INTRODUCTION
In Spain, Leishmania infantum is the only species responsible for human cutaneous and visceral leishmaniasis and also for canine leishmaniasis. Although cases of infection have been reported in foxes (Fisa et al. Reference Fisa, Gállego, Castillejo, Aisa, Serra, Riera, Carrió, Gallego and Portús1999), rats (Morillas-Márquez et al. Reference Morillas-Márquez, Benavides Delgado, Gozález Castro, Reyes Magaña and Valero López1985), equines (Solano-Gallego et al. Reference Solano-Gallego, Fernández-Bellón, Serra, Gállego, Ramis, Fondevila and Ferrer2003), cats (Martín-Sánchez et al. Reference Martín-Sánchez, Acedo, Muñoz Pérez, Pesson, Marchal and Morillas-Márquez2007) and some wild animals (Sobrino et al. Reference Sobrino, Ferroglio, Oleaga, Romano, Millan, Revilla, Arnal, Trisciouglio and Cortazar2008), the role of reservoir is basically played by the dog (Acedo-Sanchez et al. Reference Acedo-Sánchez, Martín-Sánchez, Vélez-Bernal, Sanchís-Marín, Louassini, Maldonado and Morillas-Márquez1996; Alvar et al. Reference Alvar, Cañavate, Molina, Moreno and Nieto2004). Species of the Leishmania genus are transmitted by nematocerous dipterans of the Phlebotomidae family, from the Phlebotomus genus in the Old World and the Lutzomyia genus in the New World, displaying certain specificity between the species of Leishmania and the subgenus of sandflies which transmit it (Killick-Kendrick, Reference Killick-Kendrick1990). In Spain, eleven different species of sandflies have been identified (Gil Collado et al. Reference Gil Collado, Morillas Márquez and Sanchis Marin1989; Gállego Berenguer et al. Reference Gállego Berenguer, Botet Fregola, Gállego Culleré, Portús and Vinyeta1992; Conesa Gallego et al. Reference Conesa Gallego, Romera Lozano and Martinez Ortega1999) and 2 proven Leishmania infantum vectors, Phlebotomus perniciosus and Phlebotomus ariasi, which can act in the same focus in sympatric conditions (Rioux et al. Reference Rioux, Guilvard, Gállego, Moreno, Pratlong, Portús, Rispail, Gállego and Bastien1986; Martín-Sánchez et al. Reference Martín-Sánchez, Guilvard, Acedo-Sánchez, Wolf-Echiverri, Sanchís-Marín and Morillas-Márquez1994). They are found, to a greater or lesser extent, almost throughout the country, and their relative significance as vectors depends on their abundance and density (Guevara-Benitez et al. Reference Guevara-Benitez, Ubeda-Ontiveros and Morillas-Marquez1978; Morillas-Márquez et al. Reference Morillas-Márquez, Guevara Benítez, Úbeda Ontiveros and González Castro1983; Gil Collado et al. Reference Gil Collado, Morillas Márquez and Sanchis Marin1989). Transmission of the disease is linked to the presence of the vector, whose distribution is in turn closely related to ecological and bioclimatic factors, thus being sensitive to environmental changes related to human behaviour including climate change (Rispail et al. Reference Rispail, Dereure and Jarry2002; Semenza and Menne, Reference Semenza and Menne2009).
Leishmaniasis caused by L. infantum is endemic in the province of Granada, with canine seroprevalence values varying from 1·1–20·1% according to the bioclimatic characteristics of the area (Martín-Sánchez et al. Reference Martín-Sánchez, Morales-Yuste, Acedo-Sánchez, Barón, Díaz and Morillas-Márquez2009) and the existence of sporadic cases of human leishmaniasis (Muñoz Hoyos et al. Reference Muñoz Hoyos, Morillas Marquez, Duque Alcuña, Bayes García, Rivas Moreno, Loscertales and Girón Caro1983). P. perniciosus is the most abundant species of the Phlebotomus genus and therefore the main vector in this area (Guevara-Benitez et al. Reference Guevara-Benitez, Ubeda-Ontiveros and Morillas-Marquez1978; Morillas-Márquez et al. Reference Morillas-Márquez, Martín-Sánchez, Díaz-Sáez, Barón-López, Morales-Yuste, Alves de Lima Franco and Sanchís-Marín2010).
