Introduction
Cystic echinococcosis (CE), caused by the cestode Echinococcus granulosus, is a globally distributed zoonotic disease. In endemic regions, the condition causes human and animal health-related losses as well as economic losses (Moro & Schantz, Reference Moro and Schantz2009). Intermediate hosts, which acquire parasitic cysts in the liver, lungs and other organs, include a variety of herbivorous mammals as well as humans. CE is a major public health concern in Iran, and is endemic in many areas of the country. The overall prevalence of CE in livestock (sheep, cattle, camels and goats) in Iran was reported to be 6.7%. Serological studies on humans indicated seroprevalence from 1.2 to 21.4% in different regions of the country (Rokni, Reference Rokni2009).
Echinococcus granulosus presents a high level of intraspecific variation and several host-adapted genotypes have been described in different geographical areas (Thompson, Reference Thompson2008). A revision in the classification of the genus Echinococcus has been proposed based on nuclear and mitochondrial sequence data. Studying genetic variation within and between Echinococcus populations can have significant implications for epidemiology and disease control (Nakao et al., Reference Nakao, McManus, Schantz, Craig and Ito2007; Saarma et al., Reference Saarma, Jogisalu, Moks, Varcasia, Lavikainen, Oksanen, Simsek, Andresiuk, Denegri, Gonzalez, Ferrer, Garate, Rinaldi and Maravilla2009).
Molecular epidemiological studies have been carried out on Iranian E. granulosus isolates using sequence data of mitochondrial and nuclear genes (Zhang et al., Reference Zhang, Eslami, Hosseini and McManus1998; Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Jex, Pangasa, Campbell, Kia, Eshraghian, Harandi and Gasser2009). Molecular analyses have indicated that the camel strain (G6) of E. granulosus is found in livestock and humans in different parts of Iran. However, the common sheep strain (G1) is more prevalent than the camel strain among both livestock and humans (Fasihi Harandi et al., Reference Fasihi Harandi, Hobbs, Adams, Mobedi, Morgan-Ryan and Thompson2002; Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Harandi, Rezaeian, Mohebali, Eshraghian, Rahimi and Kia2010).
To date, few sequence-based studies on E. granulosus isolates have been carried out in Iran (Rostami Nejad et al., Reference Rostami Nejad, Nazemalhosseini Mojarad, Nochi, Fasihi Harandi, Cheraghipour, Mowlavi and Zali2008; Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Jex, Pangasa, Campbell, Kia, Eshraghian, Harandi and Gasser2009). Additional sequence studies, especially of mitochondrial origin, from different intermediate hosts and geographical areas, are needed to provide a comprehensive understanding of the nature and extent of the genetic variation of E. granulosus in Iran. Due to the absence of data on E. granulosus genotypes found in south-eastern Iran, this study investigated the E. granulosus genotypes existing in livestock from this region using sequencing data of cox1 and nad1 mitochondrial genes.
Materials and methods
Collection of samples
In total, 59 E. granulosus cysts were collected from sheep (35), cattle (11), camels (9) and goats (3). The animals originated from various locations within Kerman Province, Iran and were slaughtered in abattoirs located in the cities of Rafsanjan and Kerman. A single human isolate from a female CE patient operated on at Afzalipour Medical Center in Kerman city was also included in the study. Each individual cyst was processed as an E. granulosus isolate. Protoscoleces and/or germinal layers were aspirated from cysts and were washed three times with normal saline and stored at − 20°C until used.
DNA extraction
Genomic DNA was extracted from protoscoleces removed from fertile cysts using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) according to the manufacturer's recommended protocol. DNA was extracted from the germinal layer of sterile cysts as described by Kamenetzky et al. (Reference Kamenetzky, Canova, Guarnera and Rosenzvit2000). Briefly, the germinal layers were minced and frozen and thawed four times. The germinal layers were then incubated overnight in 200 μg tissue lysis buffer and 80 μg proteinase K at 56°C (Kamenetzky et al., Reference Kamenetzky, Canova, Guarnera and Rosenzvit2000). DNA extracted from both protoscoleces and germinal layers was stored at − 20°C until polymerase chain reaction (PCR) amplification was performed.
