Introduction
Despite the recommendations of European governments and institutions to diversify fish cultures (Abellán & Basurco, Reference Abellán and Basurco1999; Anonymous, 2012), Mediterranean aquaculture is still focused predominantly on two species, the gilthead seabream, Sparus aurata L., and the European seabass, Dicentrarchus labrax L. One of the species with higher potential for aquaculture is the sharpsnout seabream, Diplodus puntazzo Cetti (see Abellán & Basurco, Reference Abellán and Basurco1999). Although catches of the sharpsnout seabream are low in Spanish fisheries (Food and Agriculture Organization, 2010), this species has good aquaculture prospects because of its easy adaptation to conditions of captivity, high growth rate and food conversion efficiency (Favaloro et al., Reference Favaloro, Lopiano and Mazzola2002; Hernández et al., Reference Hernández, Egea, Rueda, Martínez and García García2003). The culture of this sparid is still under development, with a limited number of larvae produced in Italy, Greece and Portugal (Federation of European Aquaculture Producers, 2008; Vinagre et al., Reference Vinagre, Cabral and Costa2010). In Spain this culture has been mostly experimental (Hernández et al., Reference Hernández, Egea, Rueda, Aguado, Martínez and García García2001, Reference Hernández, Martínez and García García2002, Reference Hernández, Egea, Rueda, Martínez and García García2003; Pajuelo et al., Reference Pajuelo, Lorenzo and Domínguez-Seoane2008; Nogales Mérida et al., Reference Nogales Mérida, Tomás-Vidal, Martínez-Llorens and Jover Cerdá2010; Almaida-Pagán et al., Reference Almaida-Pagán, Hernández, Madrid, De Costa and Mendiola2011). However, the introduction of the sharpsnout seabream into aquaculture has been compromised by the presence of many pathogens, often producing severe pathologies (Athanassopoulou et al., Reference Athanassopoulou, Ragias, Vagianou, Di Cave, Rigos, Papathanasiou and Georgoulakis2005; Merella et al., Reference Merella, Cherchi, Salati and Garippa2005; Katharios et al., Reference Katharios, Hayward, Papandroulakis and Divanach2006; Montero et al., Reference Montero, Cuadrado, Padrós, Crespo and Raga2007; Álvarez-Pellitero et al., Reference Álvarez-Pellitero, Palenzuela and Sitja-Bobadilla2008; Golomazou et al., Reference Golomazou, Athanassopoulou, Karagouni and Kokkokiris2009; Rigos & Katharios, Reference Rigos and Katharios2010; Sánchez-García et al., Reference Sánchez-García, Padrós, Raga and Montero2011).
Aquaculture conditions imply fish stress which favours pathogen transmission and, therefore, abnormally high infection levels (Ogawa, Reference Ogawa1996). Fish parasite diseases are often associated with important economic losses in aquaculture because of fish mortalities, production decrease or increased costs of antiparasitic treatments (Murray & Peeler, Reference Murray and Peeler2005; Rhode, Reference Rhode2005). Often infections appear in farms because many of the pathogens causing diseases are also associated with sea cages. Considerable aggregations of wild fish are usually associated with sea cages in coastal areas (Sánchez-Jerez et al., Reference Sánchez-Jerez, Bayle-Sempere, Fernandez-Jover, Valle and Dempster2007), and parasite species infecting these neighbouring fish can be transmitted, resulting in wild fish acting as reservoirs of parasite infections in farmed fish (Kent, Reference Kent2000; Mladineo et al., Reference Mladineo, Šegvic and Grubišic2009) or parasites in fish farms spreading to the surrounding environment (Rohde, Reference Rhode2005; Krkošek et al., Reference Krkošek, Ford, Morton, Lele, Myers and Lewis2007). Thirdly, different fish species farmed in neighbouring installations can experience cross-infections (Di Cave et al., Reference Di Cave, Berrilli, Cardia, De Liberato, Athanassopoulou and Orecchia2002). These situations should be considered with special attention on new cultures with a short farming history, since their pathogens are unknown or poorly known.
This situation requires the development of effective control of cross-infections, based on considerable knowledge of pathogens living in farmed and wild fish and identification of potential harmful species (Hutson et al., Reference Hutson, Ernst and Whittington2007; Rigos & Katharios, Reference Rigos and Katharios2010). The sharpsnout seabream is, in fact, one of the fish species reported by Dempster et al. (Reference Dempster, Sanchez-Jerez, Bayle-Sempere, Giménez-Casalduero and Valle2002) associated with sea cages in the Mediterranean and, therefore, wild populations of this species could be a source for pathogen transmission to farmed fish of the same or other species. Although a number of studies referring to parasites of sharpsnout seabream in the Mediterranean Sea exist (Athanassopoulou et al., Reference Athanassopoulou, Prapas and Rodger1999, Reference Athanassopoulou, Ragias, Vagianou, Di Cave, Rigos, Papathanasiou and Georgoulakis2005; Di Cave et al., Reference Di Cave, Berrilli, Cardia, De Liberato, Athanassopoulou and Orecchia2002; Vagianou et al., Reference Vagianou, Athanassopoulou, Ragias, Di Cave, Leontides and Golomazou2004; Merella et al., Reference Merella, Cherchi, Salati and Garippa2005; Katharios et al., Reference Katharios, Hayward, Papandroulakis and Divanach2006; Toksen, Reference Toksen2006; Mladineo & Maršić-Lučić, Reference Mladineo and Maršić-Lučić2007; Montero et al., Reference Montero, Cuadrado, Padrós, Crespo and Raga2007; Álvarez-Pellitero et al., Reference Álvarez-Pellitero, Palenzuela and Sitja-Bobadilla2008), these are focused on pathogens of farmed fish and no data on the parasites of sharpsnout seabream in the wild are available. From an epizootiological point of view, the description of parasite communities of this species is indispensable to prevent economic and pathological impacts of certain parasitoses.
The aim of the present study was to identify the parasites infecting wild and farmed sharpsnout seabream. The parasite fauna of wild fish from two locations in the Spanish Mediterranean are described. Furthermore, we tested the effect of culture conditions on these parasites, in order to find those species that can survive and proliferate in farms.