Nowadays, Geographical Position Systems (GPS) and Remote Sensing (RS) are being used to obtain geographical data for epidemiological purposes, whilst Geographical Information Systems (GIS) are employed in order to visualize and analyse these spatial data. These tools are enabling us to study environmental relationships and generate maps predicting the behaviour and distribution of certain vector-borne diseases (Eisen and Lozano-Fuentes, Reference Eisen and Lozano-Fuentes2009; Srivastava et al. Reference Srivastava, Nagpal, Joshi, Paliwal and Dash2009; Chamaillé et al. Reference Chamaillé, Tran, Meunier, Bourdoiseau, Ready and Dedet2010; Gálvez et al. Reference Gálvez, Descalzo, Miró, Jiménez, Martín, Dos Santos-Brandao, Guerrero and Molina2010). Our objective was to study the environmental factors associated with P. perniciosus presence in southern Granada in order to construct risk maps for the presence and absence of the vector in this province. The results obtained may help to broaden our knowledge of the epidemiology of leishmaniasis by determining the ecological patterns followed by its main vector and provide us with a base upon which to model and predict its distribution. This could help us both to determine the focus of new studies, and to design possible control programmes for this disease, as it allows us to define in a more rational way the most effective measures to be taken in each area.
MATERIALS AND METHODS
Study area
The south of the province of Granada is an area which spans altitudes ranging from 0 to 3480 m in the space of a few kilometres, thus enabling various bioclimatic levels with different environmental and vegetation features to exist within a narrow geographical range: Thermomediterranean, Mesomediterranean, Supramediterranean, Oromediterranean and Criomediterranean (Rivas Martinez et al. Reference Rivas Martínez, Bandullo, Serrada, Allue Andrade, Moreno del Burgo and González Rebollar1987). The Thermomediterranean level is defined by an altitude of 0 to 700 m above sea level, with warm temperatures in winter and an average annual temperature of 17–19°C, and rainfall of 200–300 mm a year. The Mesomediterranean level sits at altitudes of 600–900 m, with rainfall of 600–1000 mm a year, and an average temperature of 13–17°C. The Supramediterranean covers altitudes of 900–1800 m and an average annual temperature of 8–13°C, with an annual rainfall of 1000–1600 mm. The Oromediterranean and Criomediterranean levels have average annual temperatures of 4–8°C and <4°C respectively, both with annual rainfall of >1600 mm. The lower limits of the Oromediterranean level fall at altitudes of between 1600 and 2000 m, depending on the region, whilst the Criomediterranean level tends to be at above 2700 m in this part of the Iberian Peninsula.
Capture and morphological identification of the sandflies
This study was carried out in the years 2004, 2006 and 2007. Adult sandflies were captured in the month of July, which in our area is characterized by maximum richness of species and high densities of P. perniciosus (Morillas Márquez et al. Reference Morillas-Márquez, Guevara Benítez, Úbeda Ontiveros and González Castro1983). Sticky traps ((A4 sized paper: 29·7 cm×21 cm) impregnated with castor oil) were placed for 4 days in typical sandfly resting spots, such as the drainage holes in the retaining walls of roads and houses. This capture method and trap maintenance period minimizes false negatives for P. perniciosus presence.
In total, 1694 sticky traps were placed in 167 sampling sites situated at various points in the south of Granada province, such as the Granada coastline, the Alpujarra, the Lecrín valley, and an altitudinal transect from Motril, at sea level, to the University Mountain Refuge in the Sierra Nevada, at an altitude of 2500 metres (Fig. 1). The number of sticky traps placed at each site varied from 5 to 14 per site, depending on the availability of drainage holes in each retaining wall.