PCR amplification and DNA sequencing
Fragments of the cytochrome c oxidase subunit 1 (cox1) and NADH dehydrogenase 1 (nad1) genes were amplified. The JB3 (TTTTTTGGGCATCCTGAGGTTTAT) and JB4.5 (TAAAGAAAGAACATAATGAAAATG) sequences were used as cox1 forward and reverse primers and the JB11 (AGATTCGTAAGGGGCCTAATA) and JB12 (ACCACTAACTAATTCACTTTC) sequences were used as nad1 forward and reverse primers, respectively (Bowles et al., Reference Bowles, Blair and McManus1992; Bowles & McManus, Reference Bowles and McManus1993). Fifty microlitre reaction volumes containing 3.5 mm MgCl2, 250 μm of each deoxynucleoside triphosphate (dNTP), 25 pmol of each primer, 2 units Taq polymerase and 4 μl (50–100 ng/μl) of DNA template were used and amplified by PCR under the following temperature conditions: 94°C for 30 s (denaturation), 50°C for 45 s (annealing), 72°C for 35 s (extension) for 35 cycles and a final extension at 72°C for 10 min (Gasser et al., Reference Gasser, Zhu and McManus1998).
Sequencing and phylogenetic analysis
A panel of 38 PCR products from different hosts (table 1) was sequenced (ABI 3730 DNA Analyzer) after purification. Sequences were compared with each other and with reference sequences using BioEdit software (Hall, Reference Hall1999) and adjusted manually. Reference sequences of all described E. granulosus genotypes (G1–G10), Echinococcus species and Taenia saginata (as the outgroup) were obtained from previous publications (Bowles et al., Reference Bowles, Blair and McManus1992; Bowles & McManus, Reference Bowles and McManus1993, Reference Bowles and McManus1994; Gasser et al., Reference Gasser, Zhu and McManus1999) and the National Center for Biology Information (http://www.ncbi.nlm.nih.gov/).
Table 1 Echinococcus granulosus genotypes in different hosts identified by mitochondrial sequence analysis in Kerman, south-eastern Iran.

Three phylogenetic analyses of the sequence data were conducted using cox1 and nad1 sequences separately (trees 1 and 2, not shown) and together as concatenated cox1+ nad1 (tree 3, fig. 1). Each tree was run using sequences obtained in this study as well as reference sequences of all described E. granulosus genotypes (G1–G10) and Echinococcus species. Corresponding T. saginata sequences were used in the dendrogram as an outgroup. Representative GenBank accession numbers for the sequences inferred from this study and for the reference genotypes used in all analyses are shown in table 2. Sequence-based Bayesian inference methods were applied to all analyses. Bayesian inference was conducted using the program MrBayes v.3.1.2 (http://mrbayes.csit.fsu.edu/index.php). Posterior probabilities (pp) were designed for 2,000,000 generations (ngen: 2,000,000). The TreeviewX v.0.5.0 program (Page, Reference Page1996) was used to depict the resulting trees.
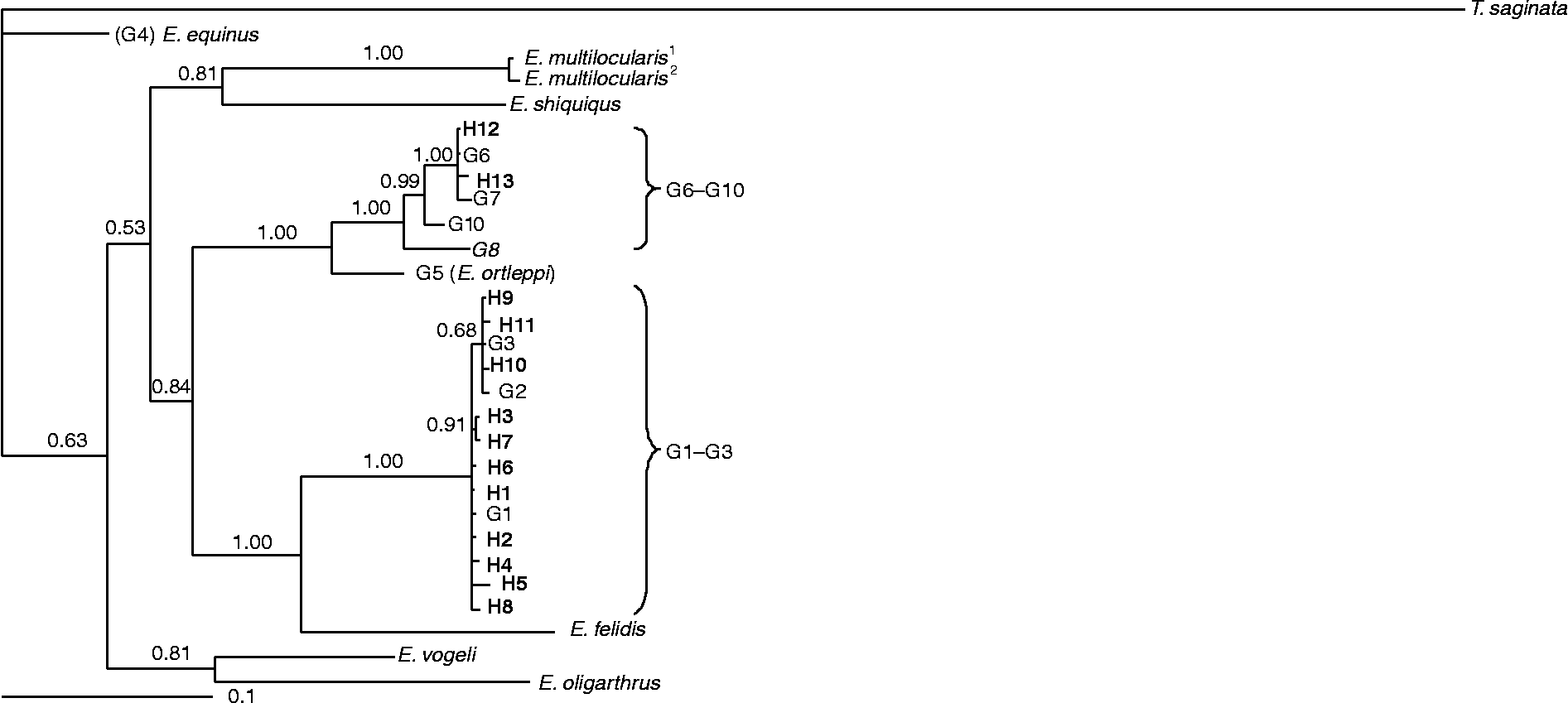
