2347 from trios). Missing data patterns did not differ by site. All 1191 probands (by definition) and 28.2% of family members had TS (1841 participants, 52.7% of the total sample). 34.2% of participants (probands and family members) had OCD, while 31.5% had ADHD (online Supplementary Table S1).
EFAs
EFA of OCD symptoms
Models containing up to 11 factors had eigenvalues ⩾1.0 (online Supplementary Fig. S1). We only examined models with up to 8 factors, as those with >8 had one or more factor(s) without any significant loadings. All models had RMSEA values <0.05, and the χ2 difference tests were significant for all comparisons up to the 8-factor model (online Supplementary Table S2). We chose the 8-factor model for further examination, which separated items into: (1) doubts/scrupulosity, (2) symmetry/exactness, (3) contamination/cleaning, (4) aggressive urges, (5) fear-of-harm, (6) need for sameness, (7) superstitions, and (8) hoarding (Table 1, online Supplementary Table S3). All factors were significantly correlated with one another (correlations between 0.21 and 0.56, all p values ⩽0.05), except the need for sameness factor, which did not correlate with any of the other OCD symptom factors (online Supplementary Table S4).
Table 1. Factor loadings and internal consistency for OCD exploratory factor model
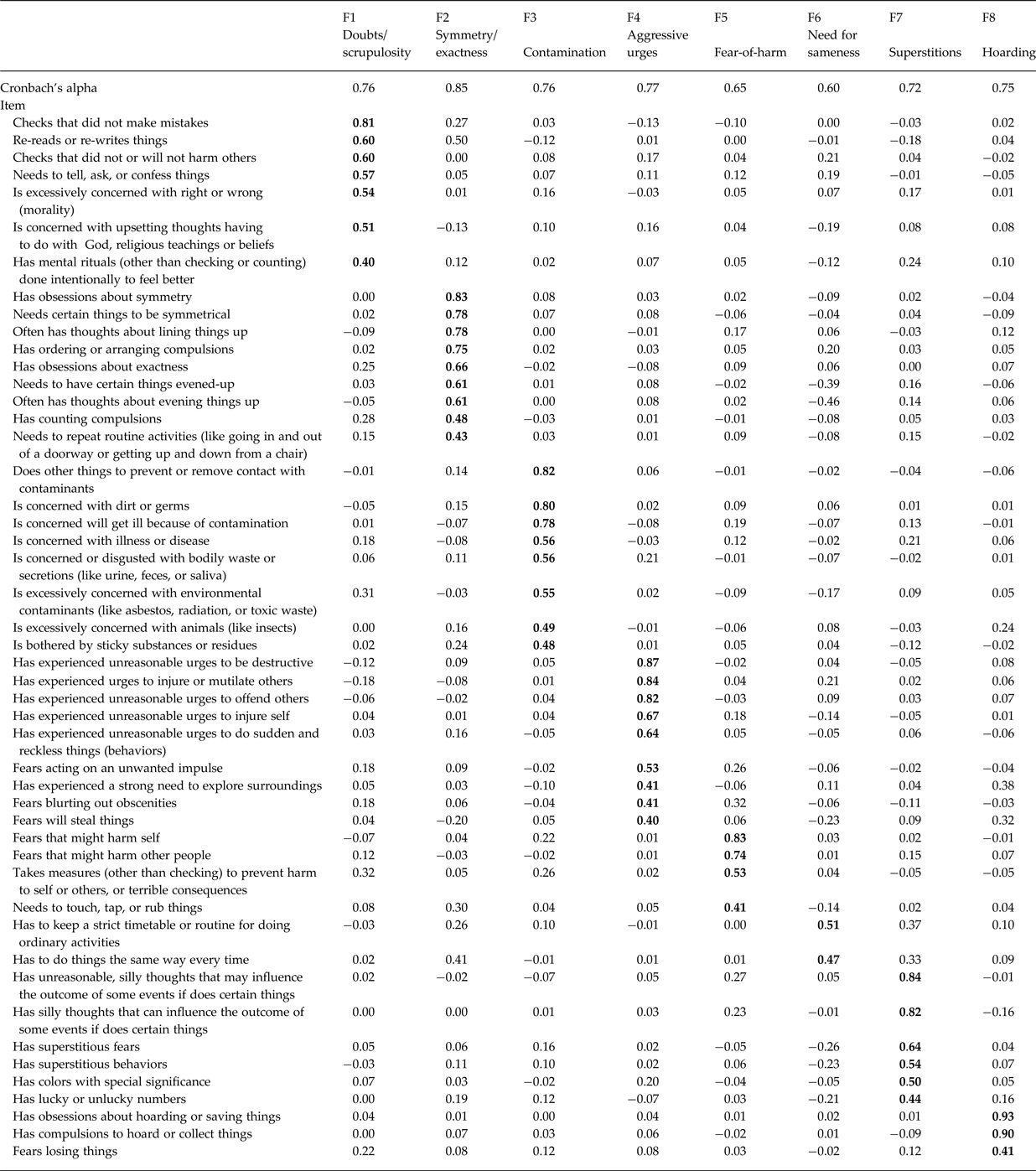
Items that loaded on 2 latent factors ⩾0.40 were assigned to the factor with the higher loading.