Materials and methods
Collection and examination of fish
Seventy sharpsnout seabream aged 1 year or more were collected alive from two different locations in the Spanish Mediterranean in 2007: 50 fish with a total length (mean ± standard deviation) of 252.1 ± 11 mm, weight 300 ± 37.7 g were collected in Mar Menor off the coast of San Pedro del Pinatar, Region of Murcia (37°41′14″ N, 0°44′10″W); and 20 fish with a total length of 188.5 ± 18 mm, weight 111.95 ± 27.73 g were collected off the coast of Santa Pola, Valencian Community (38°11′23″N, 0°33′20″W). No more fish could be obtained since, as previously stated, the sharpsnout seabream is fished in low numbers in Spain. Thirty fish from Mar Menor and all the individuals from Santa Pola were killed by medullar section and immediately frozen. In order to study the effect of culture conditions on parasites, the other 20 specimens from Mar Menor were transported alive and reared in the aquaculture facilities of the Central Service for the Support to Experimental Research of the University of Valencia (SCSIE). Fish were maintained in marine water (salinity 37‰, temperature 20°C, 8:16 h light :dark photoperiod) and fed with commercial gilthead seabream pellets. After 10 days, ten fish were killed by medullar section; the remaining ten fish were killed after 20 days of captivity.
Fish were first examined for external parasites on the skin, fins and eyes, and following a post-mortem examination internal organs, such as digestive tract, gonads, liver, gills, kidney and brain, were examined in saline solution under a stereomicroscope (100 × magnification). Parasites were removed and preserved in either 70% ethanol for morphological examination or in 100% ethanol for molecular study. Myxozoans were detected by examination of wet preparations of squeezed fresh organs, using a light microscope at magnifications of up to 1000 × with differential interference contrast (DIC).
Morphometrics
Platyhelminths fixed in 70% ethanol were stained with iron acetocarmine (Georgiev et al., Reference Georgiev, Biserkov and Genov1986), dehydrated through a graded ethanol series, cleared in dimethyl phthalate, and examined as permanent mounts in Canada balsam under a light microscope. In the case of the diplectanid monogeneans Lamellodiscus spp., representative subsamples of 30 specimens from each fish were randomly selected to be identified and counted. For those species of Lamellodiscus with no more than 30 specimens, all parasites were examined. Monogeneans were washed in distilled water and examined on semi-permanent preparations in glycerol–gelatine under a light microscope for their detailed morphological examination and identification. Crustaceans were examined under a light microscope in distilled water or in glycerine.
For some species, when the morphological identification was particularly confusing, detailed specific morphometric studies were performed. Fifteen mounted adult specimens of each of these species (i.e. Lamellodiscus spp. and Peracreadium sp.) from each location were selected for their detailed examination. Morphometric data of specimens of Lamellodiscus spp. and Peracreadium sp. were obtained by using a light microscope with a drawing tube. Haptoral parts of the diplectanids were measured according to Amine & Euzet (Reference Amine and Euzet2005) and the resulting measurements were compared with the available published data. All measurements are given in micrometres.
Molecular analysis
Those species difficult to classify morphologically (Lamellodiscus spp. and Peracreadium sp.) were also studied through genetic comparisons. Three to four specimens of these species (see table 1), previously fixed in 100% ethanol, were transferred into 300 μl TNES urea (10 mm Tris–HCl (pH 8), 125 mm NaCl, 10 mm ethylenediaminetetraacetic acid (EDTA), 0.5% sodium dodecyl sulphate (SDS), 4 m urea). Genomic DNA (gDNA) was extracted from single specimens using a phenol–chloroform standard procedure. The extracted DNA was resuspended in 20 μl of RNAse/DNAse-free water and left to dissolve overnight in the fridge. Polymerase chain reactions (PCRs) were performed with a programmable thermal cycler (Techne, TC-512, GMI, Ramsey, Minnesota, USA) in a final volume of 30 μl containing 0.3 U Taq DNA polymerase (BioLabs, Madrid, Spain) and the related 10 × Standard Taq Reaction Buffer with 1.5 mm MgCl2, 200 μm of each deoxynucleoside triphosphate (dNTP), 10 mm of each PCR primer and 20–70 ng of template.
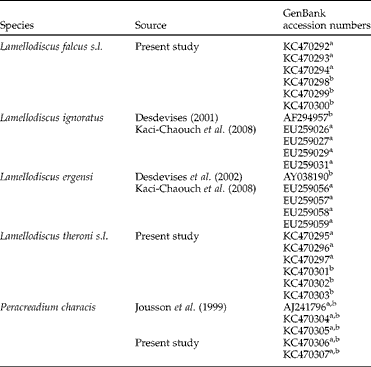
Table 1 The range of monogenean and digenean species occurring in Diplodus puntazzo with accession numbers of the ITS and 18S rDNA sequences.
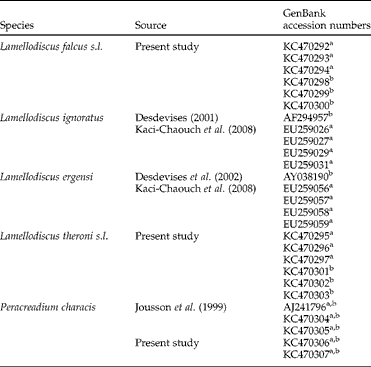
aITS; b18S.