Fig. 1 Phylogenetic tree of E. granulosus isolates from Kerman, Iran and reference sequences for E. granulosus sensu lato and other species of Echinococcus using the Bayesian Inference (BI) method (see table 2). The relationships were obtained according to phylogenetic analysis of concatenated cox1 and nad1 sequence data using BI (haplotypes 1–13 in bold type). Haplotypes 1–11 represent genotypes G1–G3 (G1–G3 complex, E. granulosus sensu stricto), whereas haplotypes 12 and 13 represent genotype G6 (within G6–G10 complex, E. canadensis). The sources and accession numbers of sequences are shown in table 2. The scale bar indicates distance. Nodal support is given as a pp value.
Table 2 Echinococcus granulosus haplotypes from Kerman Province, Iran and origins of sequences used for concatenation (cox1+ nad1) and subsequent phylogenetic analyses (see figs 1–3).

NA, not available.
a References: 1, Bowles et al. (1992); 2, Bowles & McManus (Reference Bowles and McManus1993); 3, Nakao et al. (Reference Nakao, McManus, Schantz, Craig and Ito2007); 4, Lavikainen et al. (Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2003); 5, Hüttner et al. (Reference Hüttner, Nakao, Wassermann, Siefert, Boomker, Dinkel, Sako, Mackenstedt, Romig and Ito2008); 6, Bowles & McManus (Reference Bowles and McManus1994).
Results
Fifty-nine E. granulosus isolates were obtained, of which 46 were fertile and contained protoscoleces. Cysts from sheep, goats, cattle and camels had fertility rates of 100, 66.7, 18.2, and 66.7%, respectively. PCR amplification was successfully performed on all of the isolates for both the cox1 and nad1 genes, except for six cattle isolates and two camel isolates which could not be evaluated at the nad1 region.
The G1, G3 and G6 genotypes were identified from the livestock isolates. The one human isolate produced a sequence congruent with the G6 genotype. The human isolate differed by two nucleotides compared with the cox1 (accession no: M84666) G6 reference sequence and by one nucleotide compared with the nad1 (accession no: AJ237637) G6 reference sequence. Genotype assignments for cox1 and nad1 are shown in table 1. Overall, the sequence alignments of the isolates showed eight representative profiles in cox1 sequences (considered as IRCO1 to IRCO8) and 11 representative profiles in nad1 sequences (IRND1 to IRND11) (figs 2 and 3). Sequence profiles for cox1 and nad1 were submitted to GenBank with accession numbers HM563001 to HM563022 and HM563023 to HM563037, respectively. Isolates having both cox1 and nad1 sequence profiles were concatenated and 13 haplotypes (H1 to H13) were observed. A dendrogram based on the phylogenetic analysis of cox1 and nad1 sequences and a consensus tree of the concatenated cox1+ nad1 sequences are shown in fig. 1. Isolates were grouped into two distinct clusters corresponding to the G1–G3 complex (pp = 1.00) and the G6–G10 complex (pp = 1.00). Most of the isolates (73.7%) were identified as G1 and were clustered with the G1 reference genotype (accession nos: cox1, U50464; nad1, AJ237632). Isolates identified as G3 (13.2%) clustered with the G3 (accession nos: cox1, M64663; nad1. AJ237634) reference sequences and isolates identified as G6 (13.1%) clustered with the G6 (accession nos: cox1, M84666; nad1, AJ237637) reference sequences.

