Carbapenems are effective and safe; they are the mainstay of therapy for serious Pseudomonas aeruginosa infections.Reference Pogue, Kaye, Cohen and Marchaim 1 – Reference Aloush, Navon-Venezia, Seigman-Igra, Cabili and Carmeli 3 However, there is a significant association between carbapenem nonsusceptibility and adverse clinical outcomes.Reference Lautenbach, Weiner, Nachamkin, Bilker, Sheridan and Fishman 4 Carbapenem-resistant P. aeruginosa (CRPA) is a major epidemiological threat, specifically in countries where the new antipseudomonal β-lactam agents (eg, ceftolozane-tazobactam and ceftazidime-avibactam) are not yet available.
Currently, prevention efforts frequently target CRPA strains. Contact isolation precautions are utilized for CRPA carriers, and screening programs are frequently aimed at CRPA strains.Reference Adler, Friedman and Marchaim 2 However, the mechanisms of resistance of P. aeruginosa to carbapenems are frequently mediated through chromosomal genes.Reference Adler, Friedman and Marchaim 2 Therefore, it might be appropriate to target the prevention of transmission of all P. aeruginosa strains. In addition, the studies that have reported on the association between CRPA and poor outcomes have major limitations: (1) many were not conducted using the matched case–case-control study design,Reference Lautenbach, Weiner, Nachamkin, Bilker, Sheridan and Fishman 4 (2) many did not strictly adhere to infection definitions that differentiate infection from asymptomatic carriage,Reference Aloush, Navon-Venezia, Seigman-Igra, Cabili and Carmeli 3 and (3) many have not controlled for delay in initiation of appropriate antimicrobial therapy (DAAT).Reference Lautenbach, Weiner, Nachamkin, Bilker, Sheridan and Fishman 4 All of these factors might have led to the overestimation of the independent association between resistance to carbapenems and poor clinical outcomes. For CRPA prevention programs to be effective, it is important to differentiate DAAT from the isolated impact of the resistance determinant to appropriately allocate prevention resources. Moreover, exploring the independent predictors of CRPA using an appropriate design can help guide stewardship interventions aimed at reducing DAAT and improving outcomes.
Methods
A retrospective matched case–case-control analysis was conducted among adults (>18 years old) at the Assaf Harofeh Medical Center, Israel, from 2007 to 2012. The institutional ethics committee approved the study. Resistant cases were defined as patients with bloodstream infections (BSIs) due to P. aeruginosa that were not susceptible to either meropenem or imipenem (MIC ≥ 4 µg/dL for both). 5 Susceptible cases were defined as patients with P. aeruginosa BSIs that were susceptible to both meropenem and imipenem. The uninfected control group consisted of patients without P. aeruginosa infection or BSI. Patients were included in the analysis only once. A susceptible case and an uninfected control were matched to a resistant case (1:1:1 ratio) according to time at risk,Reference Kaye, Harris, Samore and Carmeli 6 hospital unit, and calendar year. Eligible susceptible cases and uninfected controls were randomly selected (Excel software, Microsoft, Redmond WA).
Data were collected from available records. Posthospitalization deaths were captured from a national registry. The primary outcome was 14-day mortality. Assuming a 21% mortality rate among cases and 10% among controls,Reference Aloush, Navon-Venezia, Seigman-Igra, Cabili and Carmeli 3 we calculated that a population of 225 patients would be needed to provide adequate power (β = 0.8) to detect significant differences between cases and controls. Logistic and Cox regressions were used to capture predictors and outcomes of CRPA infections. All models were assessed for collinearity and were controlled for confounding. A matched analysis was performed comparing resistant cases and uninfected controls. We forced 2 variables into each outcome model: carbapenem resistance and DAAT (as a continuous variable and DAAT > 48 hours, whichever statistical association was stronger). All tests were 2-tailed.
Results
We matched 85 CRPA BSI cases to 85 case patients with carbapenem-susceptible P. aeruginosa (CSPA) BSI (from 491 eligible patients) and to 85 uninfected control patients (from 2,046 eligible patients). In total, 255 patients were enrolled. The study population consisted of elderly (69%) and functionally dependent (48%) individuals,Reference Katz, Ford, Moskowitz, Jackson and Jaffe 7 with high Charlson’s comorbidity indices (5.7 ± 3.1).Reference Charlson, Pompei, Ales and MacKenzie 8
Predictors of CRPA BSI
Most bivariate predictors associated with CRPA BSI, primarily certain demographics and background conditions, were also associated with CSPA BSI (Table 1). The distribution of the infectious syndromes was similar. Acute illness indices were more severe among patients with CRPA infections (Table 1).
