Introduction
Avocado (Persea americana Mill.) is a commercially important, evergreen fruit tree grown in tropical and sub-tropical climates (Alcaraz et al., Reference Alcaraz, Thorp and Hormaza2013). Thanks to its high-nutritional value and health-benefitting properties, the avocado is enjoying rising demand worldwide (Migliore et al., Reference Migliore, Farina, Tinervia, Matranga and Schifani2017). However, despite its growing popularity, avocado suffers from the major problem of susceptibility to frost (Ayala Silva and Ledesma, Reference Ayala Silva, Ledesma and Nandwani2014); indeed, frost damage – defined as damage to plant tissue caused by an environmental effect in which the temperature drops below the freezing point of water – is one of the major factors in impairing avocado yields (Perry, Reference Perry1998). Consequently, it also limits the geographic distribution and expansion of avocado cultivation (Charrier et al., Reference Charrier, Ngao, Saudreau and Améglio2015).
The cultivar ‘Hass’ leads global avocado commerce, accounting for over 0.85 of all avocadoes grown and sold worldwide (Ayala Silva and Ledesma, Reference Ayala Silva, Ledesma and Nandwani2014); the cultivar ‘Ettinger’ is commercially less important than ‘Hass’, but is commonly used as a pollinator in ‘Hass’ orchards in Israel. It is pear-shaped with a smooth, thin and bright green skin. In general, avocado trees of West Indian, Guatemalan and West Indian-Guatemalan hybrid varieties show severe frost injuries, whereas trees of Mexican origin are usually more cold-hardy (Cooper and Peynado, Reference Cooper and Peynado1957). ‘Hass’ avocadoes – predominantly Guatemalan with some Mexican genes – exhibit only low-to-moderate frost tolerance, whereas ‘Ettinger’ – predominantly Mexican with some Guatemalan genes – has higher frost tolerance (Rouse and Knight, Reference Rouse and Knight1991; Bergh, Reference Bergh1992; Schaffer et al., Reference Schaffer, Wolstenholme and Whiley2013; Ayala Silva and Ledesma, Reference Ayala Silva, Ledesma and Nandwani2014). The mechanism behind the greater tolerance of ‘Ettinger’ to freezing stress or frost events has not yet been elucidated.
In-field frost injury to plants may be caused by several factors. In general, the freezing temperatures during the frost event may lead to tissue damage by direct mechanical injury by ice formed in the intercellular matrix. In addition, ice formed in the extracellular space decreases its water potential, leading to water movement out of the cells and consequently shrinkage and dehydration of the protoplast (Snyder and de Melo-Abreu, Reference Snyder and de Melo-Abreu2005; Verslues et al., Reference Verslues, Agarwal, Katiyar-Agarwal, Zhu and Zhu2006; De Melo-Abreu et al., Reference De Melo-Abreu, Villalobos, Mateos, Villalobos and Fereres2016). Furthermore, freezing temperatures followed by high-morning light intensity following night frost may lead to excessive excitation of the respiratory and photosynthesis electron transport systems, thereby increasing the concentration of reactive oxygen species (ROS) inside the plant tissue (Janda et al., Reference Janda, Szalai, Rios-Gonzalez, Veisz and Páldi2003). In contrast to oxygen itself, its activated derivatives are especially reactive/toxic and, therefore, may damage cellular components such as lipids, proteins and DNA, with consequent oxidative destruction of cells (Suzuki and Mittler, Reference Suzuki and Mittler2006).
In order to withstand environmental stress, plants have developed efficient antioxidant (ROS-scavenging) mechanisms that may be categorized into two types: enzymatic components such as superoxide dismutase (SOD), ascorbate peroxidase and catalase (CAT); and non-enzymatic antioxidants such as ascorbic acid, reduced glutathione, carotenoids, flavonoids and phenolics (Das and Roychoudhury, Reference Das and Roychoudhury2014). Another freeze-protection mechanism in plants is the accumulation of osmoprotectants – low-molecular-weight metabolites such as sugars, amino acids and quaternary ammonium compounds that increase the ability of cells to retain water, by regulating their osmotic potential (Parvanova et al., Reference Parvanova, Ivanov, Konstantinova, Karanov, Atanassov, Tsvetkov, Alexieva and Djilianov2004; Pramsohler and Neuner, Reference Pramsohler and Neuner2013).
Most of the knowledge on frost-stress responses of the cultivars ‘Hass’ and ‘Ettinger’ is based on observations in orchards, of frost-related damage following winter frosts. However, there is a lack of evidence-based knowledge of the mechanism by which ‘Ettinger’ trees tolerate freezing temperatures followed by high-light intensity. Therefore, the main objective of the present study was to examine the mechanism that accounts for the difference between these two avocado cultivars in their responses to controlled frost stress.
Materials and methods
Plant material
‘Ettinger’ and ‘Hass’ branches were collected from a commercial avocado orchard located in Kibbutz Kfar Giladi in northeast Israel (33°24′56″N, 35°58′32″E, 260–263 m asl). Trees were planted in 2012 with 6 m spacing between the rows and 4 m between the trees. The rows were oriented north/south; canopy height was ~3 m. Vegetative branches 30 cm in length were removed from at least 20 different trees of each cultivar. All the trees that were selected for the study had similar external properties, i.e. the same age, canopy size, leaf density and foliage colour.
