INTRODUCTION
Every cellular organism is under constant threat of immune insults and infection by various pathogens. The immune function of host animals against pathogens, parasites and disease vectors has been studied rather widely (e.g. Boehm, Reference Boehm2012). In contrast, it is often neglected that parasitic organisms, particularly the arthropod parasites, may need immunity to survive their own infections and to successfully complete their life cycle (Lehane et al. Reference Lehane, Aksoy and Levashina2004; Taylor, Reference Taylor2006; Tian et al. Reference Tian, Gao, Fang, Ye and Zhu2010). Hematophagous ectoparasites can become infected with a wide range of micro-organisms including viruses, bacteria, protozoa and nematodes through feeding (Sonenshine, Reference Sonenshine1993; Taylor, Reference Taylor2006; Hillyer, Reference Hillyer and Söderhäll2010; Korhonen et al. Reference Korhonen, Pérez-Vera, Pulliainen, Sironen, Aaltonen, Kortet, Härkönen, Härkönen, Paakkonen, Nieminen, Mustonen, Ylönen and Vapalahti2014). They can also become infected by pathogens from the external environment, e.g. through damage to the cuticle or from each other (e.g. vertical transmission) (Korhonen et al. Reference Korhonen, Pérez-Vera, Pulliainen, Sironen, Aaltonen, Kortet, Härkönen, Härkönen, Paakkonen, Nieminen, Mustonen, Ylönen and Vapalahti2014). The immunological tolerance of hematophagous ectoparasites can affect their tendency to transmit disease agents to their hosts (Lehane et al. Reference Lehane, Aksoy and Levashina2004; Taylor, Reference Taylor2006). Capability of a parasite to resist or tolerate immune insults likely affects its ability to exploit host efficiently. Therefore, understanding environmental and intrinsic mechanisms affecting immune system of parasites and disease vectors is essential to develop pest management and other strategies to reduce the transmission of diseases.
The immune system of different types of parasitic insects has not been widely studied (see Lehane et al. Reference Lehane, Aksoy and Levashina2004; Hillyer, Reference Hillyer and Söderhäll2010). The insect immune system in general lacks adaptive immunity with specific vertebrate-like immunological memory and is based on innate responses (Hoffmann, Reference Hoffmann1995). The immune system of insects includes certain organs and tissues (exoskeleton, tracheal/alimentary epithelia, salivary glands, fat body, lymph gland), free-circulating haemocytes in the haemolymph (developed and differentiated in the lymph gland) and cell products (Lemaitre and Hoffmann, Reference Lemaitre and Hoffmann2007). Physical barriers such as the exoskeleton cuticle and the peritrophic membranes lining the digestive tract are the first lines of defence. Innate immunity forms the second line of defence that is categorized into cellular and humoral immune responses. The cellular immune response in insects is mediated by haemocytes, and the cellular immunity involves processes such as phagocytosis, nodulation and encapsulation (Hoffmann, Reference Hoffmann1995; reviewed in Marmaras and Lampropoulou, Reference Marmaras and Lampropoulou2009). Both encapsulation and nodulation are often accompanied by melanization (i.e. phenoloxidase-catalysed cascade, leading to melanin formation in the haemocytes). Humoral factors (e.g. anti-microbial peptides) are produced in the fat body and haemocytes, but also locally in epithelial tissues if wounded (Lemaitre and Hoffmann, Reference Lemaitre and Hoffmann2007).
Ecological immunology investigates the effects of environmental factors on the immune responses of organisms (e.g. Catalán et al. Reference Catalán, Wozniak, Niemeyer, Kalergis and Bozinovic2012). Ambient temperature is a major environmental factor determining the rate of metabolic processes in ectotherms (Denlinger and Lee, Reference Denlinger and Lee2010). Therefore, surrounding temperature has a great potential to regulate immune system metabolism and immune responses in insects and free-living stages of parasites. Free-living stages of parasitic insects are directly exposed to environmental conditions such as extreme temperatures and large temperature fluctuations. Especially parasites inhabiting temperate seasonal environments experience extreme temperatures during the free-living stages. A long exposure to low temperatures outside the host is known to have long-term effects e.g. on survival and development (Härkönen et al. Reference Härkönen, Hurme and Kaitala2013; Härkönen and Kaitala, Reference Härkönen and Kaitala2013). However, possible immediate and long-term effects of temperature on immunity of parasitic insects have remained virtually unstudied (but see Murdock et al. Reference Murdock, Paaijmans, Bell, King, Hillyer, Read and Thomas2012). In other insects, only immediate, short-term effects of temperature on immunity have been studied (but see Franke and Fischer, Reference Franke and Fischer2013).