Table 1 Selected Bivariable Analyses Comparing Risk Factors Associated With Resistant Case Patients, Susceptible Case Patients, and Uninfected Control Patients (n = 85 patients in each group)
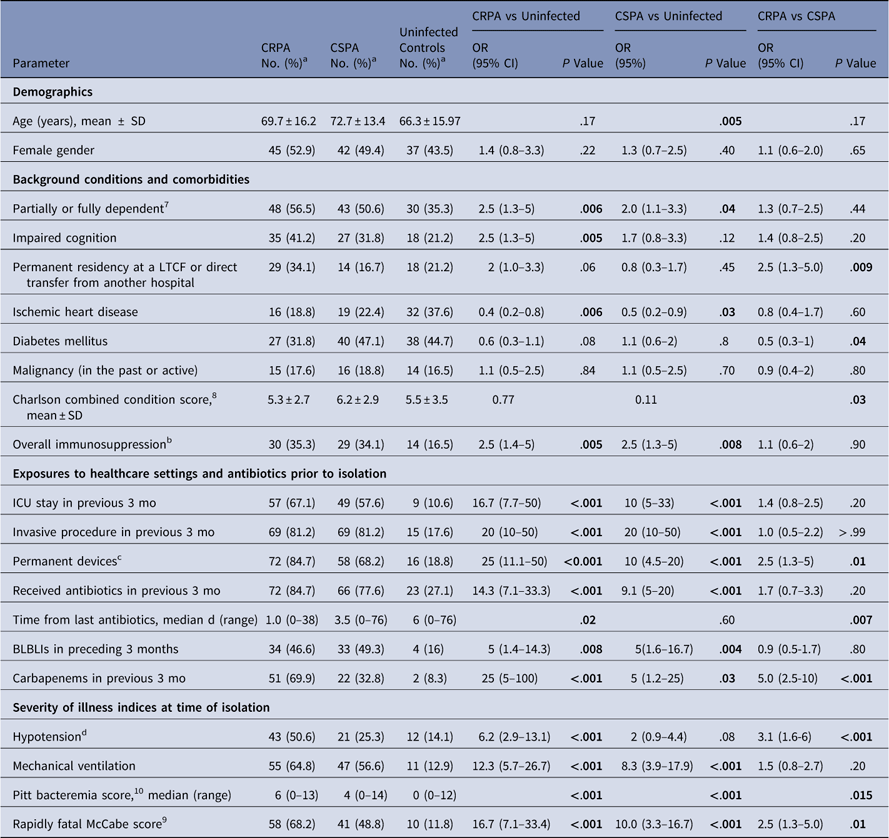
Note. Significant associations are highlighted in bold. CRPA, carbapenem-resistant Pseudomonas aeruginosa; CSPA, carbapenem-susceptible P. aeruginosa; OR, odds ratio; CI, confidence interval; SD, standard deviation; LTCF, long-term care facility. ICU, intensive care unit; BLBLI, β-lactam–β-lactamase inhibitor combination; BSI, blood stream infection.
a Valid percent: count divided by the total number of valid (ie, nonmissing) observations.
b Immunosuppression includes any of the following: neutropenia at culture date (<500 neutrophils/mm3), exposure to glucocorticoids in the previous month, chemotherapy in the previous 3 months, radiotherapy, posttransplantation of any kind, anti-TNF-α (tumor necrosis factor α) therapy in the previous 3 months, or (7) human immunodeficiency virus (HIV) infection.
c Permanent devices included tracheostomies, percutaneous endoscopic gastrostomy, pacemakers, central lines, urinary catheters, external orthopedic devices, drains. Not included: internal stents, prosthetic heart valve, and prosthetic joints. Permanent devices were in place at least 48 hours prior to the isolation.
d Hypotension was defined as a systolic blood pressure <90 mmHg, or administration of intravenous vasopressors, or an acute drop in systolic blood pressure >30 mmHg and/or diastolic blood pressure >20 mm Hg in the 48 hours preceding the culture time.
We conducted 2 multivariable risk factor analyses: resistant cases versus controls and susceptible cases versus controls. The independent predictors of CRPA were ischemic heart disease (aOR, 0.17; P = .02), recent (3 months) exposure to carbapenems (aOR, 17.4; P = .001), and rapidly fatal McCabeReference Bion, Edlin, Ramsay, McCabe and Ledingham 9 condition (aOR, 12.1; P = .001). Independent predictors of CSPA were advanced age (P = .02), ischemic heart disease (aOR, 0.13; P = .005), and recent invasive procedure (aOR, 24; P < .001). Independent predictors associated with CRPA but not CSPA infection were recent exposure to carbapenems and a rapidly fatal McCabe score.Reference Bion, Edlin, Ramsay, McCabe and Ledingham 9
Clinical outcomes of CRPA BSI
Of the 255 patients included in this study, 115 patients (45.1%) died during their index hospitalization, 66 patients (25.9%) died within 14 days (primary outcome), and 132 patients (51.8%) died within 90 days. Of patients who survived the hospitalization, the median duration of stay was 17 days (range, 0–149 days); 56 patients (40.3%) experienced functional deterioration,Reference Katz, Ford, Moskowitz, Jackson and Jaffe 7 and 45 patients (38.5%) were discharged to a long-term care facility (LTCF) after being admitted from home. Patients with CRPA or CSPA BSIs had significantly worse clinical outcomes compared to uninfected controls (Table 2). Clinical outcomes were similar between CRPA and CSPA cases. Time to initiation of appropriate therapy was significantly prolonged among CRPA BSIs compared to CSPA BSIs (Table 2).