Temperature measurements
Field air temperature data were collected from a commercial avocado orchard located in Kibbutz Regba in northwest Israel (32°58′32″N, 35°06′31″E; 20–21 m asl) during winter, from early January to mid-March 2017. The data were recorded continuously at 10-min intervals by three waterproof, single-channel, Hobo miniature temperature data loggers (UA-001-64; Onset Corp., Bourne, MA, USA) that were placed 0.5 m above the ground on the western side of the trees, shaded from direct radiation by the canopy (Bar-Noy et al., Reference Bar-Noy, Sofer-Arad, Perel, Cohen, Senesh, Noy and Rubinovich2019).
For the artificial frost treatments, air temperature was measured in the climatic test cabinet (CTC) with a single data logger.
Artificial frost-stress treatment and damage assessment
The artificial frost treatment was performed by exposing detached branches to freezing temperatures followed by high light (FHL). In detail, branches from mature ‘Ettinger’ and ‘Hass’ avocado trees were placed in 10-litre buckets containing water to a depth of 20 cm and transported immediately to a dark room for 20–30 min of dark adaptation. Five branches from each cultivar were placed in 1-litre carafes containing 20 cm of water and moved to a TK600 CTC (Nuve, Ankara, Turkey) under darkness and gradually exposed to a nine-step temperature programme: a. 13.3 °C; b. 7.4 °C; c. 4 °C; d. −1.2 °C; e. −3.3; f. −4 °C; g. −4.5 °C; h. −2.9 °C and i. 12 °C; the duration of each step was 2 h. During this programme, untreated branches remained in the dark room. Then, the carafes were transferred to a growth room at 20 °C and exposed to LED light at 700 µmol/m2/s for 5 h. For the rest of the experiment, the carafes remained in the growth room at the same temperature under fluorescent light at 30 µmol/m2/s. Twenty-four hours after the end of the FHL treatment, leaf damage on both treated and untreated branches was assessed on a 0–5 scale, with 0 representing no apparent damage and 5 maximum damage.
Chlorophyll a fluorescence analysis
After dark adaptation for at least 20 min, chlorophyll a fluorescence parameter was recorded under darkness with a FluorPen FP100 portable fluorometer (Photon Systems Instruments, Drasov, Czech Republic), and the maximum quantum yield was calculated (Schreiber et al., Reference Schreiber, Schliwa and Bilger1986; Kramer et al., Reference Kramer, Johnson, Kiirats and Edwards2004; Baker, Reference Baker2008). Controls comprised five branches of each cultivar that were not exposed to the FHL treatment. The experiment was repeated at least 10 times during all months of the year.
Browning of cut branches assay
One-year-old branches were cut from mature ‘Ettinger’ and ‘Hass’ avocado trees with pruning shears; the cut surfaces were visually assessed for browning immediately upon cutting and then every 5 min for 10–20 min. This assay was carried out at least 15 times during spring, summer and autumn, and pictures were taken with a digital camera.
In-situ antioxidant activity assays
Discs were excised from five mature leaves that had been taken during spring, summer and autumn, from each of five trees of each cultivar and immersed in 5 or 25 mm methyl viologen (MV) as described in Wei et al. (Reference Wei, Gao, Liang, Zhao, Ma and Li2018). Double-distilled water (DDW) was used as a negative control. After 24 h, tissue browning, manifesting oxidative cell damage, was assessed visually.
For the assessment of superoxide accumulation following wounding stress, leaf discs were cut (three horizontal cuts) with a scalpel. After 30 min they were placed in staining buffer containing 0.1% nitroblue tetrazolium (NBT) in 50 mm sodium phosphate (pH 7.5) for 4 h of incubation. Superoxide accumulation in leaf discs was monitored visually after foliar decolourization in hot 96% ethanol.
In-vitro antioxidant activity measurements
Leaves were removed from mature ‘Ettinger’ and ‘Hass’ avocado trees during spring, summer and autumn, or from detached branches used in controlled FHL treatments; four leaves were taken from each of five different branches. The leaves were frozen in liquid oxygen, freeze-dried with a lyophilizer and ground in the presence of liquid nitrogen with a mortar and pestle; 50 mg samples of the ground leaves were re-suspended in 1 ml of DDW.
The first assay used was the ferric reducing ability of plasma (FRAP) method (Tzulker et al., Reference Tzulker, Glazer, Bar-Ilan, Holland, Aviram and Amir2007). An intense blue colour forms when the ferric-tripyridyltriazine (Fe3+ TPTZ) complex is reduced to the ferrous (Fe2+) form. Avocado extracts were diluted 1:10 (v:v) in DDW and 50 µl aliquots of diluted samples were mixed with 285 µl of Fe3+ solutions and left to react under darkness at room temperature for 4 min. Then optical density was recorded at 593 nm with an Infinite 200 PRO fluorometer (Tecan, Männedorf, Switzerland). A standard curve was generated based on 0–1.6 mm solutions of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) in methanol. Results were expressed as Trolox equivalent.
The second assay method was the 2,2 diphenyl-1-picrylhydrazyl (DPPH) test (Payne et al., Reference Payne, Mazzer, Clarkson and Taylor2013); when the free radical DPPH in the sample is reduced by addition of antioxidants, absorbance at 517 nm decreases. Aliquots (50 µl) of the above avocado extracts or of DDW as negative control were mixed with 100 ml of 4 mm DPPH in ethanol and incubated under darkness for 45 min. Optical density was recorded at 517 nm with a fluorimeter, and the difference in absorbance between the samples and their negative controls was calculated as: $\left( {\displaystyle{{ABS_{{\rm Control}} - ABS_{{\rm Sample}}} \over {ABS_{{\rm Control}}}}} \right) \times 100$![]() .