All factors except superstitions were significantly associated with OCD severity, although only symmetry/exactness and hoarding were significantly associated with OCD diagnosis (Table 2). The symmetry/exactness, aggressive urges, and fear-of-harm factors were associated with a TS diagnosis, and the aggressive urges and hoarding factors were associated with an ADHD diagnosis. Symmetry/exactness was also associated with increased tic severity, while aggressive urges were associated with DBD diagnoses, male sex, earlier TS age-of-onset and increased tic severity. Notably, the contamination factor was only significantly associated with anxiety disorders and with OCD severity. None of the OCD factors demonstrated significant association with mood disorders, or with age-of-onset of OCD or ADHD.
Table 2. Association of OCD factor sum scores with clinical characteristics among individuals with TS and their family members
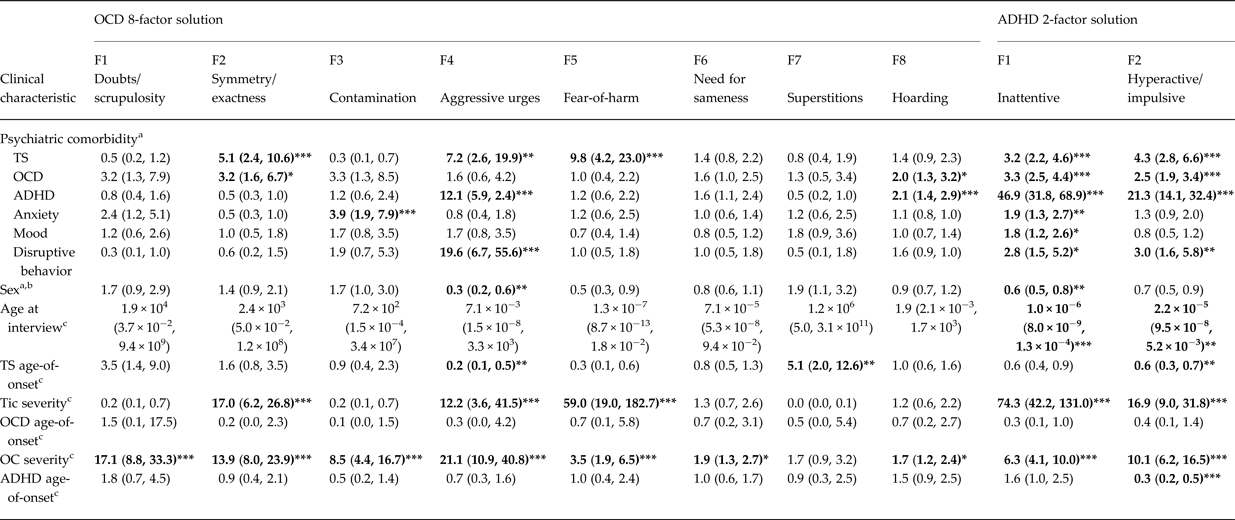
ADHD, attention-deficit/hyperactivity disorder; OC(D), obsessive-compulsive (disorder); TS, Tourette syndrome.
Generalized estimating equation models, clustering on family, simultaneously covary for all OCD or ADHD factor sum scores, age at interview (except in the model in which age at interview is the outcome), and OC severity (except in the model in which OC severity is the outcome), and define each clinical characteristic as the outcome variable in separate models.
a Values represent odds ratios (95% confidence interval).
b Odds ratios >1 indicate higher odds of female sex.
c Values represent standardized beta coefficient (95% confidence interval).
*p < 0.005, **p < 0.001, ***p < 0.0001.
EFA of ADHD symptoms
We fit models with up to 4 factors using the 18 ADHD symptoms based on RMSEA scores and clinical utility of the models (online Supplementary Table S5 and Fig. S1). A 2-factor model (online Supplementary Table S6) (inattentive and hyperactive/impulsive symptoms) was the best fit, mirroring the DSM-5 categorization of ADHD symptoms.
Both ADHD factors were significantly associated with TS, OCD, ADHD, and DBD (Table 2), and with tic and OC symptom severity. The inattentive factor was also significantly associated with anxiety and mood disorders and with male sex, while the hyperactive/impulsive factor was associated with earlier TS and ADHD age-of-onset. Neither ADHD factor demonstrated significant association with OCD age-of-onset.
Heritability analyses
Table 3 presents the heritabilities of the individual OCD and ADHD factors, and outlines the genetic relationships between the factors and TS, OCD, and ADHD. Heritability for OCD factors ranged from 0.19 to 0.37 (all p values ⩽4 × 10−12; symmetry/exactness had the highest heritability, h 2 r = 0.37, SE = 0.03), and need for sameness the lowest (h 2 r = 0.20, SE = 0.03). The ADHD factors had heritabilities of 0.41 (inattentive, SE = 0.03, p = 1.0 × 10−38) and 0.38 (hyperactive/impulsive, SE = 0.03, p = 8.5 × 10−34). While the loglikelihoods suggest that both ADHD factors are genetically related to TS, OCD, and ADHD (e.g. the heritabilities of ADHD, TS, and OCD were significantly reduced when the ADHD factors were included as covariates), the results for the OCD factors suggested more specific relationships. Although the heritability models for OCD changed when the OCD factors were included as covariates, the models for TS changed significantly with the addition of three OCD factors (symmetry/exactness, aggressive urges, and fear-of-harm), suggesting that these and not the other OCD factors are genetically related to TS. Similarly, only two OCD factors (aggressive urges and hoarding) significantly modified ADHD heritability estimates. Figure 1 shows a schematic of the relationships, both clinical and genetic, between the OCD factors and psychiatric diagnoses, including those that are OCD-specific, those associated with both OCD and TS, those that are associated with OCD and anxiety, and those that are associated with OCD, TS, and ADHD.