Fig. 2 Alignments of the eight representative profiles of cox1 sequences, among E. granulosus isolates from Kerman, Iran, with key reference sequences (for genotypes G1–G3 and G6, G7) from previous studies (Bowles et al., Reference Bowles, Blair and McManus1992; Bowles & McManus, Reference Bowles and McManus1993; Gasser et al., Reference Gasser, Zhu and McManus1999). Taenia saginata was used as the outgroup. The accession numbers of individual sequences are given in square brackets.

Fig. 3 Alignments of the eleven representative profiles of nad1 sequences, among E. granulosus isolates from Kerman, Iran with key reference sequences (for genotypes G1–G3 and G6, G7) from previous studies (Bowles et al., Reference Bowles, Blair and McManus1992; Bowles & McManus, Reference Bowles and McManus1993; Gasser et al., Reference Gasser, Zhu and McManus1999). Taenia saginata was used as the outgroup. The accession numbers of individual sequences are given in square brackets.
Discussion
The results of this study showed that the G1 genotype (E. granulosus sensu stricto) was the most commonly identified genotype from sampled livestock in Kerman Province, Iran. G1 was identified from most of the sheep (86.7%), camel (44.4%), cattle (80%) and goat (100%) isolates. The G6 (E. canadensis) and G3 genotypes were also found in sheep, cattle and camels (table 1). This finding suggests that sheep–dog and camel–dog cycles occur in this region and that cross-transmission of the two cycles occurs. G1 is the predominant genotype found in livestock globally (Casulli et al., Reference Casulli, Manfredi, La Rosa, Cerbo, Genchi and Pozio2008; Li et al., Reference Li, Ito, Nakaya, Qiu, Nakao, Zhen, Xiao, Chen, Giraudoux and Craig2008; Utuk et al., Reference Utuk, Simsek, Koroglu and McManus2008; Moro et al., Reference Moro, Nakao, Ito, Schantz, Cavero and Cabrera2009), although in some north African countries, such as Sudan, G6 is the predominant genotype found in sheep, camels and goats (Omer et al., Reference Omer, Dinkel, Romig, Mackenstedt, Elnahas, Aradaib, Ahmed, Elmalik and Adam2010).
Previous studies in Iran and the Middle East identified approximately one-third of isolates from camels as belonging to the G6–G7 complex (E. canadensis), with the remainder belonging to the G1–G3 complex (Maillard et al., Reference Maillard, Benchikh-Elfegoun, Knapp, Bart, Koskei, Gottstein and Piarroux2007; Rostami Nejad et al., Reference Rostami Nejad, Nazemalhosseini Mojarad, Nochi, Fasihi Harandi, Cheraghipour, Mowlavi and Zali2008; Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Jex, Pangasa, Campbell, Kia, Eshraghian, Harandi and Gasser2009, Reference Sharbatkhori, Mirhendi, Harandi, Rezaeian, Mohebali, Eshraghian, Rahimi and Kia2010). Another study showed that G6 occurred in camels, cattle, sheep and humans from different parts of Iran. In this same study, 75% of camel isolates were showed to be of the G6 genotype (Fasihi Harandi et al., Reference Fasihi Harandi, Hobbs, Adams, Mobedi, Morgan-Ryan and Thompson2002). However, study findings differ in the extent to which the G6 genotype is found in sheep and camel hosts, with additional studies needed, especially in areas endemic for the camel–dog transmission cycle.
Recent studies have confirmed the presence of the G3 genotype in camels in Iran (Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Jex, Pangasa, Campbell, Kia, Eshraghian, Harandi and Gasser2009). The present study also detected the G3 genotype in camels and, for the first time in Iran, identified G3 from sheep and cattle hosts. In Pakistan, the sheep strain (G1 genotype) and buffalo strain (G3 genotype) were detected among livestock (Latif et al., Reference Latif, Tanveer, Maqbool, Siddiqi, Kyaw-Tanner and Traub2010). The most prevalent genotype occurring in livestock from Turkey corresponded to the G1 genotype, while the G3 genotype was found in five isolates from sheep and cattle (Vural et al., Reference Vural, Baca, Gauci, Bagci, Gicik and Lightowlers2008). The G3 genotype has also been detected from cattle and buffalo isolates from India, Italy and Turkey (Bhattacharya et al., Reference Bhattacharya, Bera, Bera, Maity and Das2007; Casulli et al., Reference Casulli, Manfredi, La Rosa, Cerbo, Genchi and Pozio2008; Simsek et al., Reference Simsek, Balkaya and Koroglu2010). G1 was exclusively identified in Iranian livestock while the presence of G3 genotype was established in buffaloes from Aligare, northern India (Gholami et al., Reference Gholami, Irshadullah and Khan2009). It may be possible that the G3 genotype was misidentified in previous studies using molecular methods other than nucleic acid sequencing. Additional molecular investigations on E. granulosus isolates from buffalo in Iran are recommended.