.
The third assay method was total antioxidant capacity (TAC) analysis with an MAK187 TAC assay kit (Sigma Aldrich, St. Louis, MO, USA), according to the manufacturer's instructions. In brief: samples containing 5 µl of diluted (1:10 v:v) leaf extract, with or without 50 µl of protein mask solution (the use of the protein mask prevents Cu2+ reduction by protein activity), were added to DDW to a final volume of 100 µl. For positive control, the leaf extract was replaced with 10 µl of 1:10 diluted CAT (9001-05-2, Sigma Aldrich) or 33 µl of SOD (S5395, Sigma Aldrich) and for negative control, with 10 µl of 0.58 mm gallic acid (G7384, Sigma Aldrich). The samples were mixed and placed on an ELISA plate, covered with aluminium foil and incubated for 45 min at room temperature. A 100 μl aliquot of the Cu2+ working solution was then added to each sample, and these reaction solutions were incubated for an additional 90 min. Optical absorbance was measured at 570 nm with a fluorimeter and Trolox was used as a standard for calibration curve plotting.
Determination of total phenol content
Total phenol compounds (TPC) were determined by the gallic acid equivalence (GAE) method, a colorimetric method described by Nasr et al. (Reference Nasr, Ayed and Metche1996). Ten microliters of the above avocado extracts were mixed with 40 µl of 7.5% (w/v) sodium carbonate solution and 50 µl of Folin–Ciocalteu reagent diluted 1:10 in water. A standard curve was based on gallic acid (catalogue no. 149-91-7; Sigma Aldrich) at 50–500 mg/l. Optical absorbance was measured at 760 nm, and results were expressed as gallic acid equivalent.
Determination of osmotic strength
The osmolality of the leaf extract was determined with an OSM-9 osmometer (Samed, Frankfurt, Germany). Briefly: 100 µl of the leaf extracts were pipetted into 1.5-ml sample tubes, which were attached to the measuring head. Calibration was with DDW.
Statistical analysis
All results were subjected to either Student's t-test or Tukey's HSD test, by means of the JMP software, version 11.0.0 (SAS Institute, Cary, NC, USA).
Results
Comparison of artificial frost stress tolerance
To investigate the difference in frost tolerance between ‘Hass’ and ‘Ettinger’, a laboratory-controlled artificial frost-stress experiment was performed by exposing detached branches to freezing temperatures followed by high-light intensity (FHL treatment). To mimic the temperature gradient of the controlled experiments to that of an actual in-field frost event, temperature gradient that damaged ‘Hass’ leaves during a frost event in a commercial avocado orchard was monitored and recorded (Figs 1 and 2). Then, a similar stepwise temperature gradient was programmed for the CTC, and the actual air temperatures were recorded (Fig. 3).

Fig. 1. Air temperature measured at 0.5 m above the ground during a frost event on the night of −2 February 2017 in the Kibbutz Regba avocado orchard. Each data point represents a mean of three temperature data logger records.

Fig. 2. (Colour online) Tree foliage frost damage. Picture of representative ‘Hass’ tree taken a few days after the frost event that occurred on the night of 1–2 February 2017.

Fig. 3. Actual air temperature gradient during the freezing temperatures followed by high light (FHL) treatment as measured in the CTC.
Before the beginning of this experiment there was no visual difference between the two cultivars, in their leaves or stems. After 24 h from the end of the FHL treatment, ‘Hass’ leaves showed typical severe frost-damage symptoms; most of the leaves turned bronze-brown. In contrast, most of the ‘Ettinger’ leaves remained green and vital (Fig. 4(a)). Leaf damage assessment showed that ‘Hass’ leaves were significantly (P < 0.05) more severely damaged than ‘Ettinger’ leaves (Fig. 4(b)).

Fig. 4. (Colour online) Artificial frost stress response in detached ‘Hass’ and ‘Ettinger’ branches.
(a) Mature branches from both cultivars were cut and exposed to freezing temperatures in a temperature-controlled cabinet under darkness followed by an additional 5 h of exposure to high light (FHL treatment). Images of the FHL-treated branches were taken 24 h after the end of the treatment. (b) Damage on control and FHL-treated branches was assessed on a 0–5 scale. (c) Chlorophyll a fluorescence parameter recorded after dark adaptation for the calculation of the maximum quantum yield (F v/F m). Values are means ± s.e. of five biological replicates (n = 5), i.e. four leaves from each of five branches. Columns marked with an asterisk differ significantly (Student's t, * P < 0.05).
Consistent with these observations, similar maximum quantum yield (F v/F m) was observed in both cultivars in the control treatment; however, following the FHL treatment, F v/F m in both cultivars was lower than that in the control: by 36.9% in ‘Hass’ but by only 5.01% in ‘Ettinger’ (Fig. 4(c)). In both cultivars, leaves from branches that were cut and remained in the growth room for 48 h without exposure to the FHL treatment showed no leaf damage and their F v/F m values were similar, with a rate of ~0.8 (data not shown).