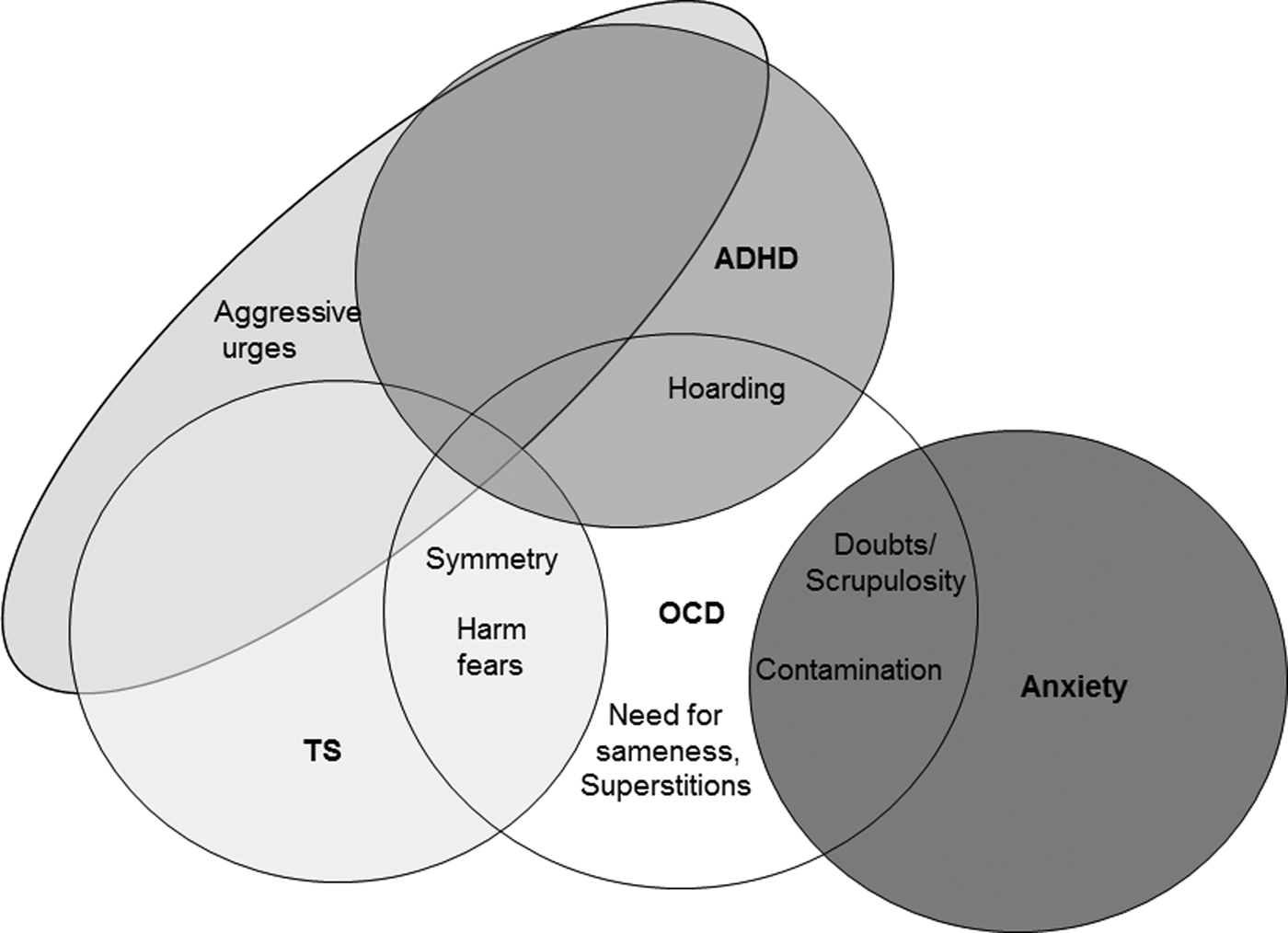
Fig. 1. Schematic representation of the relationships between the OCD factors and psychiatric diagnoses.
Table 3. Heritability of OCD and ADHD factors and their modification of the heritability of TS, OCD, and ADHD diagnoses
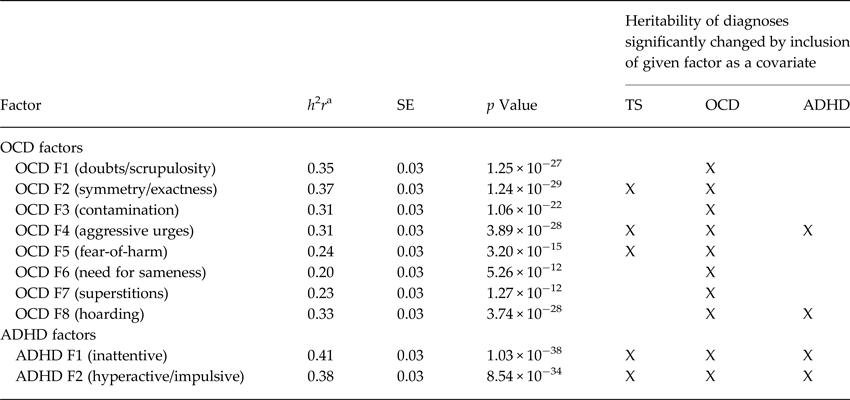
ADHD, attention-deficit/hyperactivity disorder; h 2 r, estimated heritability value; OCD, obsessive-compulsive disorder; SE, standard error; TS, Tourette syndrome.