Dramatic changes in environmental temperature and associated thermal stress can directly affect the underlying mechanisms of immune regulation. For example, heat and cold stress to a certain extent have been observed to elicit an upregulation of immune system parameters (expression of immune response genes, total hemocyte count, encapsulation capacity, melanization) in various insect species (Catalán et al. Reference Catalán, Wozniak, Niemeyer, Kalergis and Bozinovic2012; Murdock et al. Reference Murdock, Paaijmans, Bell, King, Hillyer, Read and Thomas2012; Zhao and Jones, Reference Zhao and Jones2012; Sinclair et al. Reference Sinclair, Ferguson, Salehipour-shirazi and MacMillan2013). However, although unstudied, activation of protection mechanisms against both temperature and immune stress can be energetically costly, causing potential trade-offs between the thermal tolerance and the immune tolerance in a long term. High temperature variation caused by climate change can negatively affect immune response through energetic trade-offs when, e.g. development and growth are favoured at higher temperatures at the expense of immune function (Karl et al. Reference Karl, Stoks, De Block, Janowitz and Fischer2011).
In the present study, it was investigated whether temperature variation affects immunity using one of the best studied nonspecific immune response among insects, i.e. encapsulation capacity, in the deer ked (Lipoptena cervi). Lipoptena cervi is a haematophagous insect ectoparasite of various boreal cervids. Lipoptena cervi has expanded its range in the Palearctic region, but it has also been introduced to the Nearctic region (e.g. Välimäki et al. Reference Välimäki, Madslien, Malmsten, Härkönen, Härkönen, Kaitala, Kortet, Laaksonen, Mehl, Redford, Ylönen and Ytrehus2010; Samuel et al. Reference Samuel, Madslien and Gonynor-McGuire2012). Adult L. cervi may cause negative effects on its cervid hosts and on accidental hosts such as humans (Kortet et al. Reference Kortet, Härkönen, Hokkanen, Härkönen, Kaitala, Kaunisto, Laaksonen, Kekäläinen and Ylönen2010; Kynkäänniemi et al. Reference Kynkäänniemi, Kettu, Kortet, Härkönen, Kaitala, Paakkonen, Mustonen, Nieminen, Härkönen, Ylönen and Laaksonen2014). Lipoptena cervi is also known to act as a potential vector for haemotrophic Bartonella bacteria infecting ruminants (Korhonen et al. Reference Korhonen, Pérez-Vera, Pulliainen, Sironen, Aaltonen, Kortet, Härkönen, Härkönen, Paakkonen, Nieminen, Mustonen, Ylönen and Vapalahti2014). Adult L. cervi individuals feed on the host's blood and reproduce on the host throughout the year, but the immobile pupal stage occurs outside the host and lasts up to 10 months (i.e. until the emergence of non-fed, host-searching adults). Lipoptena cervi pupae are produced one at a time by a female (after which a pupa drops almost immediately of the host) (Haarløv, Reference Haarløv1964). Female reproductive output is dispersed evenly across the whole reproductive phase, which lasts from autumn to the following spring (Härkönen et al. Reference Härkönen, Hurme and Kaitala2013). The pupae can encounter extreme temperatures and fluctuating weather conditions during winter and early spring. Here, it was studied whether a sudden low temperature or high temperature exposure during pupal diapause after leaving the host, has effects on immunity of newly emerged, non-fed adults. These thermal conditions can increasingly occur in the future, when environmental temperature variation is more likely due to ongoing climate change. Also potential main and interaction effects of weight at birth, pupal collection time (i.e. birth time) and temperature on adult immunity were analysed. It was hypothesized that both low- and high-temperature exposures during pupal diapause can negatively affect encapsulation capacity in adulthood (see Karl et al. Reference Karl, Stoks, De Block, Janowitz and Fischer2011). Present work adds important information to the earlier work on cold tolerance of L. cervi pupae (see Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012).