Partial 18S and entire internal transcribed spacer 1 (ITS1) of Lamellodiscus spp. were amplified and sequenced using the primers L7 (5′-TGA TTT GTC TGG TTT ATT CCG AT-3′ (Verneau et al., Reference Verneau, Renaud and Catzeflis1997)) and IR8 (5′-GCT AGC TGC GTT CTT CAT CGA-3′ (Kaci-Chaouch et al., Reference Kaci-Chaouch, Verneau and Desdevises2008)) that anneal to the 18S and 5.8S rRNA genes, respectively. It has been shown that ITS1 is highly variable and not useful for inferring evolutionary relationships among Lamellodiscus spp., but it can be used to differentiate species (Desdevises et al., Reference Desdevises, Jovelina, Jousson and Morand2000). Complete sequences of ITS1, 5.8S and ITS2 of Peracreadium sp. were amplified and sequenced using primers 18dF (5′-CAC ACC GCC CGT CGC TAC TAC CGA TTG-3′ (Hillis & Dixon, Reference Hillis and Dixon1991)) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′ (Anderson & Barker, Reference Anderson and Barker1993)). The thermocycling profile used for the amplification of sequences of Lamellodiscus spp. consisted of denaturation of DNA (95°C for 3 min); 38 cycles of amplification (94°C for 50 s, 50°C for 50 s and 72°C for 1 min 20 s); and 4 min extension hold at 72°C. The same profile was used for gene amplification of Peracreadium sp. but with an annealing temperature of 55°C. After checking the presence of DNA in a 1% agarose gel in sodium acetate buffer and detection following GelRedTM Nucleic Acid Gel Stain staining and ultraviolet transillumination (VWR, Genoview, Barcelona, Spain), the PCR products were purified for sequencing using the GFX PCR DNA and Gel Band purification Kit (GE Healthcare UK Ltd, Pollards Wood, Bucks, UK). Cycle sequencing was conducted in a 48 capillary ABI 3730 sequencer (Applied Biosystems, Madrid, Spain) using the BIG Dye terminator v 3.1 Ready Sequencing Kit (Applied Biosystems) according to the manufacturer's instructions, using the same primers as those used for the PCR. The contiguous sequences were aligned using BioEdit v. 7.0.5. (Hall, Reference Hall1999) and compared for similarities with sequences lodged in GenBank (detailed in table 1) using BLAST (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) and MEGA 4.1. (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007).
In the case of the species of Lamelodiscus, not all sequences were available in GenBank. In particular, no information on L. falcus and L. theroni was found, and the sequences of L. falcus s.l. and L. theroni s.l. obtained in the present study were aligned and compared with the morphologically similar and phylogenetically closest species with available sequences, i.e. L. ignoratus Palombi, 1943 and L. ergensi Euzet et Oliver, 1966, respectively (Amine & Euzet, Reference Amine and Euzet2005).
Data analysis
For all parasite species, mean abundance and prevalence (P%) were calculated as defined by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997), except for the myxozoans for which only prevalence was determined. Confidence intervals for the prevalence were also calculated. A comparison of prevalences and abundances between the samples of the two locations studied, and between wild and captive sharpsnout seabream, were calculated to identify significant differences. Statistical boostrap t-test for abundance and Fisher's exact test for prevalence were carried out with Quantitative Parasitology 3.0 (Rózsa et al., Reference Rózsa, Reiczigel and Majoros2000).
Results
All sharpsnout seabream analysed were infected by at least four parasite species. In total, 19 parasite species were found, 15 in the fish sampled in Mar Menor and 11 in the fish sampled off Santa Pola (table 2). Seven species of the parasites were shared by fish from the two localities. All parasites were identified to species level, except for the myxozoan Ceratomyxa sp., the digenean brain parasite Galactosomum sp. met. and the monogenean Microcotylidae gen. sp. The first two of these parasites were identified only up to genus level as their species identification requires further molecular or morphological analyses. Regarding Microcotylidae gen. sp., one single specimen was found and its morphological traits were not assignable to any known microcotylid species: (1) genital atrium armed with three types of spines (14 long peripheral, 9 small posterior and 4 long central); (2) vaginal pore dorsal and single, with narrow and sinuous conspicuous vaginal duct; and (3) 104 symmetrical clamps in the haptor.
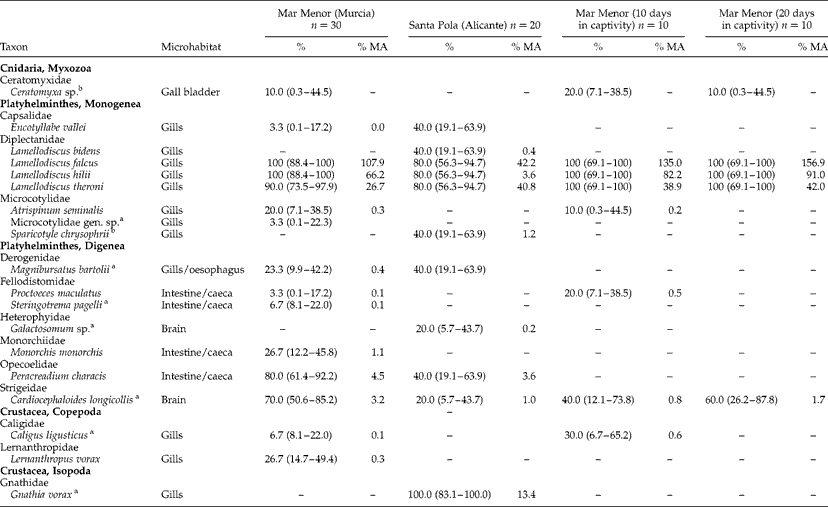
Table 2 The prevalence (%) and mean abundance (% MA) of myxozoan, heminth and crustacean parasites from Diplodus puntazzo in two localities of the Spanish Mediterranean, and from fish maintained in captivity for 10 and 20 days; n=number of fish examined, CI (95% confidence intervals) given in parentheses.

a New records for D. puntazzo.
b New records for wild D. puntazzo.