Comparison of in-situ antioxidant activity
There are numerous mechanisms by which plants may acquire enhanced tolerance to frost stress and other abiotic stresses. One well-described mechanism is the accumulation of active antioxidants, which provide high-antioxidant activity (AA) for scavenging free radicals and thus reduces cellular oxidative damage (Das and Roychoudhury, Reference Das and Roychoudhury2014). Hence, it would be reasonable to consider that the observed difference in frost tolerance between ‘Hass’ and ‘Ettinger’ is related to their AA. To investigate this hypothesis, in-situ assays to evaluate general AA were carried out. First, leaf discs were excised from both cultivars and immersed for 24 h in MV at various concentrations, to artificially induce oxidative stress. Dose-dependent tissue browning attributed to oxidative cell damage was observed in leaves from both cultivars immersed in MV at 5 or 25 mm, but not in leaves immersed in water (Fig. 5(a)). Interestingly, at both MV concentrations, tissue browning was much lower in ‘Ettinger’ than in ‘Hass’. Second, excised leaf discs were wounded by making three horizontal cuts in order to elicit an oxidative burst. The NBT assay, which enables in-situ visualization of superoxide, revealed that an accumulation of this radical, i.e. an oxidative burst, was induced in the cuts on the leaf discs (Fig. 5(b)). Strong colouration, indicating high accumulation of superoxide molecules, was observed at the cut sites of ‘Hass’ leaves, but this colouration was much lower in ‘Ettinger’ leaves.
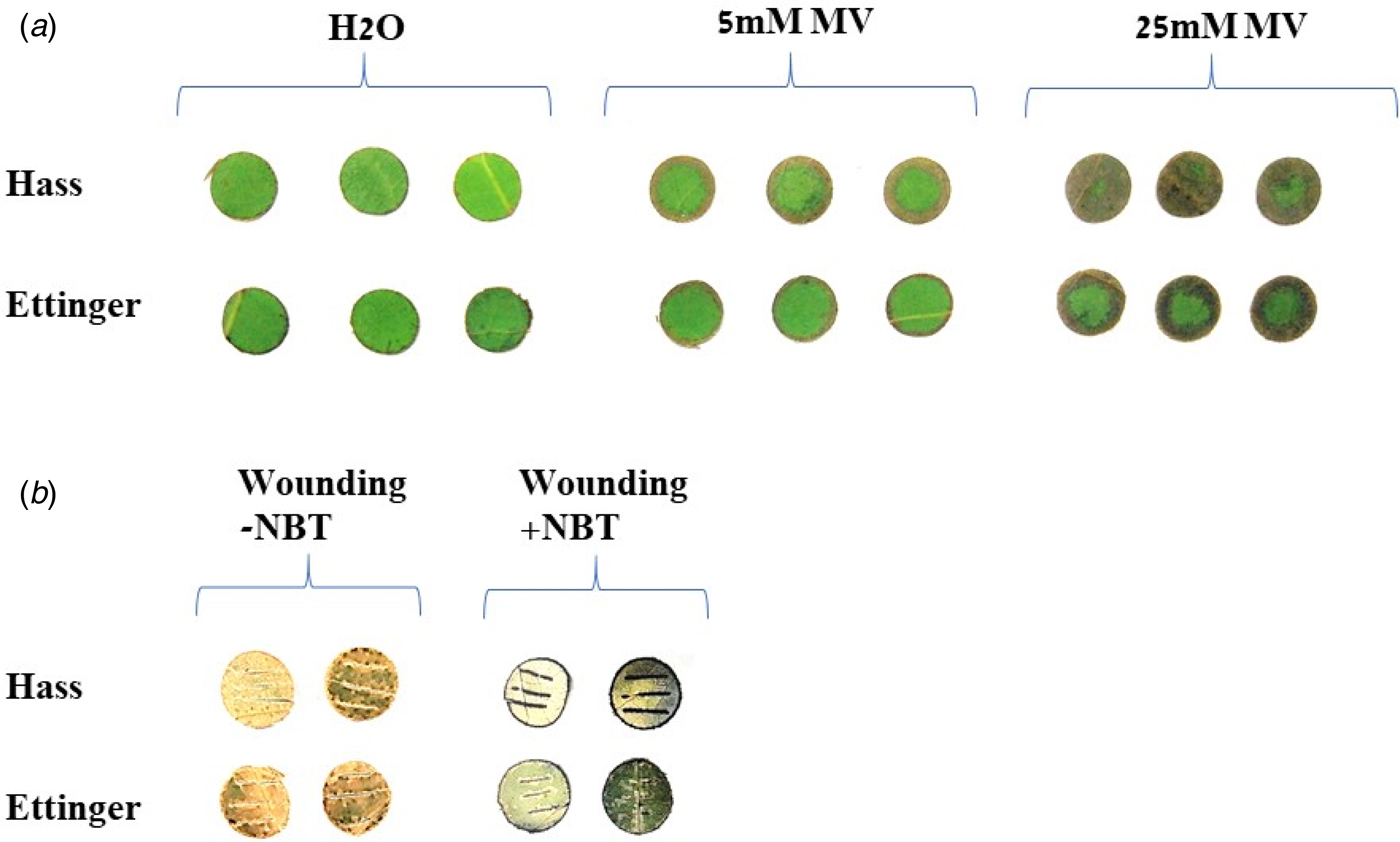
Fig. 5. (Colour online) In-situ AA assays. (a) Leaf discs were excised from mature leaves of ‘Hass’ and ‘Ettinger’ trees and immersed in water (H2O) or in 5 or 25 mm MV for 24 h, and browning was visually analysed. (b) Detached mature leaves from both cultivars were cut, immersed in staining buffer with or without 0.1% NBT for 3 h. Leaves were de-colourized in hot ethanol and visually analysed for superoxide staining.
To further validate the hypothesis that ‘Ettinger’ harbours enhanced AA over ‘Hass’ a simple test was performed in the field; branches from mature trees of each cultivar were cut and the cut surfaces were monitored for browning, which would indicate oxidation of the sap. Interestingly, although the cut surface on ‘Hass’ branches gradually turned brown over 10 min, it remained almost unchanged on ‘Ettinger’, implying rapid oxidation of ‘Hass’ sap but not of that of ‘Ettinger’ (Fig. 6).