MATERIALS AND METHODS
Pupal collection and weight measurement
We collected 264 L. cervi pupae from winter bedding sites of the moose (Alces alces) in the Siikalatva area, Central Finland (64°30′20″N, 25°39′00″E; 60 m AMSL) in 2009. A. alces is the main host species in the study area (Kaunisto et al. Reference Kaunisto, Kortet, Härkönen, Härkönen, Ylönen and Laaksonen2009). Only the fresh (<24 h old) beddings were used, in which the pupae were detectable by naked eye on the snow cover. In the study area, temperatures range from +7 to −30 °C from December to February. In April, temperatures can potentially vary from −22·8 to +22·3 °C in Central Finland (e.g. in 2011), although usually the thermal range is more narrow. Both collection dates (26th of February and 3rd of April) occurred after 3-day-long thawing period (from +1 to +4 °C; Finnish Meteorological Institute). Collected pupae were not covered by snow. Therefore, all pupae had experienced only mild air temperatures before collection. Immediately after the collections, pupal weight was measured with a precision balance (Mettler Toledo MT 5, accuracy of 0·001 mg). All pupae were kept in a cold room (from +3 to +5 °C) until the temperature experiments, which took place within 2 weeks. The cold room temperature mimicked temperatures that pupae had experienced in the field before collection. Temperatures near +5 °C have been observed to maintain pupal diapause of L. cervi in laboratory conditions (Härkönen and Kaitala, Reference Härkönen and Kaitala2013).
Temperature experiments
Our experimental set up mimicked temperature variation that can occur in the wild (fluctuations from −15 °C/−20 °C to +5 °C are realistic in February and sometimes also in April). Moreover, one of the aims was also to explore possible effects of increasing, climate change-related thermal variation on the deer ked immune defence. To study the effects of a sudden low and high temperature peak during pupal diapause on adult encapsulation response, the experiment included four temperature treatments (−20, −15, +5 and +20 °C). All diapausing pupae (collected on 26th of February and 3rd of April; kept at near +5 °C) were randomly divided into the temperature groups (see Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012). For the two sub-zero treatments (−15 and −20 °C), the pupae were placed individually in Eppendorf tubes and were sunk into boxes filled with sand to stabilize possible temperature fluctuations in the freezer rooms. The pupae were exposed to −15 °C (n = 66) and −20 °C (n = 67) for only 3 days, because longer exposure time for these low temperatures have been observed to cause severe or total mortality of pupae (i.e. no adult emergence) (see Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012). The exposure of −20 °C for 3 days represents very demanding conditions. After the two sub-zero treatments, the diapausing pupae were returned back to the cold room (~+5 °C) for 4 days to stabilize. After stabilizing, they were transferred to a climate chamber (+20 °C, 60% relative humidity and a photoperiod of 19/5 h for day/night) to start post-diapause development and after development to measure the immunity of emerged adults. A third group of pupae (n = 65) did not experience severe sub-zero temperatures, but instead, these diapausing pupae were exposed to a sudden high temperature peak (+20 °C) for 3 days, and then returned back to the cold room temperature (~+5 °C) for 4 days. After the recovering time, the pupae were transferred to a climate chamber (see above) to start definite post-diapause development together with cold-treated pupae. A fourth, control group of pupae (n = 66) was held only in a cold room (~+5 °C; 21 days after the collections) to maintain diapause (see Härkönen and Kaitala, Reference Härkönen and Kaitala2013) until they were transferred to a climate chamber to develop simultaneously with the other treatment groups. The control treatment (from +5 to +20 °C) as well as the rearing temperature of +20 °C has been previously observed to be suitable for post-diapause development and for achieving high survival percentages (i.e. adult emergence from pupae) (e.g. Härkönen et al. unpublished data; Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012). The aim was to achieve adequate survival percentages per treatments, because the adult encapsulation capacity could not be measured from dead or unemerged individuals. The survival rates (i.e. adult emergence rate) for the four temperature treatments were counted from the larger data by Härkönen et al. (Reference Härkönen, Kaitala, Kaunisto and Repo2012).