‘X’ indicates p ⩽ 0.05, uncorrected.
a Controlling for age, sex, and age × sex.
LCAs
LCA of EFA-based ADHD and OCD factor sum scores
The results of the nested LCA, incorporating both ADHD and OCD factor sum scores for all participants supported a 3-class solution:
(LC1) few symptoms, (LC2) OCD&ADHD symptoms, and (LC3) symmetry/exactness, hoarding, and ADHD symptoms (online Supplementary Table S7 and Fig. S2). The probability of endorsement was <30% and <10% for the ADHD and OCD factors, respectively, in LC1, and >50% for all factors in LC2. The probability of endorsement was >40% for the symmetry/exactness, hoarding, inattentive, and hyperactive/impulsive factors for LC3. Significant differences in comorbidity rates were observed between classes (Fig. 2) for OCD (χ2 = 2454.35, df = 2, p ⩽ 0.001), ADHD (χ2 = 1943.89, df = 2, p ⩽ 0.001), mood disorders (χ2 = 61.61, df = 2, p ⩽ 0.001), anxiety disorders (χ2 = 86.69, df = 2, p ⩽ 0.001), and disruptive behavior disorders (χ2 = 21.16, df = 2, p ⩽ 0.001). LC1 (few/no OCD/ADHD symptoms) had the lowest rates of all psychiatric disorders; the most common was TS, endorsed by ~40% of individuals in LC1. LC2 (OCD&ADHD symptoms) was characterized by high rates of all psychiatric disorders, with OCD present in >90%, TS present in >80%, and ADHD present in 50%. This class also had higher rates of mood, anxiety, and disruptive behavior disorders than LC1 and LC3 (symmetry, exactness, hoarding and ADHD symptoms). LC3 was characterized by high rates of TS, OCD, and ADHD (over 75% of individuals in this class had these three disorders) and comparatively low rates of other psychiatric diagnoses.
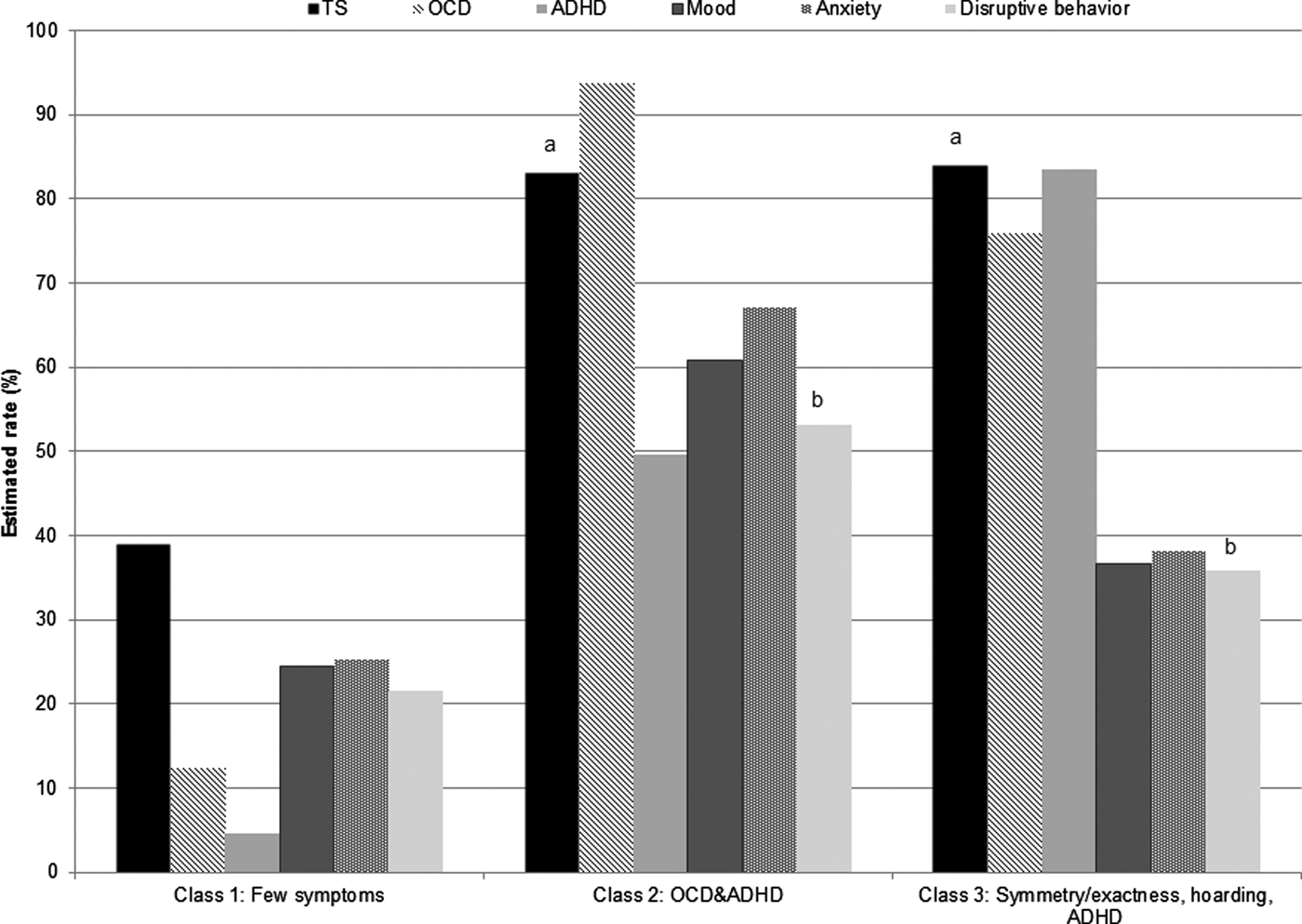
Fig. 2. Rates of comorbid psychiatric disorders among latent classes using OCD and ADHD factor sum scores in probands and family members. ADHD, attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder. Letters above bars indicate pairwise comparisons that are not significantly different at p < 0.05.