Fig. 6. (Colour online) Browning of the cut surface area. One-year-old branches from both cultivars were cut, and the browning of the cut surface was assessed visually over 10 min. The experiment was repeated at least 15 times throughout the year, with similar results.
In-vitro comparison of antioxidant activity and osmolarity
To further support these observations and to investigate the origin of the greater AA of ‘Ettinger’, several quantitative assays were performed in-vitro. First, to test the hypothesis that ‘Ettinger’ harbours higher AA than ‘Hass’, the FRAP assay was used to measure this activity in leaf extracts from both cultivars. The results showed that, in line with previous observations, the AA of ‘Ettinger’ was significantly (P < 0.001) higher, by about 127%, than that of ‘Hass’ (Fig. 7(a)). Similar significant (P < 0.001) differences were revealed by the DPPH assay (Fig. 8).
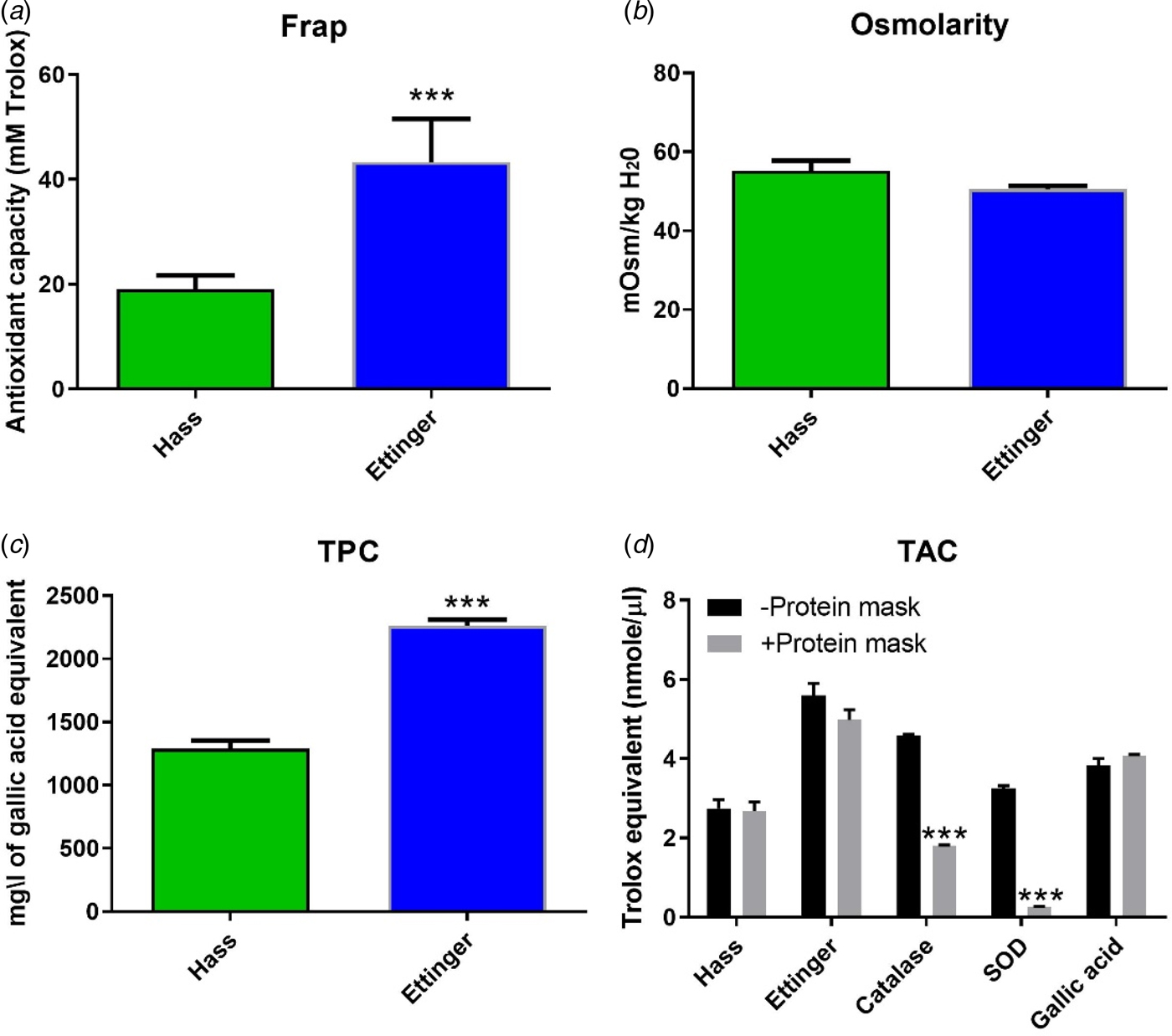
Fig. 7. (Colour online) In-vitro biochemical assays in ‘Ettinger’ and ‘Hass’ leaf extracts. (a) AA measured with the FRAP assay. (b) Osmotic strength of cell solute measured with an osmometer. (c) Total phenolic content measured by the GAE method. (d) AA measured by using the TAC assay with or without addition of a protein mask. CAT and SOD were used as a positive controls and gallic acid as a negative control. Values are means ± s.e. of five biological replicates (n = 5), i.e. four leaves from each of five trees. Each experiment was repeated at least three times, with similar results. Significant differences are indicated by asterisks above the columns (Student's t, *** P < 0.001).