Encapsulation measurements
Encapsulation capacity was measured randomly for most of the non-fed adults, who emerged 3 months after the temperature treatments; +20 °C (n = 40), +5 °C (n = 47), −15 °C (n = 55), −20 °C (n = 46). Immune response was measured in terms of encapsulation, in which free-circulating haemocytes recognize and bind to foreign multicellular targets (e.g. parasites, protozoa and nematodes) in the hemolymph (e.g. Yourth et al. Reference Yourth, Forbes and Smith2002; Rantala and Roff, Reference Rantala and Roff2007). As a result, haemocytes form a multilayer melanin capsule around the invader, suppressing and immobilizing it (Marmaras and Lampropoulou, Reference Marmaras and Lampropoulou2009). During the encapsulation measurements the adults were approximately 7–11 days old.
Encapsulation response was measured against a novel object (i.e. implant) as a proxy for cellular immune response of adult L. cervi. As a foreign invader, a sterile nylon monofilament capsule (2 mm in length; 0·16 mm in diameter; knotted at one end; Stroft GTM, Germany) was used to activate and measure the level of encapsulation response (see e.g. Krams et al. Reference Krams, Daukšte, Kivleniece, Krama and Rantala2013). The ability to encapsulate the nylon monofilament is strongly associated with the ability to resist real pathogens (Rantala and Roff, Reference Rantala and Roff2007). The surface of the nylon monofilament was scratched with sandpaper P600 before it was used to make implants. This was done to enhance the likelihood of hemocytes sticking to the implant. Each scratched and knotted implant was sterilized in 96% ethanol prior to use.
Prior to implantation, the L. cervi adults (n = 188) were anesthetized with CO2. While anesthetized, the implants were set through a puncture into the pleural membrane between the third and fourth abdominal sternite. During the 5-h exposure to implants the L. cervi adults were kept in individually numbered, small cylindrical translucent plastic canisters (30 mm diameter, 50 mm height) at 22 ± 0·5 °C (Rantala et al. Reference Rantala, Jokinen, Kortet, Vainikka and Suhonen2002; Krams et al. Reference Krams, Daukšte, Kivleniece, Krama and Rantala2013). After the exposure time, the knot allowed us to non-destructively remove the monofilament. Monofilaments were then dried and examined under a light microscope, and the implants were photographed from two different angles. These images were analysed with an image analysis software ImageJ (Abramoff et al. Reference Abramoff, Magalhaes and Ram2004). The amount of reflecting light from the implants was measured. The encapsulation rate was analysed as grey values of reflected light from the implants. The data were transformed so that the darkest grey values correspond to the highest encapsulation rates. This transformation was performed by subtracting the observed grey values from the control grey value (clear implant, having grey value 236). The repeatability of this method for estimating the encapsulation rate has been proven to be high (e.g. Rantala et al. Reference Rantala, Jokinen, Kortet, Vainikka and Suhonen2002).
Statistical analyses
The data were analysed with SPSS for Windows (version 21.0). The level of significance (P-value) for the statistical analyses was set at P < 0·05. A general linear model (GLM) was fitted for the data on encapsulation response. In the full model, temperature treatment (+20, +5, −15, −20 °C) and collection i.e. birth date (February 26/April 3), were set as fixed factors. Pupal mass at birth was set as covariate in the full model. All possible interactions were first included in the full model but, in order to obtain the most parsimonious model, the non-significant terms were later removed from the model. A custom hypothesis test was performed using a contrast analysis (K Matrix) within the model to test how the three temperature treatments (+20, −15, −20 °C) differ from the control treatment of +5 °C.
The interaction effects of 3-day cold exposure and pupal size on survival rate of L. cervi have already been analysed by Härkönen et al. (Reference Härkönen, Kaitala, Kaunisto and Repo2012). Since the survival at low temperatures (−15 and −20 °C) was significantly biased towards larger individuals (see Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012), in the present study an additional regression analysis on the effects of pupal size on adult encapsulation rate was carried out only among the control individuals (+5 °C).