The results of the LCA limited to probands only also supported a 3-class solution. In this solution, the ‘symmetry/exactness, hoarding, and ADHD symptoms’ and ‘ADHD&OCD symptoms’ classes paralleled those derived from the entire sample; the remaining class was similar to the original ‘few symptoms’ class, but had higher rates of ADHD symptoms (46% had inattentive symptoms and 36% had hyperactive/impulsive symptoms) (data not shown).
Discussion
Although OCD, ADHD, and TS are hypothesized to share underlying genetic factors (Davis et al. Reference Davis, Yu, Keenan, Gamazon, Konkashbaev, Derks, Neale, Yang, Lee, Evans, Barr, Bellodi, Benarroch, Berrio, Bienvenu, Bloch, Blom, Bruun, Budman, Camarena, Campbell, Cappi, Cardona Silgado, Cath, Cavallini, Chavira, Chouinard, Conti, Cook, Coric, Cullen, Deforce, Delorme, Dion, Edlund, Egberts, Falkai, Fernandez, Gallagher, Garrido, Geller, Girard, Grabe, Grados, Greenberg, Gross-Tsur, Haddad, Heiman, Hemmings, Hounie, Illmann, Jankovic, Jenike, Kennedy, King, Kremeyer, Kurlan, Lanzagorta, Leboyer, Leckman, Lennertz, Liu, Lochner, Lowe, Macciardi, McCracken, McGrath, Mesa Restrepo, Moessner, Morgan, Muller, Murphy, Naarden, Ochoa, Ophoff, Osiecki, Pakstis, Pato, Pato, Piacentini, Pittenger, Pollak, Rauch, Renner, Reus, Richter, Riddle, Robertson, Romero, Rosario, Rosenberg, Rouleau, Ruhrmann, Ruiz-Linares, Sampaio, Samuels, Sandor, Sheppard, Singer, Smit, Stein, Strengman, Tischfield, Valencia Duarte, Vallada, Van Nieuwerburgh, Veenstra-Vanderweele, Walitza, Wang, Wendland, Westenberg, Shugart, Miguel, McMahon, Wagner, Nicolini, Posthuma, Hanna, Heutink, Denys, Arnold, Oostra, Nestadt, Freimer, Pauls, Wray, Stewart, Mathews, Knowles, Cox and Scharf2013; McGrath et al. Reference McGrath, Yu, Marshall, Davis, Thiruvahindrapuram, Li, Cappi, Gerber, Wolf, Schroeder, Osiecki, O'Dushlaine, Kirby, Illmann, Haddad, Gallagher, Fagerness, Barr, Bellodi, Benarroch, Bienvenu, Black, Bloch, Bruun, Budman, Camarena, Cath, Cavallini, Chouinard, Coric, Cullen, Delorme, Denys, Derks, Dion, Rosario, Eapen, Evans, Falkai, Fernandez, Garrido, Geller, Grabe, Grados, Greenberg, Gross-Tsur, Grunblatt, Heiman, Hemmings, Herrera, Hounie, Jankovic, Kennedy, King, Kurlan, Lanzagorta, Leboyer, Leckman, Lennertz, Lochner, Lowe, Lyon, Macciardi, Maier, McCracken, McMahon, Murphy, Naarden, Neale, Nurmi, Pakstis, Pato, Pato, Piacentini, Pittenger, Pollak, Reus, Richter, Riddle, Robertson, Rosenberg, Rouleau, Ruhrmann, Sampaio, Samuels, Sandor, Sheppard, Singer, Smit, Stein, Tischfield, Vallada, Veenstra-VanderWeele, Walitza, Wang, Wendland, Shugart, Miguel, Nicolini, Oostra, Moessner, Wagner, Ruiz-Linares, Heutink, Nestadt, Freimer, Petryshen, Posthuma, Jenike, Cox, Hanna, Brentani, Scherer, Arnold, Stewart, Mathews, Knowles, Cook, Pauls, Wang and Scharf2014; Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016), the reasons behind their significant comorbidity, and the clinical and etiological relationships between them, are complex and not yet clearly elucidated. This study extends upon previous work (Eapen et al. Reference Eapen, Fox-Hiley, Banerjee and Robertson2004; Storch et al. Reference Storch, Murphy, Geffken, Soto, Sajid, Allen, Roberti, Killiany and Goodman2004; Grados & Mathews, Reference Grados and Mathews2009; Cavanna et al. Reference Cavanna, Critchley, Orth, Stern, Young and Robertson2011; Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017; Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016; Huisman-van Dijk et al. Reference Huisman-van Dijk, Schoot, Rijkeboer, Mathews and Cath2016) to examine ADHD and OCD symptoms separately using factor analysis, and jointly, using LCA, in a sample of TS families. Taken together, our results both confirm and extend the growing body of literature that suggests that individual OCD symptom groups are differentially associated with specific psychopathologies, while ADHD symptoms may represent more general global underlying psychopathology.