Fig. 1. Map showing the 167 locations where traps were set with white and black dots for drainage hole with or without PVC piping, respectively.
Captured specimens were removed from the sticky traps using 96% alcohol and were then conserved in 70% alcohol. After eliminating any remaining traces of oil in a 5% detergent solution, they were placed in Marc André solution and heated to boiling point to clear the external cuticle of the sandfly, after which they were kept at 37°C for 2 days. Following this they were mounted on slides under a cover-slip using Berlese solution. Morphological classification was carried out in accordance with Gil Collado et al. (Reference Gil Collado, Morillas Márquez and Sanchis Marin1989), and Gállego Berenguer et al. (Reference Gállego Berenguer, Botet Fregola, Gállego Culleré, Portús and Vinyeta1992) via examination under an optical microscope, paying particular attention to the spermatheca in females and the external genitalia in males.
Variables studied
Data were collected from each of the sampling sites via GIS and notes made on-site with a Personal Digital Assistant (PDA) for use as independent variables. Abundance/density data for P. perniciosus at the 167 sampling sites was reduced down to data for the presence or absence of this vector, thus generating the dependent variable. Given that this can only have values of 0 or 1, the multivariate statistical technique chosen to estimate the probability of occurrence of the event was logistic regression.
To standardize data collection in our fieldwork with the PDA, we used the program Pendragon Form v.5.0.7, through which a database was constructed for use by the leishmaniasis group (EDEN-LEI) of the EDEN project (Emerging Diseases in a changing European environment, GOCE-2003-010284). The following data were collected by this method: location of sampling site (geographical coordinates), number of traps placed and collected, meteorological data (rain, wind) and ecological and environmental factors (site relative to settlement, situation of site, site category, aspect, shelter, water course, drainage hole wall features, drainage hole features, hole interior, wetness of holes, vegetation on wall, well present, refuse bin present, bioclimatic level, predominant natural vegetation adjacent zone 0–100 m, predominant natural vegetation nearby zone 100–1000 m, second predominant natural vegetation nearby zone 100–1000 m; predominant adjacent land cover, second predominant adjacent land cover; presence of different animals in the immediate surroundings). Through GIS we obtained data for altitude, land use (CORINE), slope, Normalized Difference Vegetation Index (NDVI) and Land Surface Temperature (LST), provided by the Junta de Andalucía [Regional Government of Andalusia] (http://www.juntadeandalucia.es/medioambiente/site/web/...) or the European EDEN network within which this study was carried out.
Spatial statistical analysis and maps
Using the SPSS 15.0 software for Windows, univariate logistic regression studies were carried out with all the independent variables plotted against the presence/absence of P. perniciosus as the dependent variable. Particular attention was paid to the detection of collinearity and also interaction or confusion between the independent variables through the construction and comparison of different logistic regression models. In the case of closely correlated variables, we retained the one which was easiest to represent on a map or the most significant one. Continuous variables such as altitude or LST were categorized, and categorical variables were re-categorized in the search for association with the dependent variable. To construct the multivariate model we used all the variables with P⩽0·100 in the univariate study.
In the final model, variables with P<0·05 were retained. The variables which could not be represented in the map were included in the constant of the predictive model. The maps for exposure to P. perniciosus were generated with the software package ArcGis 9.2, through the Raster Calculator option, with which we were able to enter the multivariate statistical model obtained into the program. This enabled us to interpolate precise data to surfaces of risk and extrapolate it to a wider area, which in our case is represented by the province of Granada.
In order to check the usefulness of the spatial risk model for the presence and absence of P. perniciosus to predict the risk of leishmaniasis in the area, we applied linear regression with the seroprevalence of canine leishmaniasis in 14 localities in southern Granada as the dependent variable and the estimated average risk of presence and absence of P. perniciosus in these localities as the independent variable.