RESULTS
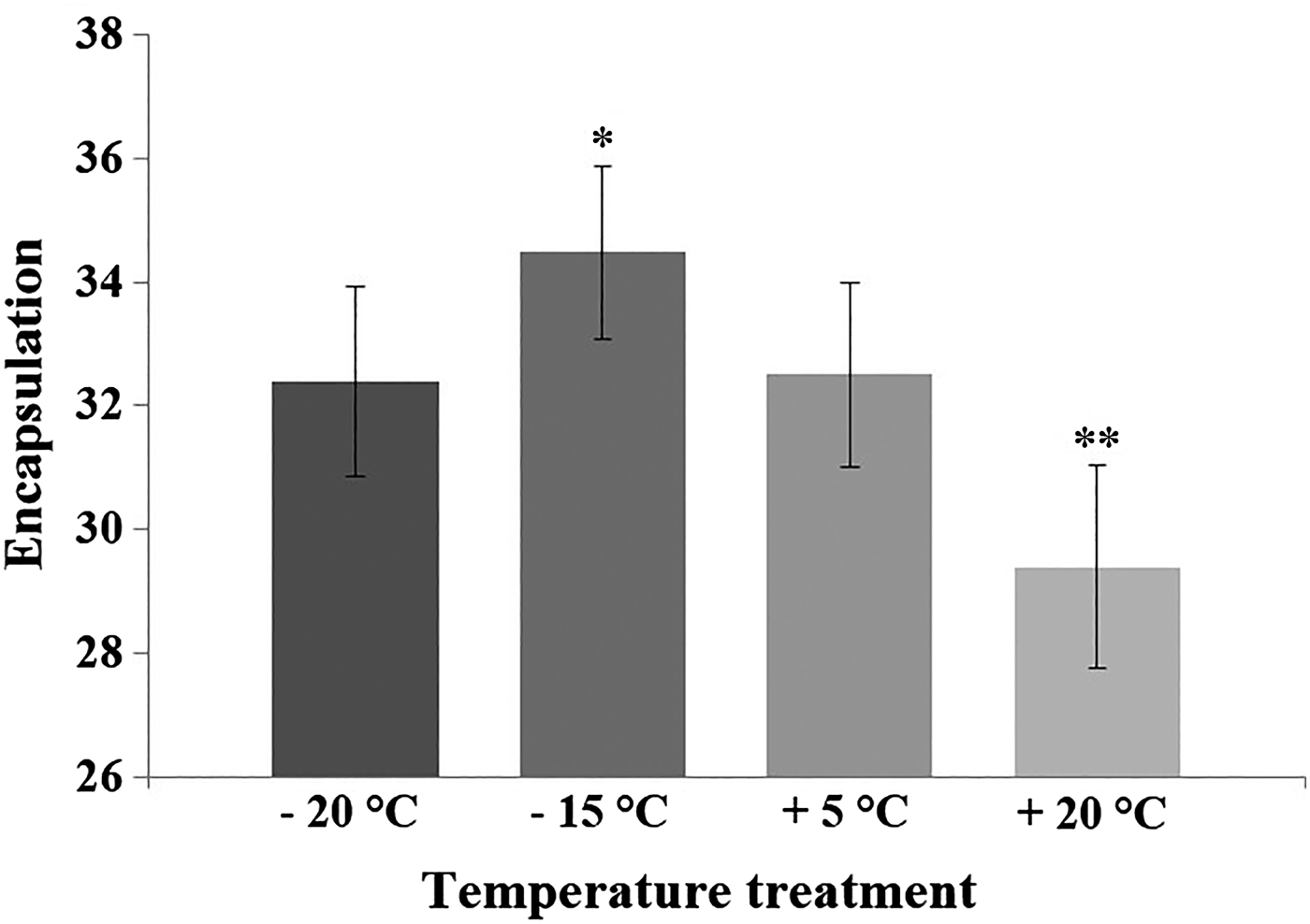
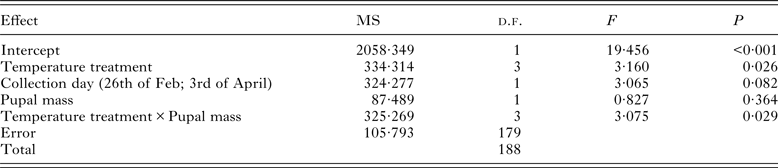
Results of the GLM indicated that temperature treatment of diapausing pupae affected adult immunity 3 months later (see Table 1). The custom hypothesis test showed that both the +20 and −15 °C treatments differed from the control treatment (+5 °C) (Fig. 1). The contrast estimate of the test between the +20 °C and the control treatment was −67·88 (95% CI: −117·687 to −18·073; P = 0·008). The contrast estimate between the −15 °C and the control treatment was −50·234 (95% CI: −96·605 to −3·862; P = 0·034). Treatment of −20 °C and the control did not statistically differ from each other (the contrast estimate was −14·304; 95% CI: −62·377 to 33·769; P = 0·558). The adult encapsulation response was lower among the individuals that were suddenly exposed to high temperature (+20 °C) during pupal diapause compared with the control group (+5 °C) (Fig. 1). On the contrary, the −15 °C -treatment during pupal diapause enhanced adult encapsulation capacity when compared with the control treatment. Estimated marginal means (GLM) and standard deviations (for raw data) for the encapsulation values in the control and temperature treatments were: control (32·5; s.d. = 10·15), +20 °C (29·4; s.d. = 10·37), −15 °C (34·48; s.d. = 10·06), −20 °C (32·4; s.d. = 11·39).