Table 2 Bivariable Analyses Comparing Clinical Outcomes of Resistant Case Patients, Susceptible Case Patients, and Uninfected Control Patients (n = 85 patients in each group).

Note. CRPA, carbapenem-resistant Pseudomonas aeruginosa; CSPA, carbapenem-susceptible P. aeruginosa; OR, odds ratio; CI, confidence interval; LTCF, long-term care facility; CDI, Clostridium difficile infection. Significant associations are highlighted in bold.
a Valid percent: count divided by the total number of valid (ie, nonmissing) observations.
b Examples of invasive procedure other than surgery include endoscopy, percutaneous procedure, central line, urinary catheter, and drain insertion.
Multivariable models were calculated for 2 clinical outcomes: 14-day mortality and discharge to an LTCF (Table 2). Independent factors associated with 14-day mortality were time to appropriate antimicrobial therapy (P = .008), malignancy (aOR, 4.1; 95% confidence interval [CI], 1.5–11.5; P = .007), rapidly fatal McCabe score (aOR, 3.7; 95% CI, 1.1–12.1; P = .03),Reference Bion, Edlin, Ramsay, McCabe and Ledingham 9 and a Pitt bacteremia score ≥4 (aOR, 1.3; 95% CI, 1.1–1.5; P = .004).Reference Paterson, Ko and Von Gottberg 10 Independent factors associated with discharge to an LTCF were impaired cognition at baseline (aOR, 14.7; 95% CI, 1.2–176; P = .03), ICU stay during current hospitalization (aOR, 24.3; 95% CI, 2.2–266; P = .009), and Pitt bacteremia score ≥4 (aOR, 1.8; 95% CI, 1.03–3.1; P = .04).Reference Paterson, Ko and Von Gottberg 10 Carbapenem resistance was not associated with these outcomes.
Discussion
Debate continues as to whether the associations between poor clinical outcomes and resistance are due to DAAT or to inherent properties of the resistance determinant of the offending strain.Reference Pogue, Kaye, Cohen and Marchaim 1 This study addressed methodological limitations from prior studies evaluated CRPA infections and clinical outcomes.
This investigation confirmed that DAAT impacts outcomes of patients with multidrug-resistant organism (MDRO) infections.Reference Pogue, Kaye, Cohen and Marchaim 1 This impact has been demonstrated in the past with Acinetobacter baumannii, carbapenem-resistant enterobacteriaceae (CRE), vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA).Reference Pogue, Kaye, Cohen and Marchaim 1 There is an urgent need to develop genuine measures to shorten DAAT (eg, via use of rapid diagnostics, efficacious predictive tools) to improve the outcomes of MDRO infections.Reference Pogue, Kaye, Cohen and Marchaim 1
In contrast to prior investigations,Reference Aloush, Navon-Venezia, Seigman-Igra, Cabili and Carmeli 3 , Reference Lautenbach, Weiner, Nachamkin, Bilker, Sheridan and Fishman 4 patients with CSPA BSIs did not suffer significantly worse outcomes compared to patients with CRPA BSI (Table 1). We believe this was due to the study design, which resulted in selecting a control group which was truly representative of the source population and also due to limitations in sample size and power in the current study (though it was powered to detect differences in the primary outcome).Reference Kaye, Harris, Samore and Carmeli 6
Our study had several limitations. It was a single-centered, retrospective with a relatively small sample size. However, by overcoming prior limitations, this study generated valuable data regarding stewardship and infection control aspects, pertaining to the management of P. aeruginosa infections in hospitalized patients. Results should be validated in other centers and on larger populations.
Based on these results, infection control programs should not focus solely on carbapenem resistance. The case–case-control study design identified independent predictors for CRPA (ie, recent exposure to carbapenems and a rapidly fatal McCabe score). This information can be used to develop successful stewardship interventions and to reduce DAAT and improve patient outcomes.
Acknowledgments
This work was performed in partial fulfillment of the M.D. thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Financial support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors reported no conflicts of interest relevant to this article.