Significant differences in the total mean abundance were found between wild fish from Mar Menor and Santa Pola (bootstrap t-test, P < 0.01), although the only parasites with significantly different mean abundance were the monogeneans L. falcus and L. hilii (bootstrap t-test, P < 0.05). Fish from both localities also showed significant differences in the prevalence of these monogenean species and of the digeneans Cardiocephaloides longicollis (Rudolphi, 1819) and Peracreadium characis (Stossich, 1886) (Fisher tests, P < 0.05). A greater variety of species was found in the fish from Mar Menor, which harboured eight parasite species not found in the fish from off Santa Pola. These include one myxozoan (Ceratomyxa sp.), two monogeneans (Microcotylidae gen. sp. and Atrispinum seminalis Euzet et Maillard, 1973), three digeneans (Proctoeces maculatus Looss, 1901; Steringotrema pagelli (van Beneden, 1871); and Monorchis monorchis Stossich, 1890) and two copepods (Caligus ligusticus Brian, 1906 and Lernanthropus vorax Richiardi, 1880). Fish from Santa Pola also harboured four parasite species not found in fish from Mar Menor, including two monogeneans (Lamellodiscus bidens Euzet, Reference Euzet1984 and Sparicotyle chrysophrii (van Beneden et Hesse, 1863)), one digenean (Galactosomum sp.) and one isopod (Gnathia vorax Lucas, 1849).
Parasite species richness and total abundance in the fish samples from Mar Menor gradually decreased in captivity conditions. Total parasite abundance was significantly lowered after 10 days of captivity (bootstrap t-test, P < 0.01), and reached even lower levels after 10 more days (bootstrap t-test, P < 0.01). In 10 days, many monogenean species (A. seminalis, L. falcus s.l., L. hilii Euzet, Reference Euzet1984 and L. theroni s.l.) and the myxozoan Ceratomyxa sp. were still observed. In contrast, most digenean species had disappeared, and only two species were found: P. maculatus and the metacercariae of C. longicollis. The last 10 fish examined after 20 days in captivity were parasitized by the same three species of Lamellodiscus, the abundance of these species being higher (although not significantly different) than in the fish examined immediately after capture. Digeneans of the digestive tract were not found at this time, whereas parasites in strictly internal microhabitats (C. longicollis in the brain and Ceratomyxa sp. in the gall bladder) were still present.
Morphometrics
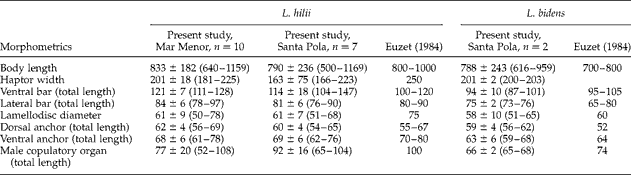
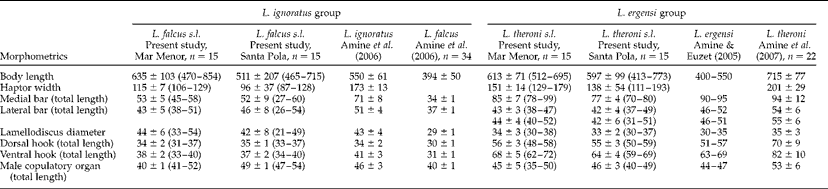
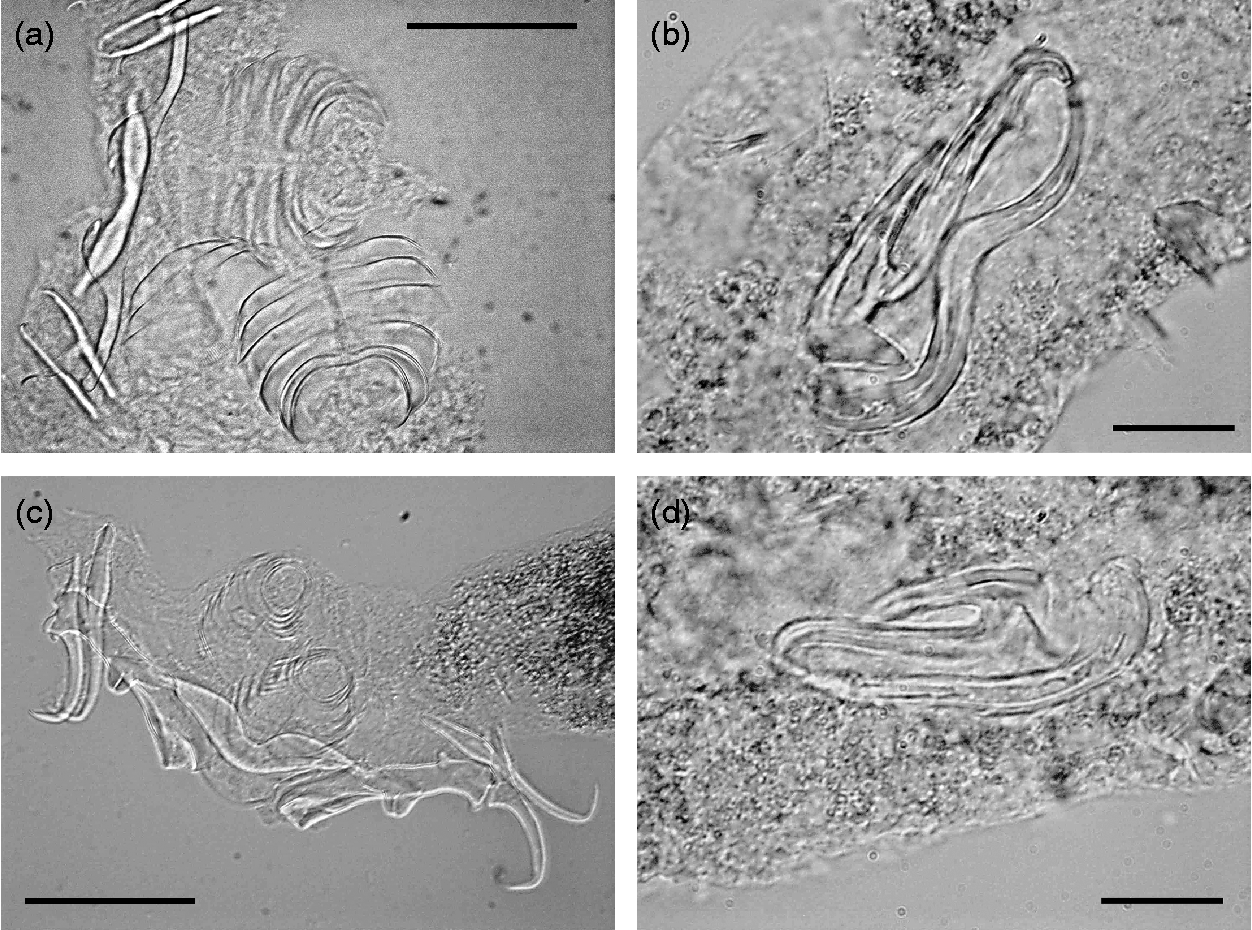
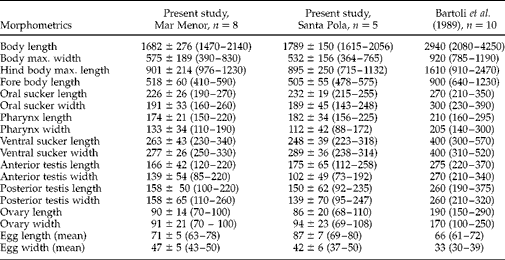
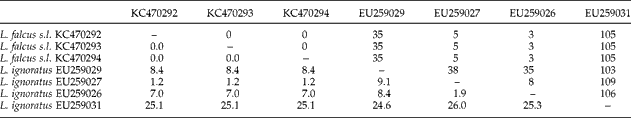
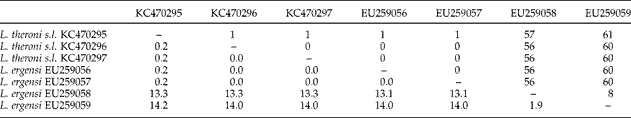