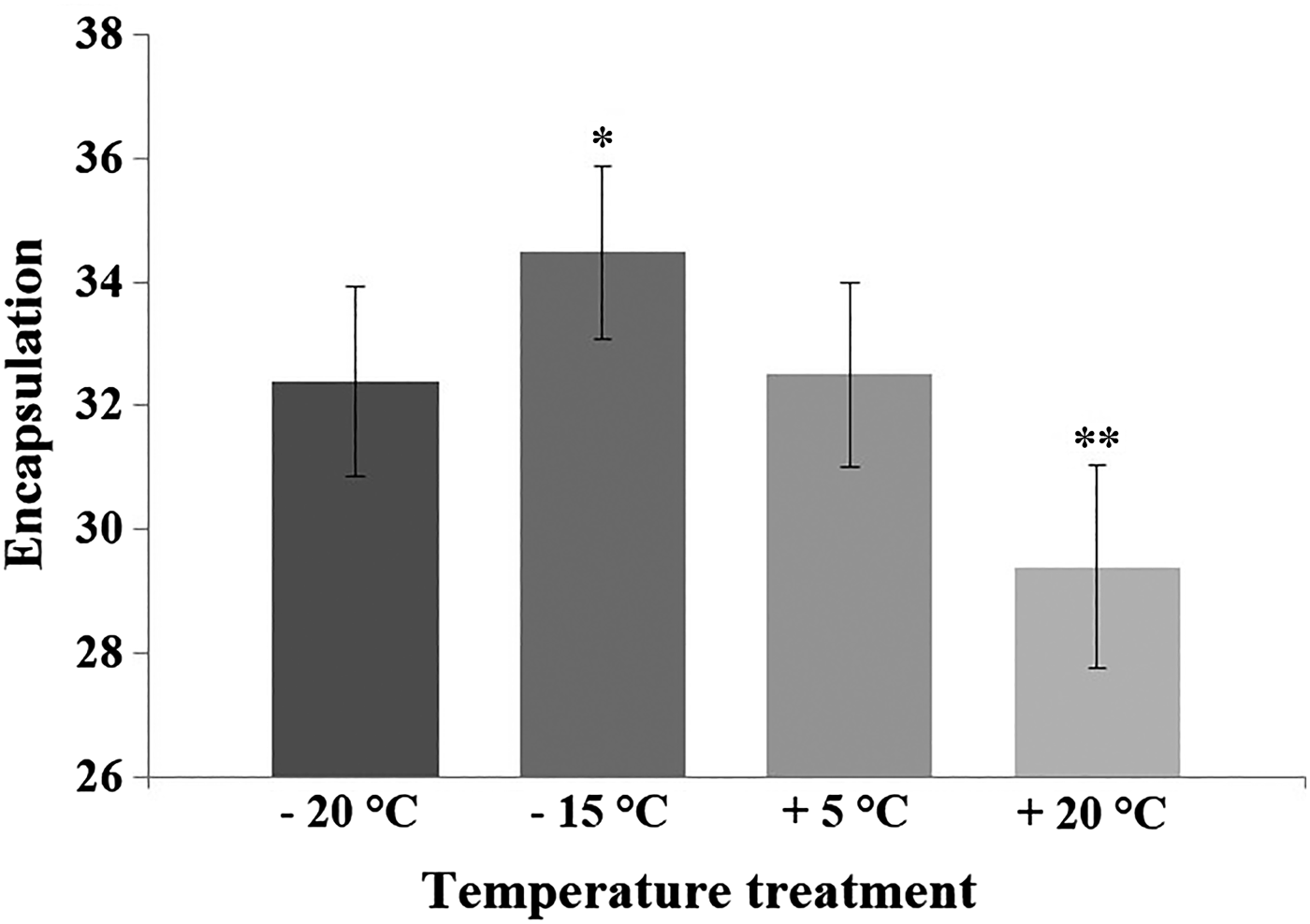
Fig. 1. The encapsulation rate of the L. cervi adults after being exposed to the temperature treatments and control temperature during their pupal dormancy. The GLM -estimated marginal means and standard errors (bars) of the encapsulation rate for each temperature treatment (+20 °C, n = 40; −15 °C, n = 55; −20 °C, n = 46) and control (+5 °C, n = 47). Significant differences with control group (*=P < 0·05; **=P < 0·01) are shown by asterisks (custom hypothesis test, a contrast analysis). Encapsulation response was determined by subtracting the mean of the implant grey value measures from the grey value of a clear implant (having grey value 236).