RESULTS
Capture and species identification data
Of the 1694 sticky traps placed, a total of 1633 were collected, representing a loss of 3·6%. We captured 30 019 sandflies belonging to 6 different species: 5 of the Phlebotomus genus (P. perniciosus, P. ariasi, P. papatasi, P. sergenti and P. alexandri) and 1 of the Sergentomyia genus (S. minuta). At least 1 species was captured in 154 of the 167 sites sampled (92·22%). The most abundant species was S. minuta (89·90%), which was found in 151 of the 167 sites sampled (90·42%), whilst P. alexandri is the least abundant (0·003%) and least common, having been found in only 1 site (0·60%) at the Thermomediterranean bioclimatic level. A total of 2256 specimens were classified morphologically as P. perniciosus, representing 7·51% of the total number captured. This species has an average density of 11·03 sandflies/m2 and was present in 112 of the 167 sites sampled (67·07%). Both males with typical bifurcated aedeagus and males with an incurved aedeagal tip were classified as P. perniciosus (Pesson et al. Reference Pesson, Ready, Benabdennbi, Martín-Sánchez, Esseghir, Cadi-Soussi, Morillas-Márquez and Ready2004), although the latter category only made up 0·13% of the total number of cases. We identified 225 specimens (0·75%) of the species P. ariasi, which we found in 55 of the 167 sites sampled (32·93%), with an average density of 1·10 sandflies/m2 (Table 1). With regard to altitude, we captured S. minuta in this study at 60–2490 m, P. perniciosus at 76–1534 m and P. ariasi at 85–1694 m. P. perniciosus abundance is highest in the Mesomediterranean (P⩽0·005) with 9·70%. P. ariasi abundance is also greater in the Mesomediterranean than in the Thermomediterranean (P<0·001) but does not show statistically significant differences with the Supramediterranean (P=0·187). At the Oromediterranean level no specimens of any vector species were captured.
Table 1. General data on capture and abundance/density of the two vector species, Phlebotomus perniciosus and P. ariasi organized by bioclimatic level

Statistical study and construction of predictive models
Univariate logistic regression models were constructed between the dependent variable (P. perniciosus presence/absence) and each of the independent variables, finding a notable number of associations (Table 2). Some of the independent variables are closely correlated and cannot be in the same multivariate model.
Table 2. Possible factors associated with the presence of Phlebotomus perniciosus in the Granada province, southeast Spain: univariate analysis by logistic regression

The multivariate logistic regression analysis shows that the best predictors for P. perniciosus presence/absence are altitude (a. 0–384 m, b. 385–768 m and 1154–1537 m, c. 769–1153 m, d. 1538–2691 m), land use (a. other uses, b. urban area) and presence/absence of PVC piping in the drainage holes of retaining walls (a. plastic pipe, b. other holes). The category of ‘other holes’ includes the following types of holes: unlined, which was the most frequent, having been found at 123 sites, and was initially used as reference, brick lined (8 sites), cement pipe (5), plaster lined (4) and other (2). As there was no statistically significant difference in P. perniciosus presence between each of the above types and the unlined holes, they were all included within the reference category.
The altitude and land use variables were obtained via GIS and therefore could be used in the construction of the risk map, whilst the PVC piping variable was recorded with the PDA and had to be included in the constant of the model in order to be represented, for which reason 2 different models were constructed, one with and the other without this variable. In Table 3 and Fig. 2 respectively, we can see the model and the risk map for exposure to P. perniciosus depending on the variables of altitude and land use, without taking the type of drainage hole into account. This map shows us the probability of finding this vector in the province of Granada without the type of drainage hole affecting the model. Using the model in Table 4, we then generated 2 risk maps in which the influence of PVC piping on the presence/absence of P. perniciosus is established. The first of these maps shows the risk of exposure to P. perniciosus in the hypothetical case that PVC piping was used for drainage holes in all retaining walls (Fig. 3), whilst the second shows the risk of exposure in the absence of PVC piping (Fig. 4). These maps show that, all other variables in the model being equal, the probability of encountering P. perniciosus in Granada province varies from 2·3% to 91·8% when PVC piping is absent whilst in the opposite scenario (only PVC piping is present), the probability varies from 0·4% to 66·5%. Furthermore, the highest risk of exposure to P. perniciosus occurs at altitudes of 769–1153 m, which means that all other variables in the model being equal, the risk is 7·8 times higher than at altitudes of 0–384 m. Altitudes of 1538–2691 m and the use of land as an urban area are both factors that serve to protect against exposure to P. perniciosus. Altitude and land use being equal, the lack of PVC piping in drainage holes entails a risk of exposure to P. perniciosus which is 5·7 times higher than if the drainage holes had PVC piping in them.