Table 3 summarizes the metrical data for the diplectanid monogeneans found in this study identified to the species level, compared with the original descriptions of Lamellodiscus bidens Euzet, Reference Euzet1984 and L. hilii. The identification of the other two morphotypes of ‘Lamellodiscus’ was controversial (morphometric data in table 4). These morphotypes were similar to L. falcus or to L. theroni (further referred respectively to as L. falcus sensu stricto (s.s.) and L. theroni sensu stricto, for the original descriptions, and L. falcus sensu lato (s.l.) and L. theroni sensu lato, for the specimens of the present study). Both morphotypes in sharpsnout seabream had similar morphology and body length, but the haptor of L. falcus s.l. was, in general, narrower, with sclerotized structures thinner than those of L. theroni s.l. (fig. 1a). Moreover, the dorsal bar of L. falcus s.l. was undivided while that of L. theroni s.l. was divided in two pieces (fig. 1a, b). The copulatory organs of both species were both lyre-shaped, although the single piece of the copulatory organ of the specimens of L. falcus s.l. was observed to be markedly hooked (fig. 1c), and that of L. theroni s.l. was more straightened (fig. 1d).
Table 3 Comparative morphometrics (μm) of the monogenean species Lamellodiscus hilii and L. bidens infecting Diplodus puntazzo; n=number of specimens examined and range in size given in parentheses.
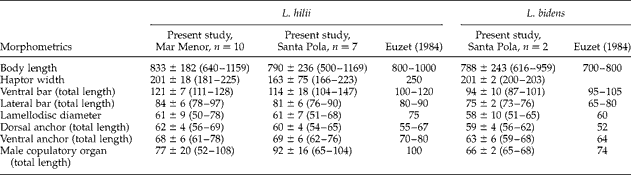
Table 4 Comparative morphometrics (μm) of the monogenean species Lamellodiscus ignoratus and L. ergensi groups infecting Diplodus puntazzo; n= number of specimens examined and range in size given in parentheses.


Fig. 1 The haptors of (a) Lamellodiscus falcus s.l. and (b) L. theroni s.l. and copulatory organs of (c) L. falcus s.l. and (d) L. theroni s.l. from Diplodus puntazzo from the Spanish Mediterranean. Scale bars: a, b = 500 μm; c, d = 100 μm.
The morphometric measurements of the specimens of the digenean Peracreadium sp. found in both localities are given in table 5.
Table 5 Comparative morphometrics (μm) of the digenean species Peracreadium characis infecting Diplodus puntazzo; n=number of specimens examined and range in size given in parentheses.

Molecular analysis
For molecular markers, ITS1 and 18S, the sequences of L. falcus s.l. and L. theroni s.l. obtained here were aligned and compared with the available sequences in GenBank for L. ignoratus and L. ergensi, respectively (accession numbers in table 1). There was no variation in the length of the 18S sequences (525 bp). The 18S sequences for isolates of L. falcus s.l. were identical with the sequences of L. ignoratus retrieved from GenBank. The 18S sequences for the isolates of L. theroni s.l. were identical and exhibited 0.2% divergence from the sequence for L. ergensi retrieved from GenBank. In case of the ITS1 region, the sequences varied in length from 441 to 563 bp. ITS1 sequences obtained in the present study were also aligned and sequence divergences (% of p-distances and number of differences) computed are given in tables 6 and 7.
Table 6 The range of nucleotides (above the diagonal) and genetic distances calculated as percentages (below the diagonal) in ITS1 sequences of Lamellodiscus ignoratus and L. falcus s.l. analysed in the present study (L. ignoratus complex).
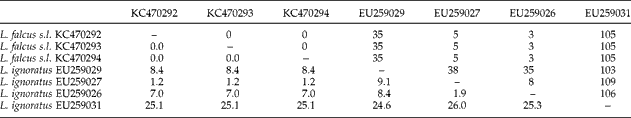
Table 7 The range of nucleotides (above the diagonal) and genetic distances calculated as percentages (below the diagonal) in ITS1 sequences of Lamellodiscus ergensi and L. theroni s.l. analysed in the present study (L. ergensi complex).