Table 1. Result of the GLM-model testing the main effects of temperature treatment, collection time (i.e. birth time) of pupae and pupal mass on adult encapsulation capacity in L. cervi, as well as interactions between treatment and pupal mass on adult encapsulation. MS is mean square and d.f. refers to the degrees of freedom. P-value is considered significant at <0·05

Pupal mass had no significant main effect on encapsulation response (Table 1). Instead, there was a significant pupal mass × temperature interaction. Within the treatments of +20 and −15 °C, large size was found to increase the encapsulation response, whereas at temperatures of +5 and −20 °C, high encapsulation was found in small individuals. In the model, collection time had no significant main effect on encapsulation rate (Table 1). Encapsulation capacity value for individuals collected in February was 31·37 (s.d. = 11·04; s.e. = 1·24) and for individuals collected in April the encapsulation value was 33·53 (s.d. = 10·16; s.e. = 0·97).
Survival rates of the L. cervi pupae with respect to temperature treatment were: +20 °C (64%), −15 °C (85%) and −20 °C (68%), and in the control treatment +5 °C (87%) (see also Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012). Additional analysis on effects of pupal mass on adult encapsulation among control individuals (i.e. no significant size-selective mortality assumed) revealed that encapsulation response is negatively correlated with size (B ± s.e. = −3·429 ± 1·555; F 1·44 = 4·860, P = 0·033). The mean weight for pupae born in February was 9·78 mg (s.d. = 0·902; s.e. = 0·102) and for pupae born in April the mean weight was 10·35 mg (s.d. = 0·776; s.e. = 0·074).
DISCUSSION
The present study was conducted to assess whether exposure to different temperatures has long-term effects on immunity in L. cervi. Specifically, it was explored whether a low temperature peak (−15 or −20 °C) or a high temperature peak (+20 °C), experienced during pupal dormancy, would have significant effects on immunity of the host-seeking, non-fed adults emerging several months later. Surprisingly, it was found that exposure to the naturally occurring low temperature (−15 °C) during pupal diapause boosted immunity of emerged non-fed adults, whereas even harsher frost of −20 °C had no effect when compared with control treatment. Instead, a high temperature peak (+20 °C) during pupal diapause deteriorated significantly the encapsulation capacity. Among the control individuals the adult encapsulation response was negatively correlated with pupal size, but in general, the effect of pupal mass on encapsulation response varied between the temperature treatments.
A low temperature of −15 °C experienced during pupal diapause enhanced encapsulation capacity of the L. cervi adults, when compared with the control treatment temperature (i.e. no exposure to extreme temperature peaks) (Fig. 1). During the long, free-living pupal stage in northern Europe, L. cervi has to face such a low temperatures as well as large temperature fluctuations in their natural environment. Insects that are exposed to tolerable low temperatures may exhibit slower metabolic processes resulting in higher fitness consequences and energy reserves for immune responses in later life (Catalán et al. Reference Catalán, Wozniak, Niemeyer, Kalergis and Bozinovic2012; Mandrioli, Reference Mandrioli2012). In addition, there is evidence of cold exposure -related immunity upregulation in insects (reviewed by Sinclair et al. Reference Sinclair, Ferguson, Salehipour-shirazi and MacMillan2013). However, in the present study the other low temperature peak (−20 °C) did not boost immunity compared with the control treatment. The lack of a positive immune response may be explained if the −20 °C represents already too severe frost for L. cervi. Cold hardening mechanisms required for surviving such an extremely low temperature exposure are energetically costly and there is likely no energy left for additional immunity boosting in adulthood. In the present data, after exposing pupae to −20 °C for 3 days the mean survival was 68%, i.e. notably lower compared with the survival percentages in the −15 and +5 °C treatments (85 and 87%, respectively). Thus, the poorer survival suggests that −20 °C represents energetically demanding conditions. Because the survival at −20 °C was already notably lowered, but the later immunity was not, it is possible that there are no trade-offs between encapsulation and cold-hardening in L. cervi.