We identified eight OCD symptom subgroups (instead of the four or five typically identified in OCD samples (Bloch et al. Reference Bloch, Landeros-Weisenberger, Rosario, Pittenger and Leckman2008)), some of which appear to be differentially related to TS (symmetry/exactness, fear-of-harm), ADHD (hoarding), and anxiety disorders (doubts/scrupulosity, contamination), some of which are OCD-specific (superstitions, need for sameness), and one that appears to be related to multiple manifestations of psychopathology in this sample (aggressive urges). In contrast, the ADHD factor analysis identified two symptom subgroups that parallel the DSM-5 classifications (inattentive and hyperactive/impulsive symptoms) (Pillow et al. Reference Pillow, Pelham, Hoza, Molina and Stultz1998; Collett et al. Reference Collett, Crowley, Gimpel and Greenson2000; Dumenci et al. Reference Dumenci, McConaughy and Achenbach2004; Toplak et al. Reference Toplak, Pitch, Flora, Iwenofu, Ghelani, Jain and Tannock2009) and are also globally associated with measures of increased psychopathology. We discuss each of these observed patterns below.
Tics
Two of the eight identified OCD factors (symmetry/exactness and fear-of-harm) were clinically and genetically related to TS, as indicated by the change in TS heritability when either factor was added as a covariate to the model. One of these, symmetry/exactness, not only replicates and expands on earlier work in this sample that also found a relationship between symmetry symptoms and tics (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017; Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016), but is also in line with the new DSM-5 classification system that recognizes a specific tic-related subtype of OCD (Leckman et al. Reference Leckman, Denys, Simpson, Mataix-Cols, Hollander, Saxena, Miguel, Rauch, Goodman, Phillips and Stein2010). The symmetry/exactness factor had the highest internal reliability and heritability of all the OCD factors, and these symptoms were also a core feature of LC2, suggesting that this phenotype is robust and of relevance to genetic studies of TS. Previous studies of TS samples also identified a relationship between tics and symmetry symptoms (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017; Huisman-van Dijk et al. Reference Huisman-van Dijk, Schoot, Rijkeboer, Mathews and Cath2016), and recent work in the current sample indicates that symmetry symptoms in the absence of tics are associated with TS genetic susceptibility but not with OCD genetic susceptibility (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017).
The other factor that was genetically and clinically associated with TS was fear-of-harm. These symptoms, which include fears of harming oneself or others, taking measures to prevent harm, and touching, tapping and rubbing, typically cluster with aggressive urges and taboo fears in OCD samples (Bloch et al. Reference Bloch, Landeros-Weisenberger, Rosario, Pittenger and Leckman2008; Katerberg et al. Reference Katerberg, Delucchi, Stewart, Lochner, Denys, Stack, Andresen, Grant, Kim, Williams, den Boer, van Balkom, Smit, van Oppen, Polman, Jenike, Stein, Mathews and Cath2010). However, our analysis suggests that further investigation of these symptoms as a separate phenotype in individuals with TS may also be important. Fears-of-harm were among the most highly endorsed OCD symptoms in our TS sample, and also showed evidence of genetic relationships with TS.
OCD
Two symptom subgroups, superstitions and need for sameness, were OCD-specific, and did not show any strong clinical or genetic associations with other psychiatric diagnoses or severity measures. Although often excluded from factor analyses as ‘miscellaneous’ symptoms, superstitious symptoms have been previously identified as a distinct subgroup in at least one item-level factor analysis of OCD-affected individuals (Katerberg et al. Reference Katerberg, Delucchi, Stewart, Lochner, Denys, Stack, Andresen, Grant, Kim, Williams, den Boer, van Balkom, Smit, van Oppen, Polman, Jenike, Stein, Mathews and Cath2010). The current study indicated good heritability for the superstitions factor, although it had no genetic relationships with the other OCD symptom factors, or with OCD symptom severity. The second OCD-specific symptom subgroup, need for sameness, comprises two items, need to do things in exactly the same way every time, and the need to keep a strict timetable for routine activities. These symptoms are also typically not included in OCD factor analyses; in our cross-disorder analysis in this sample, the first symptom was included in the symmetry factor, while the second did not reliably load on any factor (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017). It is likely that the sameness factor is more closely associated with obsessive-compulsive personality disorder than frank OCD, accounting for its nonsignificant correlation with the remaining OCD factors. Neither superstitions nor need for sameness were associated with OCD diagnosis or age-of-onset, nor with any other psychiatric diagnosis, although need for sameness was associated with increased OCD severity, and superstitions were associated with a later TS age-of-onset. In the LCA, superstitions were the least frequently endorsed symptom group for all classes, while need for sameness was the third most commonly endorsed symptom group in LC3 (symmetry/exactness, hoarding and ADHD), and the fifth most frequently endorsed in LC2 (OCD&ADHD). In addition, while superstitions were positively correlated with other OCD symptom subgroups, need for sameness was not, suggesting that this symptom subgroup may tap into a different clinical phenomenon than the other OCD symptoms (e.g. obsessive compulsive personality disorder).