The ITS1 isolates of L. falcus s.l. were identical, but exhibited 1.2–26.0% divergence with the four sequences for L. ignoratus retrieved from GenBank (table 6). The divergences between the ITS1 sequences for isolates of L. theroni s.l. ranged between 0 and 0.2% whereas dissimilarities between them and the sequence for L. ergensi from GenBank ranged between 0.2 and 14.0% (table 7). The mean inter-individual uncorrected genetic distances for ITS1 sequences ( ± SD and range in parentheses) of ‘L. ignoratus complex’ (i.e. the new sequences for L. falcus s.l. obtained in the present study together with the sequence for L. ignoratus from GenBank) was 10.5 ± 10.0 (0.0–26), and of ‘L. ergensi complex’ (i.e. the new sequences for L. theroni s.l. obtained here together with the sequence for L. ergensi from GenBank) was 6.6 ± 6.9 (0.0–14.2). The mean intraspecific distances for each species separately were: L. falcus s.l., 0; L. theroni s.l., 0.1 ± 0.1 (0.0–0.2); L. ignoratus, 15.4 ± 8.2 (9.1–26.0); L. ergensi, 16.1 ± 5.7 (0.0–14.0).
Three specimens from Santa Pola and one from Mar Menor identified as Peracreadium sp. were sequenced (see GenBank accession numbers in table 1). The sequences obtained were aligned and compared with sequences of the partial 18S rDNA and complete ITS1–5.8S–ITS2 for P. characis from GenBank (AJ241896). The length of the 18S sequences was 120 bp in all sequences examined. The length of the complete ITS1–5.8S–ITS2 sequences was 1081 bp. The 18S sequences for isolates of Peracreadium sp. were identical with the GenBank sequence for P. characis. The ITS sequences for the isolates of Peracreadium sp. were identical, whereas the divergence between these and the sequence for P. characis in GenBank was 0.3% in all cases.
Discussion
We present here the first survey on the parasite fauna of the sharpsnout seabream in the wild, finding 19 different parasites species. The sharpsnout seabream harboured a diverse community of metazoan parasites, many of them previously reported in this species in the Mediterranean Sea (Radujkovic & Euzet, Reference Radujkovic and Euzet1989; Sasal et al., Reference Sasal, Niquil and Bartoli1999; Bartoli et al., Reference Bartoli, Gibson and Bray2005; Mladineo, Reference Mladineo2006; Gargouri Ben Abdallah & Maamouri, Reference Gargouri Ben Abdallah and Maamouri2008). The current study also provides new parasite records in the wild fish populations. The sharpsnout seabream is a new host record for seven species, including the polyopisthocotylean monogenean Microcotylidae gen. sp., the digeneans Magnibursatus bartolii Kostadinova, Power, Fernández, Balbuena, Raga et Gibson, 2003, Steringotrema pagelli, Cardiocephaloides longicollis and Galactosomum sp., the copepod Caligus ligusticus and the isopod Gnathia vorax. In addition, two parasite species, the polyopisthocotyleans, Atrispinum seminalis and Sparicotyle chrysophrii, which have already been reported in farmed fish, were found for the first time in wild populations of sharpsnout seabream. Most of these species were reported previously in other sparid fish which cohabit with sharpsnout seabream. Among these new records, ten parasite species were common in this fish, as they were noticeably frequent (prevalence ≥ 40%, in at least one locality, see table 2). The remaining parasite species were infrequent (prevalence ≤ 20%) and, therefore, they could be more specific to other hosts in the area (mainly in other sparids, see Álvarez-Pellitero et al., Reference Álvarez-Pellitero, Sitja-Bobadilla, Franco-Sierra and Palenzuela1995; Golomazou et al., Reference Golomazou, Athanassopoulou, Vagianou, Sabatakou, Tsantilas, Rigos and Kokkokiris2006).
Most parasites were monoxenous (11 species, 8 monogeneans and 3 crustaceans). The heteroxenous species were mainly digeneans (7 species), together with the only species of myxozoan. Interestingly, no nematodes have been recorded in the current study or in previous studies of sharpsnout seabream (Gibson et al., Reference Gibson, Bray and Harris2005). This fact is surprising, especially considering that the species of genera Hysterothylacium Ward et Magath, 1917 and Contracaecum Railliet et Henry, 1912 exhibit very low host specificity and have been reported frequently in many fish species in the Mediterranean (e.g. Petter & Maillard, Reference Petter and Maillard1987; Petter & Radujkovic, Reference Petter and Radujkovic1989; Fernández et al., Reference Fernández, Aznar, Montero and Raga2005; Pérez-del Olmo et al., Reference Pérez-del Olmo, Fernández, Gibson, Raga and Kostadinova2007).
Morphological and molecular identification
The identification of the species of Lamellodiscus is particularly complicated, as species taxonomy is often based on subtle morphological differences. Lamellodiscus falcus and L. theroni belong to two species groups (‘ignoratus’ and ‘ergensi’ group, respectively) composed of species with very similar morphological traits; hooks, lateral bars and copulatory organs of the species within each group are highly similar (Amine et al., Reference Amine, Euzet and Kechemir-Issad2007). In fact, L. falcus differs from L. ignoratus only in the smaller size of all sclerotized pieces and by slight differences in the morphology of the single piece of the male copulatory organ (referred as to ‘impair piece’ in Amine et al., Reference Amine, Euzet and Kechemir-Issad2006). The material of L. falcus s.l. examined in the present study showed mixed morphological features of both L. falcus and L. ignoratus (table 4). The shape of the sclerotized structures was clearly similar to that described for L. falcus by Amine et al. (Reference Amine, Euzet and Kechemir-Issad2006), especially in relation to the hooked shape of the single piece of the copulatory organ (see fig. 1). However, the range of total body lengths and the lengths of the medial and lateral haptor bars overlapped the ranges described for L. falcus and L. ignoratus (table 4). In contrast, the hooks in the Mediterranean material were larger than those described for L. falcus, and more similar to those of L. ignoratus (see Amine et al., Reference Amine, Euzet and Kechemir-Issad2006). A similar situation occurred in the case of the specimens of L. theroni s.l. compared with its original description (Amine et al., Reference Amine, Euzet and Kechemir-Issad2007). Lamellodiscus theroni differs from L. ergensi in the size of the body and the sclerotized structures, particularly those of the medial bar. In the case of the specimens of L. theroni s.l. found in the present study, the medial bar morphology was closer to that described for L. theroni and the total body length fell within the range described by Amine et al. (Reference Amine, Euzet and Kechemir-Issad2007). However, the dimensions of the sclerotized haptor structures were smaller than those of L. theroni, and more similar to those of L. ergensi (table 4). In summary, these results show a distinct similarity between the morphology of the species of Lamellodiscus found in the present study and the descriptions of L. falcus and L. theroni but, in contrast, their dimensions were mostly similar to those of L. ignoratus and L. ergensi, respectively.