The only human isolate evaluated in this study belonged to the G6 genotype. Occurrence of the G6 genotype in humans is considered rare (Simsek et al., Reference Simsek, Kaplan and Ozercan2011). Fasihi Harandi et al. (Reference Fasihi Harandi, Hobbs, Adams, Mobedi, Morgan-Ryan and Thompson2002) first documented human G6 infection in Iran, with other studies only identifying the G1 genotype from human isolates (Zhang et al., Reference Zhang, Eslami, Hosseini and McManus1998; Jamali et al., Reference Jamali, Ghazanchaei and Asgharzadeh2004; Sharbatkhori et al., Reference Sharbatkhori, Mirhendi, Jex, Pangasa, Campbell, Kia, Eshraghian, Harandi and Gasser2009). Similar studies in other countries showed that most human CE cases were infected by the sheep strain (G1 genotype). In a recent study conducted in Kenya, 83% of human isolates belonged to the G1 genotype (Casulli et al., Reference Casulli, Zeyhle, Brunetti, Pozio, Meroni, Genco and Filice2010). In addition, all of the human isolates from Spain and Tunisia were identified as belonging to the G1 genotype (Daniel Mwambete et al., Reference Daniel Mwambete, Ponce-Gordo and Cuesta-Bandera2004; M'Rad et al., Reference M'Rad, Filisetti, Oudni, Mekki, Belguith, Nouri, Sayadi, Lahmar, Candolfi, Azaiez, Mezhoud and Babba2005). It is believed that humans are less susceptible to the G6 genotype (E. canadensis) than to the G1 genotype (Thompson & McManus, Reference Thompson, McManus, Eckert, Gemmell, Meslin and Pawlowski2001). This may be due to increased exposure to the G1 genotype as well as the G1 genotype being more prevalent in dogs and various intermediate hosts.
Thirty-seven animal isolates, along with the reference genotypes, were phylogenetically analysed using Bayesian inference. The isolates were clustered in two main groups corresponding to the G1–G3 and G6–G10 genotype complexes. Thirty-three isolates were nested within the G1–G3 reference genotypes and four isolates were nested within the G6–G10 reference genotypes. Five isolates identified as belonging to the G3 genotype were grouped together with the G3 reference genotype (fig. 1) and the G1 isolates, providing further evidence that the G1 and G3 genotypes should be considered as E. granulosus sensu stricto (Nakao et al., Reference Nakao, McManus, Schantz, Craig and Ito2007; Saarma et al., Reference Saarma, Jogisalu, Moks, Varcasia, Lavikainen, Oksanen, Simsek, Andresiuk, Denegri, Gonzalez, Ferrer, Garate, Rinaldi and Maravilla2009). Comparing topologies of the dendrograms showed that the G1–G3 isolates were clearly distinct from the G6–G7 isolates, indicating the presence of at least two cryptic species in E. granulosus sensu lato (i.e. E. granulosus sensu stricto (G1–G3) and E. canadensis (G6–G8 and G10)). Based on epidemiological, geographical and host-range data for the G6–G8 plus G10 complex, it has been proposed that the complex could be broken into two different species, namely E. intermedius (for G6 and G7) and E. canadensis (for G8 and G10) (Tappe et al., Reference Tappe, Kern and Frosch2010). One human isolate identified as G6 clustered in the phylogenetic tree together with other animal G6 isolates as well as the reference G6 genotype.
In conclusion, our study demonstrated that the G3 genotype is transmitted among cattle, camels and sheep in Iran. In addition, this study showed that G1 is the predominant genotype circulating among livestock in south-eastern Iran and that the camel–dog and sheep–dog cycles have interactions in the region.
Acknowledgements
The authors would like to thank all the people who helped to accomplish this study, with special thanks to H. Kamyabi, J. Beigzadeh and M.Sangi for their help with sample collection and to Dr Christine Budke for her time to read and comment on the manuscript. This study was supported financially by a grant from Kerman University of Medical Sciences, Project No. 88-43.