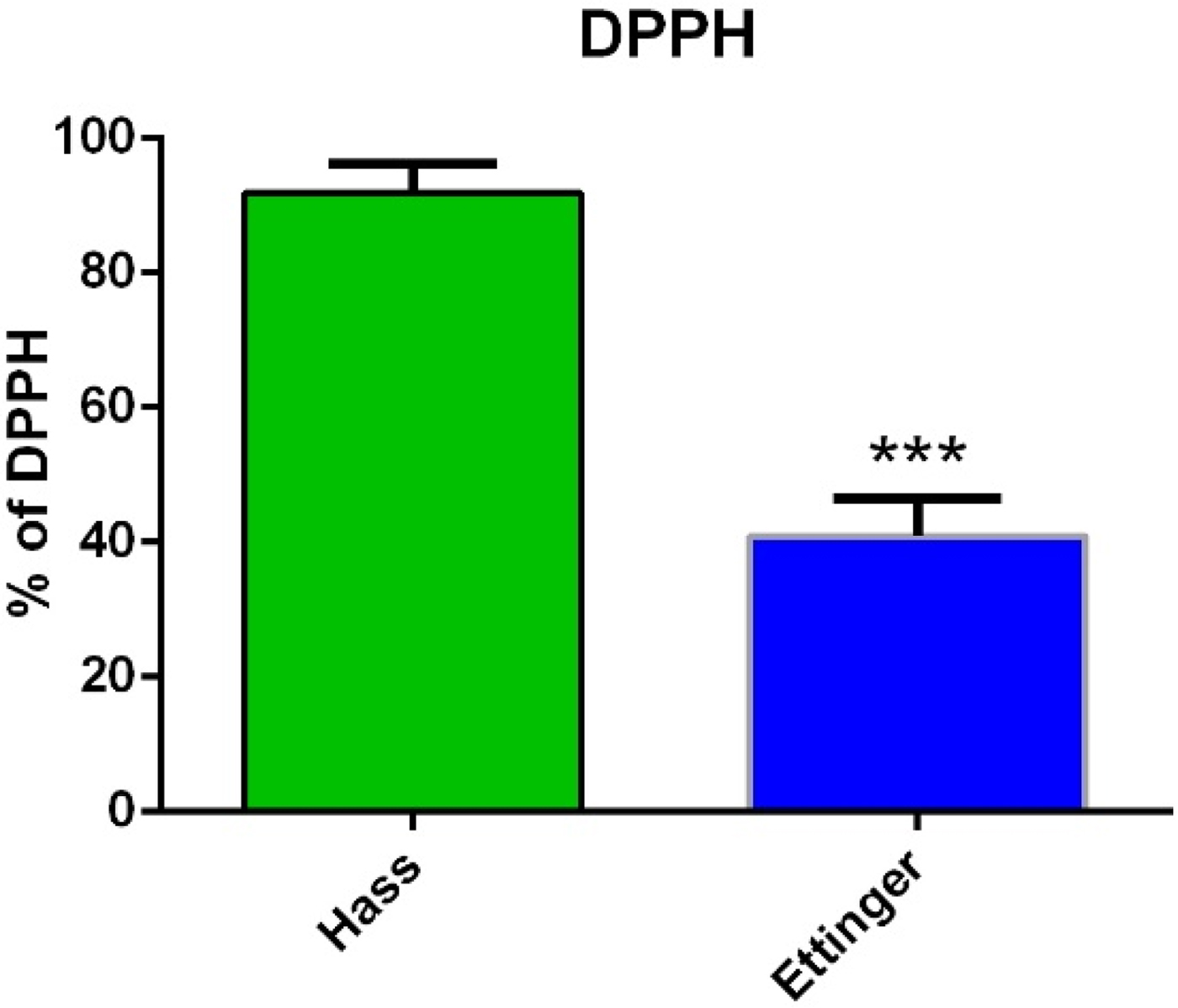
Fig. 8. (Colour online) ‘Ettinger’ and ‘Hass’ leaf extracts AA measured with the DPPH assay. Leaf AA was measured with the DPPH assay. Values are means ± s.e. of five biological replicates (n = 5), i.e. four leaves from each of five different trees. Each experiment was repeated at least three times, with similar results. Significant differences are indicated by an asterisk above the columns (Student's t, *** P < 0.001).
To investigate whether osmotic strength differences might account for differences between the two cultivars in frost tolerance, the cell solute osmotic strength of ‘Hass’ and ‘Ettinger’ was measured with an osmometer. Interestingly, in contrast to their AAs, there were no significant differences between the osmolarity of the two cultivars (Fig. 7(b)). To further investigate why the AA of ‘Ettinger’ was greater than that of ‘Hass’, the total polyphenolic content of the two cultivars was then compared by using the GAE assay. The results showed that TPC levels were significantly (P < 0.001) higher, by about 75%, in ‘Ettinger’ than in ‘Hass’, and were positively correlated with the AA of both cultivars (Fig. 7(c)). To assess the contribution of enzymatic factors to the AA of both cultivars, the commercial TAC kit assay was used to compare the AA of leaf extracts of ‘Ettinger’ and ‘Hass’ with or without the addition of a protein mask, which is used to reduce enzymatic activity of the tested solution. The AA of CAT and SOD, which were used as positive controls, decreased significantly (P < 0.001) on addition of the protein mask. In contrast, with gallic acid (which served as a negative control) and the leaf extracts there was only a minor, insignificant change in the AA following addition of the protein mask (Fig. 7(d)).