Fig. 2. Risk map for presence and absence of Phlebotomus perniciosus according to altitude and land use (corine), without taking type of drainage hole into account. Black represents lack of data.
Table 3. Multivariate model in which the variable drainage hole features have been excluded due to not being a GIS variable


Fig. 3. Risk map in the hypothetical scenario that all retaining walls have PVC piping in their drainage holes. Black represents lack of data.
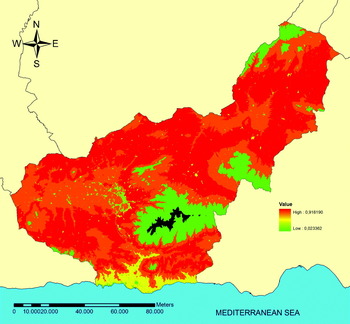
Fig. 4. Risk map in the hypothetical scenario in which none of the retaining walls have PVC piping in their drainage holes. Black represents lack of data.
Table 4. Factors associated with the presence of Phlebotomus perniciosus in Granada province, southeast Spain

With respect to the validation of the predictive model, the linear regression analysis between the seroprevalence of canine leishmaniasis and the risk of presence/absence of P. perniciosus indicates a positive linear association between the two parameters (P=0·032), so that an increase in risk of 0·1 would entail an increase in seroprevalence in the area of 2·6% (R2=0·272). The risk among affected localities varies from 0·137 to 0·855 whilst seroprevalence varies from 0 to 40%.
DISCUSSION
Using sticky traps as our capture method, we found that the most common species in the south of Granada province is S. minuta, followed by P. perniciosus, P. papatasi, P. sergenti, P. ariasi and P. alexandri. We are aware that the capture method can influence the number and relative abundance of the species captured, so if light traps are used, the number of S. minuta captured is considerably lower (Gállego et al. Reference Gállego Berenguer, Botet Fregola, Gállego Culleré, Portús and Vinyeta1992). However, the herpetophilic nature of this species means that it is of no importance in the epidemiology of leishmaniasis due to L. infantum. We can confirm that P. perniciosus is dominant in its role as vector in comparison with P. ariasi, on showing higher figures in density, abundance and distribution (67·07% of sites sampled as opposed to 32·93% for P. ariasi), thus justifying the central role it plays in this study.