An exposure to high temperature peak during pupal diapause was found to significantly decrease the adult encapsulation capacity. Also, the survival percentage (64%) was substantially deteriorated after the pupae were exposed to the +20 °C (compared with the control treatment, 87%). Such a high temperature peak is quite unusual to occur during pupal diapause in nature conditions between February and early April. The high temperature peak may have disturbed the pupal diapause syndrome in unpredictable ways leading to lower survival and reduced encapsulation response in adulthood. During the diapause, metabolic rate is reduced and such a high temperature in general can accelerate biochemical processes, when immune response can quickly be enhanced or even reach the maximum (Haynie, Reference Haynie2008; Catalán et al. Reference Catalán, Wozniak, Niemeyer, Kalergis and Bozinovic2012). However, a maximal response might not always be the optimal, because of possible long-term energetic trade-offs between other high temperature - accelerated traits and immunity (Karl et al. Reference Karl, Stoks, De Block, Janowitz and Fischer2011; Catalán et al. Reference Catalán, Wozniak, Niemeyer, Kalergis and Bozinovic2012; reviewed in Mandrioli, Reference Mandrioli2012).
Large pupal size has been shown to increase survival rate at low temperatures until adult emergence in L. cervi (Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012, Reference Härkönen, Hurme and Kaitala2013). Because the encapsulation could be measured only for those individuals that survived the temperature treatments, there is a bias towards larger adults in the cold treated, survived L. cervi individuals (for detailed size-dependent survival analyses see Härkönen et al. Reference Härkönen, Kaitala, Kaunisto and Repo2012). Therefore, in the present study, only the control individuals were looked to determine the size-immune relationship, which drove the result that small individuals had better immune response. It is possible that the negative relationship between size and encapsulation could reflect a trade-off between investment in immune response and growth and development (Vogelweith et al. Reference Vogelweith, Dourneau, Thiéry, Moret and Moreau2013). Increase in encapsulation with pupal size at +20 and −15 °C, accompanied with negative relationship between encapsulation and size at +5 and −20 °C, could implicate complex trade-offs between investment in cold-hardening/survival, growth and immune response.
Temperature effects on insect immunity have been a focus of study lately (e.g. Mourya et al. Reference Mourya, Yadav and Mishra2004; Murdock et al. Reference Murdock, Paaijmans, Bell, King, Hillyer, Read and Thomas2012), but the work presented in the present study is the first to explore the interplay between temperature and immune function in blood-feeding, semi-permanent ectoparasites. Moreover, long-term effects of temperature on immunity have remained virtually unstudied in insects (but see Benelli, Reference Benelli1998; Mourya et al. Reference Mourya, Yadav and Mishra2004; Schmid-Hempel, Reference Schmid-Hempel2005). Thus, our study is among the first to emphasize that environmental temperature may have long-term effects on insect immunity through developmental stages, highlighting the need for more detailed study on the topic. The present experimental set up includes extreme temperature variation that could possibly occur under ongoing climate change. However, the results of this study should be interpreted cautiously within limitations of our approach. A ramped temperature protocol (in which temperature would increase slowly over a longer time period), or daily fluctuating, natural rearing temperature regimes would be fruitful approaches in future work.
ACKNOWLEDGEMENTS
We thank Nick DiRienzo and anonymous referees for their feedback on the manuscript. This study was conducted in accordance with the Finnish legislation.
FINANCIAL SUPPORT
This study was partly funded by EnviroNet (L. H.) and several Finnish foundations: the North Karelia Regional Fund (S. K.), the Ella and Georg Ehrnrooth Foundation (S. K., L. H.), the Biological Society of Finland Vanamo (S. K., L. H.), Societas Pro Fauna et Flora Fennica (S. K., L. H.), and the Alfred Kordelin Foundation (S. K.)