In-vitro comparison of antioxidant activities following FHL treatment
In order to associate the greater frost tolerance of ‘Ettinger’ than that of ‘Hass’ with its greater AA, as was shown in the field observations and laboratory experiments, the AA of both cultivars was measured in-vitro, following the artificial frost treatment. The results show that the AA of extracts from FHL-treated ‘Hass’ leaves was similar to that of their corresponding control (Fig. 9(a)). However, AA of FHL-treated ‘Ettinger’ leaf extracts was 31.8% greater than that of their control. In contrast, for both cultivars, the osmotic strengths of cell solute from the control and the FHL-treated leaves were similar (Fig. 9(b)).
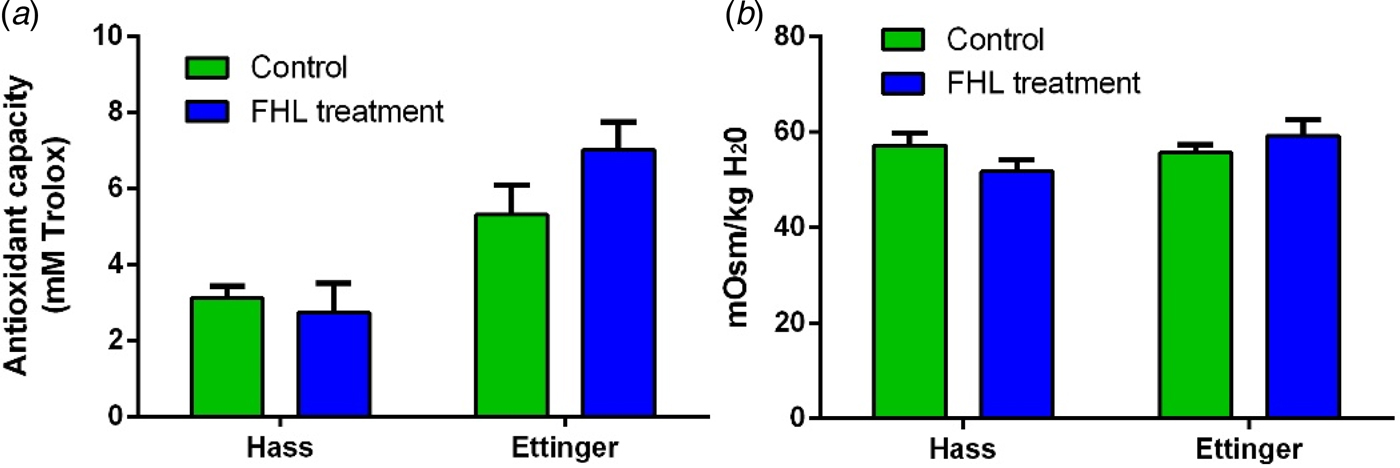
Fig. 9. (Colour online) In-vitro AA and osmolarity of detached branches following the artificial frost treatment. Mature branches from both cultivars were exposed to the freezing temperatures followed by high light (FHL) treatment. (a) AA from freeze-dried leaf extracts measured with the FRAP assay. (b) Osmotic strength of cell solute measured with an osmometer. Values are means ± s.e. of four biological replicates (n = 4), i.e. four leaves from each of four branches. The experiments were repeated at least three times, with similar results.
Discussion
The responses of ‘Hass’ and ‘Ettinger’ avocado cultivars to artificial frost stress were investigated and compared in controlled experiments in the laboratory, by exposing them to freezing temperatures followed by high-light intensity. Their leaf AA, TPC and osmolarity were also assessed, in order to identify a possible mechanism for the greater frost tolerance of ‘Ettinger’ than that of ‘Hass’. Measurements of physiological parameters following the controlled artificial frost treatments in the laboratory showed that detached ‘Ettinger’ branches had greater tolerance of the FHL treatment than ‘Hass’ branches, as their visible damage rate was lower and F v/F m rate, a reliable quantitative indicator of frost damage and other stress damage (Rizza et al., Reference Rizza, Pagani, Stanca and Cattivelli2001; Ehlert and Hincha, Reference Ehlert and Hincha2008; Stern, Reference Stern2015), was significantly higher. These results are consistent with previously described frost-hardy characteristics of ‘Ettinger’ trees – descriptions mostly based on growers' observations over the years (data not shown). Having said that, it should be noted that the artificial frost treatments conducted in the current study simulate only partially an actual in-field natural frost event, as various environmental factors, such as humidity rate, may affect plant reactions (Snyder and de Melo-Abreu, Reference Snyder and de Melo-Abreu2005).
One of the major factors leading to plant cell injury under frost stress is the accumulation of ROS and resulting oxidative damage (Janda et al., Reference Janda, Szalai, Rios-Gonzalez, Veisz and Páldi2003). Therefore, it was hypothesized that the frost tolerance of ‘Ettinger’ might arise from enhanced AA. Previous studies have shown that wounding induces the production of ROS in plants (Le Deunff et al., Reference Le Deunff, Davoine, Le Dantec, Billard and Huault2004) and enhanced AA reduced MV-mediated oxidative damage and ROS accumulation in wounded leaves. For example, in transgenic arabidopsis over-expressing GASA4 and in petunia over-expressing GIP2, both cysteine-rich redox active proteins, hydrogen peroxide (H2O2) accumulation was inhibited in leaves following wounding (Wigoda et al., Reference Wigoda, Ben-Nissan, Granot, Schwartz and Weiss2006; Rubinovich and Weiss, Reference Rubinovich and Weiss2010). In another study, following exposure to MV, oxidative damage symptoms in transgenic Brassica napus plants overexpressing a manganese superoxide dismutase, an important enzyme of the antioxidant pathway, were reduced compared with those in WT leaf discs (Basu et al., Reference Basu, Good and Taylor2001). Thus, both in-situ and in-vivo, the occurrence in ‘Ettinger’ of enhanced tolerance to oxidative damage in the MV assay, reduced accumulation of superoxide following wounding, and only minor browning of the wound surface in cut branches, indicate greater AA in ‘Ettinger’ than in ‘Hass’ leaves, and this could account for the greater tolerance of ‘Ettinger’ to various abiotic stresses.