ADHD
Only one OCD factor was associated with ADHD both clinically and genetically—hoarding (hoarding obsessions and compulsions and fear of losing things). Hoarding symptoms were also clinically and genetically related to OCD and to OCD symptom severity, although not to TS, anxiety, mood or DBDs. Hoarding symptoms were elevated in both LC2 and LC3, as were inattentive and hyperactive symptoms. These findings parallel recent literature that finds elevated rates of ADHD (Frost et al. Reference Frost, Steketee and Tolin2011) and executive dysfunction patterns that mimic ADHD (Tolin et al. Reference Tolin, Villavicencio, Umbach and Kurtz2011) among individuals with hoarding symptoms; furthermore, there is complementary evidence supporting a genetic relationship between hoarding and ADHD (Fullana et al. Reference Fullana, Vilagut, Mataix-Cols, Adroher, Bruffaerts, Bunting, de Almeida, Florescu, de Girolamo, de Graaf, Haro, Kovess and Alonso2013). It should be considered that the presence of ADHD symptoms in this sample may, in part, result from an ‘executive-overload model’ of OCD (Abramovitch et al. Reference Abramovitch, Dar, Mittelman and Wilhelm2015), in which disrupted neuronal maturation found in pediatric OCD leads to ADHD-like symptoms (Abramovitch et al. Reference Abramovitch, Dar, Mittelman and Schweiger2013). This model has been used to explain the mis-diagnosis of ADHD in OCD pediatric samples.
Anxiety
Two OCD symptom groups, are arguably among the most pathognomonic and recognizable OCD symptoms, were clinically associated with anxiety disorders—contamination/cleaning and doubts/scrupulosity (which includes religious and morality obsessions, checking for mistakes and for inadvertent harm, need to confess, mental rituals, and re-reading/re-writing). Although we do not have sufficient power to examine the genetic relationships between these factors and anxiety disorders, a recent twin study demonstrated strong genetic relationships between washing and religious/sexual obsessions (parallel to our contamination and doubts factors) and anxiety disorders (Lopez-Sola et al. Reference Lopez-Sola, Fontenelle, Verhulst, Neale, Menchon, Alonso and Harrison2016).
Global psychopathology
Finally, one OCD symptom group, aggressive urges, and both ADHD symptom subgroups were associated with multiple forms of psychopathology in our sample, and were genetically related to the three core diagnoses, TS, OCD, and ADHD. The OCD aggressive urges factor, which in this study is comprised primarily of unreasonable (ego-dystonic) urges to be destructive or harm one's self or others, has been identified previously in TS samples (Alsobrook & Pauls, Reference Alsobrook and Pauls2002; Storch et al. Reference Storch, Murphy, Geffken, Soto, Sajid, Allen, Roberti, Killiany and Goodman2004), and parallels symptom subtypes identified in the tic-only analysis (which identified socially inappropriate or disinhibited tics) (Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016) and the cross-disorder analysis (a disinhibition factor comprised socially inappropriate tics plus aggressive and hoarding symptoms, but not ADHD symptoms) (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017).
Both of the ADHD factors (inattentive and hyperactive/impulsive) were also non-specifically, and strongly, associated with the majority of the severity and age-of-onset measures of psychopathology, as well as with TS, OCD, ADHD, and DBD diagnoses (the inattentive factor was additionally associated with mood and anxiety disorders). ADHD symptoms were elevated in all three latent classes, although to a lesser extent in the ‘unaffected’ class, LC1, and the heritability analyses suggested that both factors had strong genetic relationships with ADHD, TS, and OCD. As with the aggressive urges factor, this pattern suggests that ADHD symptoms, at least in this TS sample, may be manifestations of a global underlying psychopathology rather than being specific for any particular DSM-based categorical disorder (including ADHD). Together with previous tic-only and cross-disorder analyses (Darrow et al. Reference Darrow, Hirschtritt, Davis, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2017; Hirschtritt et al. Reference Hirschtritt, Darrow, Illmann, Osiecki, Grados, Sandor, Dion, King, Pauls, Budman, Cath, Greenberg, Lyon, Yu, McGrath, McMahon, Lee, Delucchi, Scharf and Mathews2016), the current findings also suggest a common underlying failure of top-down cognitive control represented by symptoms from all three diagnostic categories (e.g. multiple ADHD symptoms, copro- and echo-phenomena, aggressive obsessions). Specifically, evidence from functional neuroimaging and neuropsychological paradigms in ADHD (Friedman-Hill et al. Reference Friedman-Hill, Wagman, Gex, Pine, Leibenluft and Ungerleider2010), OCD (Zhang et al. Reference Zhang, Wang, Yang, Wu, Li, Chen, Yue, Tang, Yan, Lui, Huang, Chan, Zang, He and Gong2011), and TS (Wang et al. Reference Wang, Maia, Marsh, Colibazzi, Gerber and Peterson2011) suggest the presence of disrupted connectivity between various regions of the prefrontal cortex and posterior cortical regions that corresponds to dysregulated behavioral control (e.g. failure of dorsolateral prefrontal cortical inhibition of involuntary movements regulated by the basal ganglia in TS) (Arnsten & Rubia, Reference Arnsten and Rubia2012).