Finally, although the comparative sequence analysis showed intraspecific differences in ITS1, this variation was within the range of the intraspecific variation provided by Kaci-Chaouch et al. (Reference Kaci-Chaouch, Verneau and Desdevises2008) for both L. ergensi and L. ignoratus. We agree with Poisot & Desdevises (Reference Poisot and Desdevises2010) and Poisot et al. (Reference Poisot, Verneau and Desdevises2011) who suggested that this degree of molecular divergence in specimens of Lamellodiscus does not reinforce the separation of species despite their morphometric variations (L. falcus versus L. ignoratus and L. theroni versus L. ergensi). Recent studies emphasized that generalist Lamellodiscus species (i.e. members of L. ergensi and L. ignoratus groups) show higher morphometric variability on their different hosts than specialist species (e.g. L. bidens and L. hilii) (Poisot & Desdevises, Reference Poisot and Desdevises2010). This intraspecific variability induced by the host, combined with the possibility of morphometric differentiations caused by geographical variation, could explain many morphological variations. Studies incorporating morphological, morphometrical and genetic characterization are thus essential to understand the Lamellodiscus species classification.
With reference to digenean identification, specimens of Peracreadium sp. collected from both localities apparently belong to P. characis (see Bartoli et al., Reference Bartoli, Gibson and Bray1989), since the general morphology mostly corresponded to this species but our individuals were somewhat smaller. The results obtained from the molecular analyses confirm that all isolates examined belonged to the same species, because the ITS sequences for isolates from Santa Pola and Mar Menor were identical. The low divergence (0.3%) between these sequences and the sequence for P. characis in GenBank fall well below the range for intraspecific sequence divergence given by Jousson et al. (Reference Jousson, Bartoli and Pawlowski1999) for the Opecoelidae, thus supporting the suggestion that specimens from the Mediterranean sharpsnout seabream also belong to P. characis. The present study therefore extends the range of the morphometric variation in this species.
Diversity of parasite fauna
The only myxozoan species found in the present study was Ceratomyxa sp. from the gall bladder. To date, two species of this genus have been reported in sharpsnout seabream: C. diplodae Lubat, Radujkovic, Marques et Bouix, 1989 and C. puntazzi Alama-Bermejo, Raga et Holzer, 2011 (Lubat et al., Reference Lubat, Radujkovic, Marques and Bonix1989; Alama-Bermejo et al., Reference Alama-Bermejo, Raga and Holzer2011). Both species parasitize the gall bladder and are very similar morphologically. Some myxozoan species are known to be highly damaging for aquaculture. In fact, one of the most pathogenic parasites for the culture of sharpsnout seabream has been the intestinal myxozoan, Enteromyxum leei Diamant, Lom et Dyková, Reference Diamant, Lom and Dyková1994 (Montero et al., Reference Montero, Cuadrado, Padrós, Crespo and Raga2007; Muñoz et al., Reference Muñoz, Cuesta, Athanassopoulou, Golomazou, Crespo, Padrós, Sità-Bobadilla, Albiñana, Esteban, Alvarez-Pellitero and Meseguer2007; Álvarez-Pellitero et al., Reference Álvarez-Pellitero, Palenzuela and Sitja-Bobadilla2008). Species of Ceratomyxa such as C. sparusaurati Sitjà-Bobadilla et Álvarez-Pellitero, 1995 in the gilthead seabream Sparus aurata L. (Palenzuela et al., Reference Palenzuela, Sitjá-Bobadilla and Álvarez-Pellitero1997) and C. diplodae in the sharpsnout seabream (Lubat et al., Reference Lubat, Radujkovic, Marques and Bonix1989; Katharios et al., Reference Katharios, Garaffo, Sarter, Athanassopoulou and Mylonas2007) have also been related to severe pathologies in Mediterranean sparids.
The most abundant group was the Monogenea, including eight species; all of them from the orobranchial cavity, usually on the gills (see table 2). Five of these species were monopisthocotyleans, four belonging to the genus Lamellodiscus. This genus is specific for the family Sparidae which can be parasitized by one or more species of Lamellodiscus (see Desdevises et al., Reference Desdevises, Morand, Jousson and Legendre2002). The fifth monopisthocotylean found in this study was the capsalid Encotyllabe vallei Monticelli, 1907, previously reported from the gills and pharynx of sharpsnout seabream and other sparids, including S. aurata (Radujkovic & Euzet, Reference Radujkovic and Euzet1989). The remaining three monogenean species were microcotylid polyopisthocotyleans. Of these, Atrispinum seminalis has been recorded on the gills of five different species of Diplodus (see Gibson et al., Reference Gibson, Bray and Harris2005), including the farmed sharpsnout seabream (Di Cave et al., Reference Di Cave, Berrilli, Cardia, De Liberato, Athanassopoulou and Orecchia2002; Athanassopoulou et al., Reference Athanassopoulou, Ragias, Vagianou, Di Cave, Rigos, Papathanasiou and Georgoulakis2005). Sparicotyle chrysophrii is often recorded in wild and farmed S. aurata (e.g. Euzet & Audouin, Reference Euzet and Audouin1959; Radujkovic & Euzet, Reference Radujkovic and Euzet1989; Antonelli et al., 2010a). Although this parasite has been found occasionally in farmed fish (Di Cave et al., Reference Di Cave, Berrilli, Cardia, De Liberato, Athanassopoulou and Orecchia2002), this study provides the first record in wild populations of sharpsnout seabream. The third microcotylid reported here, Microcotylidae gen. sp., has not been classified beyond family level since only one specimen was found, and its morphology does not correspond to any of the three genera previously reported in sharpsnout seabream, or to any other genus within the family (Mamaev & Parukhin, Reference Mamaev and Parukhin1976; Maillard & Noisy, Reference Maillard and Noisy1979; Mamaev, Reference Mamaev1986; Radujkovic & Raibaut, Reference Radujkovic and Raibaut1989). Among the microcotylid species reported to date in sharpsnout seabream, S. chrysophrii appears most similar due to the presence of genital armature with three types of spines and a single vagina, and the lack of sclerotized plate. However, the number of the peripheral spines and the number of the haptoral clamps of S. chrysophrii are distinctly higher (Antonelli et al., Reference Antonelli, Quilichini and Marchand2010a). More specimens of this morphotype should be examined in order to describe this possible new species.