We obtained data for the presence and absence of P. perniciosus at 167 sampling sites distributed across the southern part of Granada province, from which we also took a series of ecological and climate-related data. Through logistic regression we estimated the probability of P. perniciosus presence at each sampling site as a function of these environmental variables. Although in the initial univariate analyses, the probability of P. perniciosus presence at a particular sampling site appeared to be associated with a large variety of factors, in the multivariate logistic regression model only altitude, land use and drainage hole features (with/without PVC piping) were retained as predictors for the distribution of this vector species. Among the variables which lose their significance we have bioclimatic level, which is justifiable given that this variable comprises the influence of other factors such as altitude, relative humidity and vegetation (Rivas Martínez et al. Reference Rivas Martínez, Bandullo, Serrada, Allue Andrade, Moreno del Burgo and González Rebollar1987). The risk of P. perniciosus presence was found to be 2·4 times higher in the Mesomediterranean than in the Thermomediterranean, which is in accordance with the higher prevalence of canine leishmaniasis at this bioclimatic level (Martín-Sánchez et al. Reference Martín-Sánchez, Morales-Yuste, Acedo-Sánchez, Barón, Díaz and Morillas-Márquez2009). However, we must take into account that capture data may not be the same when annual sampling is carried out. Variation in altitude is one of the strongest determinants for variation in temperature and bears a heavy influence on rainfall, which would justify the loss of significance of the variable Land Surface Temperature (LST). In the univariate analysis, the range 23·7–25°C shows a risk of P. perniciosus presence 4·2 times greater than at lower temperatures. Thomson et al. (Reference Thomson, Elnaiem, Ashford and Connor1999) found that temperature, alongside land type, is an important ecological determinant for the distribution of P. orientalis in Sudan. Land cover is partially determined by altitude and is closely correlated with other variables such as land use and main vegetation, which also showed association with P. perniciosus presence. We found that these dipterans prefer areas where there is some kind of scrub vegetation, especially if alongside olive groves and not located in the proximities of certain vegetation types – riverside, garden or deciduous trees together with chestnut trees. Areas with this type of vegetation tend to be wetter than those occupied only by scrub, and this humidity would not be well tolerated by this species which is typical of semi-arid areas (Rioux et al. Reference Rioux, Akalay, Prereires, Dereure, Mahjour, Le Houerou, Leger, Desjeux, Gállego, Saddiki, Barkia and Nachi1997). In the sampling sites facing north and west there were fewer P. perniciosus present. Conversely, the presence of any animal near the sampling site increases the risk of encountering the vector by 89·6%, although not all animals have the same effect. Thus equines, sheep, cattle, goats and rabbits increase the probability of encountering this dipteran. Various authors have noted the preferences of P. perniciosus and P. ariasi for cattle (Rioux et al. Reference Rioux, Guilvard, Gállego, Moreno, Pratlong, Portús, Rispail, Gállego and Bastien1986; De Colmenares et al. Reference De Colmenares, Portús, Botet, Dobaño, Gállego, Wolff and Seguí1995; Rossi et al. Reference Rossi, Bongiorno, Ciolli, Di Muccio, Scalone, Gramiccia, Gradoni and Maroli2008). The presence of dogs in the immediate vicinity of the sampling site turned out not to be statistically significant, just as happened in a study carried out in central Spain (Gálvez et al. Reference Gálvez, Descalzo, Miró, Jiménez, Martín, Dos Santos-Brandao, Guerrero and Molina2010). The loss of significance of these and other variables in the multivariate model is a response to reasons which are difficult to determine.
It is common knowledge that the distribution of sandflies, including P. perniciosus, is heavily influenced by altitude (Guernaoui et al. Reference Guernaoui, Boumezzough and Laamrani2006; Maroli et al. Reference Maroli, Rossi, Baldelli, Capelli, Ferroglio, Genchi, Gramiccia, Mortarino, Pietrobelli and Gradoni2008). In this study, the altitudinal range that reveals the greatest presence of P. perniciosus is 769–1153 m, covering part of the Mesomediterranean and Supramediterranean, which agrees along general lines with the data provided by Guevara-Benitez et al. (Reference Guevara-Benitez, Ubeda-Ontiveros and Morillas-Marquez1978). The range in which one is least likely to encounter this vector species is 1538–2691 m, which corresponds to the Oromediterranean. Changes in land use will bring about alterations to the microhabitat surrounding the sampling sites, affecting sandfly populations, one