Limitations
The data for this study were gathered over multiple years, and for ADHD, using different symptom surveys, and we only had psychiatric diagnoses for a subset of the sample. Nonetheless, we arrived at the conventional two-factor solution for ADHD that demarcated inattentive from hyperactive/impulsive symptoms, suggesting the robust nature of this factor model. Factor and LCA are inherently subjective; however, the use of a priori criteria for choosing among models reduced the arbitrary nature of these psychometric techniques. Last, although we analyzed OCD and ADHD symptoms separately to identify subtle symptom patterns for future research, in TS families, OCD and ADHD are highly etiologically related to one another. Although it is possible that such relationships could confound our results, our previous cross-disorder analyses suggests that, in fact, OCD and ADHD symptoms factor separately, somewhat alleviating this concern.
Implications
This work suggests that OCD and ADHD symptom subgroups in TS families may represent markers for distinct underlying patterns of psychopathology. Symptom-based phenotypes could be exploited in future research to identify additional genes or gene pathways relevant to the etiology of neuropsychiatric disorders. For example, converging evidence from multiple studies suggests that OCD symmetry symptoms may represent a robust endophenotype of TS (rather than an endophenotype of OCD) that could be an independent target for genetic studies, whereas contamination and scrupulosity symptoms may be relevant to understanding the pathophysiology of anxiety disorders. Finally, the genetic overlap between OCD and TS in disinhibition symptoms supports further investigation of ‘top-down’ cortical control in subsequent neuroimaging studies among family members with high familial TS loading. From a clinical perspective, the association between aggressive and superstitious symptoms (and to a lesser extent symmetry and fear-of-harm symptoms) and increased tic severity or earlier age-of-onset, and between contamination symptoms and increased risk for anxiety disorders may prove useful in predicting and monitoring the course of TS in children and adolescents. Additionally, the identification of contamination symptoms might then lead to ongoing monitoring for, and thus earlier identification of and intervention for subsequent anxiety disorders.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001672
Acknowledgements
We wish to thank the families who participated in this research, the TSAICG study coordinators at each site for their assistance in study logistics, as well as Dr Lea Davis (Division of Genetic Medicine, Department of Medicine, Vanderbilt University) for her advice on heritability analyses. An earlier version of this report was presented as a poster at the Society of Biological Psychiatry Annual Meeting on May 20, 2017 (San Diego, CA, USA).This work was supported by the National Institutes of Health, grant numbers R01MH096767 (‘Refining the Tourette syndrome phenotype across diagnoses to aid gene discovery,’ PI: Carol Mathews), U01NS040024 (‘A genetic linkage study of GTS,’ PI: David Pauls/Jeremiah Scharf), K23MH085057 (‘Translational phenomics and genomics of Gilles de la Tourette syndrome,’ PI: Jeremiah Scharf), K02MH00508 (‘Genetics of a behavioral disorder: Tourette syndrome,’ PI: David Pauls), and R01NS016648 (‘A genetic study of GTS, OCD, and ADHD,’ PI: David Pauls), and from the Tourette Syndrome Association.
Declaration of Interest
Drs Darrow, Hirschtritt, Illmann, Greenberg, McGrath, and Delucchi, and Ms. Osiecki reported no biomedical financial interests or potential conflicts of interest. Drs Grados, Sandor, McMahon, Pauls, Dion, King, Budman, Cath, Lyon, and Lee received research support from the Tourette Association of America (TAA). Dr Cath has received speakers’ honoraria from Pfizer BV. Dr Budman reported funding for clinical research studies from Psyadon Pharmaceuticals, Assurex/TEVA Pharmaceuticals, Synchroneuron Pharmaceuticals, Neurocrine Pharmaceuticals, and Otsuka Pharmaceutical; she also serves as a consultant for Bracket and a speaker for the Tourette Association of America-Center for Disease Control Partnership. Dr Lyon serves on advisory boards for GenePeeks, Inc. and Omicia, Inc., and has served a consultant to Good Start Genetics. Dr Sandor has received unrestricted educational grants from Purdue and Shire, clinical research from Otsuka Pharmaceutical, a speaker fee from Purdue, and was a member of the data safety monitoring committee for Psyadon Pharmaceuticals. Drs Scharf and Mathews have received research support, honoraria and travel support from the TAA. Dr Scharf is a member of the TAA Scientific Advisory Board and has received consulting fees from Nuvelution Pharma, Inc. Dr Mathews is the co-chair of the TAA Scientific Advisory Board. None of the funding agencies for this project (NINDS, NIMH, TAA) had any influence or played any role in (a) the design or conduct of the study; (b) management, analysis or interpretation of the data; (c) preparation, review or approval of the manuscript. The views expressed in this publication are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.