Damage from a frost event or freeze treatment in plants may also be prevented by inhibiting extracellular ice formation, which can be achieved by increasing cell solute content and consequently reducing osmotic potential (Pramsohler and Neuner, Reference Pramsohler and Neuner2013; Arias et al., Reference Arias, Bucci, Scholz and Goldstein2015). For example, increased total solute accumulation was observed in the freezing tolerant arabidopsis mutant eskimo1 (Xin and Browse, Reference Xin and Browse1998). However, in the present study osmometer measurements that found no significant differences between the osmotic strength of cell solutes from the respective cultivars imply that this parameter was not a major contributor to the difference between the frost tolerance of ‘Ettinger’ and ‘Hass’, which supports the hypothesis that the frost-resistance trait in ‘Ettinger’ derives mainly from its AA.
The antioxidant mechanism that helps mitigate oxidative-stress-induced damage in plants has two components: enzymatic and non-enzymatic antioxidants (Das and Roychoudhury, Reference Das and Roychoudhury2014). The reason(s) for the greater AA in ‘Ettinger’ than in ‘Hass’ were further examined; it was found that TPC levels were significantly higher in ‘Ettinger’ than in ‘Hass’ and were positively correlated with the AA in both cultivars. Moreover, the addition of a protein mask did not significantly change the AA in either cultivar. These results suggest that the greater AA of ‘Ettinger’ has a mainly non-enzymatic cause, e.g. accumulation of phenolic compounds, rather than enhanced enzymatic activity. Similar results were obtained in previous studies in pomegranates and cereals, which showed that their AA was derived mainly from their total phenolic contents and that these two parameters were highly correlated (Tzulker et al., Reference Tzulker, Glazer, Bar-Ilan, Holland, Aviram and Amir2007; Deng et al., Reference Deng, Xu, Guo, Xia, Li, Wu, Chen, Ling and Li2012).
The AA and osmolarity of both cultivars were also investigated following the FHL-stress experiments, and the results suggest that ‘Ettinger’ but not ‘Hass’ responded to this stress by elevating its AA, possibly as a protection mechanism against frost-derived oxidant damage. This post-FHL elevation in the AA of ‘Ettinger’, in addition to its already high-basal AA level, on the one hand, and its unchanged osmolarity, on the other hand, may indicate that AA is the major contributing factor to the greater frost tolerance of ‘Ettinger’. Enhanced AA as a facilitator of frost tolerance was previously described in other plants: for example, elevated levels of ROS-scavenging activities in pine trees were found to be associated with increased freezing tolerance (Tao et al., Reference Tao, Öquist and Wingsle1998). Another study suggested that treating olive plants with salicylic acid (SA) enhanced their freezing tolerance by increasing antioxidant enzymatic activities and total phenolic content (Hashempour et al., Reference Hashempour, Ghasemnezhad, Fotouhi Ghazvini and Sohani2014).
All in all, the above results imply that the major contributor to the greater frost tolerance of ‘Ettinger’ than of ‘Hass’ is its elevated AA and not an osmotic-related factor, and that this activity may be derived mainly from enhanced polyphenolic content and not directly from an enzymatic activity. To the best of our knowledge, the present study is the first that suggests a possible frost-tolerance mechanism in avocado trees. Thus, in addition to the contribution to the basic understanding of the frost-tolerance mechanism in ‘Ettinger’ provided by the knowledge acquired in the current study, several potential applications are suggested. For example, in breeding programmes, selection of the enhanced-AA trait in avocado hybrids could be used to develop new frost-tolerant ‘Hass’-like cultivars. Another possible opportunity may lie in the use of state-of-the-art techniques such as genome editing (Li et al., Reference Li, Teng, Li and Zhou2013; Nishitani et al., Reference Nishitani, Hirai, Komori, Wada, Okada, Osakabe, Yamamoto and Osakabe2016) for developing avocado trees with enhanced-redox-active phenolics to impart in-field frost tolerance. Moreover, agrotechnical practices could be developed to elevate the AA of ‘Hass’ to levels similar to that in ‘Ettinger’ and thereby to prevent frost damage in the orchard. These putative future developments would, in turn, enable expansion of the geographic range of avocado orchards and help to minimize financial losses caused by frost events in avocado-growing countries worldwide. However, further research is required to deepen our understanding of the frost-tolerance mechanism of ‘Ettinger’ and to validate this suggested mechanism in other frost-hardy avocado cultivars.
Acknowledgements
The authors extend their thanks to the Regba avocado team and Mr. Hadar Cohen for their efforts in performing this study, and Ms. Dikla Dawari and Ms. Farah Raslan for their assistance in the field and laboratory measurements.
Financial support
The authors wish to thank the Israeli Avocado Growers Table for their financial support.
Conflict of interest
None
Ethical standards
Not applicable