of the most notable changes being the urbanization of land. The results indicate that P. perniciosus shows less affinity for these urban environments, the opposite phenomenon to that which occurs in the case of P. papatasi and P. alexandri in the Middle East (Colacicco-Mayhugh et al. Reference Colacicco-Mayhugh, Masuoka and Grieco2010). Several studies have highlighted the influence of land use on the distribution of sandflies or other vectors (King et al. Reference King, Campbell-Lendrum and Davies2004; Cecchi et al. Reference Cecchi, Mattioli, Slingenbergh and de la Rocque2008; Claborn et al. Reference Claborn, Masuoka, Morrow and Keep2008). One important limitation detected in the use of this variable, and which in general can be extrapolated to other GIS variables, concerns the resolution of the data, which affects the classification of the land and determines the introduction of a significant bias. This limitation has also been pointed out by other authors (King et al. Reference King, Campbell-Lendrum and Davies2004; Cecchi et al. Reference Cecchi, Mattioli, Slingenbergh and de la Rocque2008). This can make it much more difficult to create risk maps to predict the behaviour of the event in wide areas. Another limiting factor, also pointed out by other authors, is the difficulty of including variables in the study which do not represent spatial data but which are nevertheless of great epidemiological interest (Cecchi et al. Reference Cecchi, Mattioli, Slingenbergh and de la Rocque2008; King et al. Reference King, Campbell-Lendrum and Davies2004). This is the case with the presence/absence of PVC piping in drainage holes, which is presented in this study as a feasible control measure against P. perniciosus, the dominant L. infantum vector in the area. The walls which have PVC in their drainage holes are generally more recently built than the typical terrain retaining walls, and they generally retain less soil. Also, fewer animals are found inside (reptiles, micromammals, arthropods, etc…). The absence of these animals not only limits potential food sources for adult females, but also reduces the presence of organic matter produced by the waste of these animals. This, together with the scarce soil retention which makes it impossible for plants to grow, means that the holes containing PVC offer unfavourable conditions for the proliferation of sandfly larvae, which need this organic matter in order to develop. All in all, this makes these holes less apt for sandfly resting and breeding sites. It has been demonstrated how the drainage hole features produce significant variations in the probability of P. perniciosus presence, oscillating from 2·3 to 91·8% if PVC piping is absent and from 0·4 to 66·5% if all holes have PVC piping. Altitude and land use being equal, the use of PVC piping in the construction of drainage systems for walls would reduce the probability of encountering P. perniciosus in them by up to 25·3%, thus demonstrating its potential usefulness as a protection factor against the presence of this vector and therefore against leishmaniasis. As already pointed out, there is a positive linear association between the risk of P. perniciosus presence and the seroprevalence of canine leishmaniasis, although according to the model, the variability in the risk only explains 27·2% of the variability in the prevalence. Among the other factors involved, the age and weight of the animal, its activity and its location at night have been pinpointed (Martín-Sánchez et al. Reference Martín-Sánchez, Morales-Yuste, Acedo-Sánchez, Barón, Díaz and Morillas-Márquez2009).
The control of sandflies is perceived as a measure to reduce the transmission of L. infantum which is more efficient and more readily accepted by society than that of putting down infected dogs (Alexander and Maroli, Reference Alexander and Maroli2003). Among the methods employed, the use of insecticides plays an important role, both in houses, animal shelters and surrounding vegetation and in common sandfly resting and feeding places. However, for environmental reasons its prolonged use would not be allowed. It is precisely in this aspect where measures such as the use of PVC piping begin to acquire importance, because it is a simple non-toxic way to significantly reduce the presence of P. perniciosus. Given that this type of retaining wall may be located near inhabited areas or passing places, it would be an effective control method which, in conjunction with other methods, could help to reduce the transmission of this disease.
ACKNOWLEDGEMENTS
To the E.U. for EDEN (GOCE-2003-010284-2).
FINANCIAL SUPPORT
Regional Government of Andalusia [Junta de Andalucía] subsidize the group CIV-176; the Spanish Ministry of Education and Science [Ministerio de Educación y Ciencia] the projects AGL2004-06909-CO2-O2/GAN and CGL2007-66943-CO2-O2/BOS; EDEN provided S.D.B. with a Ph.D. Student grant.