Monopisthocotylean monogeneans represent a minor problem for the sparid cultures (Antonelli et al., Reference Antonelli, Quilichini and Marchand2010b; Sánchez-García et al., Reference Sánchez-García, Padrós, Raga and Montero2011). There are exceptional cases where some Lamellodiscus species have been related to severe lesions and problems in some cultured sparids (Roubal, Reference Roubal1994), including the sharpsnout seabream (Katharios et al., Reference Katharios, Hayward, Papandroulakis and Divanach2006). However, Sánchez-García et al. (Reference Sánchez-García, Padrós, Raga and Montero2011) indicated that although the attachment mechanism of Lamellodiscus spp. seems quite traumatic (mostly due to the hooks piercing the gill epithelium) the damage provoked by these tiny parasites is mild and localized. In fact, despite the fact that some wild or captive sharpsnout seabream harboured more than 500 individuals of Lamellodiscus spp., fish were apparently healthy. In contrast, microcotylids, and S. chrysophrii in particular, represent a major parasitological problem in sparid cultures. This species is a well-known parasite of S. aurata, very often related to lethal epizootics in Mediterranean cultures (Faisal & Imam, Reference Faisal, Imam, Perkins and Cheng1990; Sanz, Reference Sanz1992; Sitjà-Bobadilla & Álvarez-Pellitero, Reference Sitjà-Bobadilla and Álvarez-Pellitero2009). Sparicotyle chrysophrii has also been reported to cause severe mortalities in sharpsnout seabream (Di Cave et al., Reference Di Cave, Berrilli, Cardia, De Liberato, Athanassopoulou and Orecchia2002). The finding of S. chrysophrii in wild sharpsnout seabream not only extends our knowledge on the occurrence of this emerging parasite, but also confirms that infected wild sharpsnout seabream can act as reservoirs of this monogenean for cultured gilthead seabream (Athanassopoulou et al., Reference Athanassopoulou, Ragias, Vagianou, Di Cave, Rigos, Papathanasiou and Georgoulakis2005). No data exist on epizootics related to A. seminalis, but this parasite also seems potentially pathogenic, as its attachment and feeding strategies are similar to those of S. chrysophrii. Moreover, being monoxenous, it could also reach high loads in cultures.
With reference to digeneans, the derogenid Magnibursatus bartolii was found on gills or the oesophagus. As gills are not a usual infection site for digeneans, these parasites could have been regurgitated by fish; however, other hemiuroids, such as Aponurus spp. are frequently found on gills (Carreras-Aubets et al., Reference Carreras-Aubets, Repullés-Albelda, Kostadinova and Carrassón2011). Magnibursatus bartolii has not been reported previously in sharpsnout seabream, but this parasite has been found in other sparids, such as Boops boops L. (the type-host) from the Spanish Mediterranean (Pérez-del-Olmo et al., Reference Pérez-del Olmo, Fernández, Gibson, Raga and Kostadinova2007) or S. aurata from Tunisia (Gargouri Ben Abdallah & Maamouri, Reference Gargouri Ben Abdallah and Maamouri2008). More recently, a new species of Magnibursatus, M. diplodii Bayoumy & Abu-Taweel, Reference Bayoumy and Abu-Taweel2012 was described in Diplodus sargus L. (Bayoumy & Abu-Taweel, Reference Bayoumy and Abu-Taweel2012). The intestinal fellodistomids Steringotrema pagelli and Proctoeces maculatuswere only found in fish from Mar Menor. This is the first record of S. pagelli in the sharpsnout seabream. Proctoeces maculatus has been cited previously in a survey of this fish off the Tunisian coast (Gargouri Ben Abdallah & Maamouri, Reference Gargouri Ben Abdallah and Maamouri2008). This parasite has been recorded previously in other sparids (and interestingly also in one gobiid and pleuronectiforms, see Gibson et al., Reference Gibson, Bray and Harris2005). The monorchid Monorchis monorchis and the opecoelid Peracreadium characis were previously reported in sharpsnout seabream, although only P. characis is strictly specific to this host (Bartoli et al., Reference Bartoli, Gibson and Bray1989, Reference Bartoli, Gibson and Bray2005).
Metacercariae of Cardiocephaloides longicollis and Galactosomum sp. are reported for the first time from the brain of sharpsnout seabream. Although this is the first record of both parasites in sharpsnout seabream, it is not surprising since both exhibit low specificity towards second intermediate hosts (Naidenova & Mordvinova, Reference Naidenova and Mordvinova1997; Gibson et al., Reference Gibson, Bray and Harris2005; Osset et al., Reference Osset, Fernández, Raga and Kostadinova2005, Culurgioni et al., 2007). In heavy infections, parasites of the brain (including that of C. longicollis) have significant pathological effects as they can influence host behaviour in favour of parasite transmission to the final host (Chappel et al., Reference Chappel, Hardie, Secombes, Pike and Lewis1994; Lafferty, Reference Lafferty2008; Fredensborg & Longoria, Reference Fredensborg and Longoria2012). These species must be monitored, as they infect a vital organ, and could display synergic effects in heavy mixed infections.
The low number of crustacean parasites may be related to the loss of the individuals attached to skin during fish collection and processing (including handling or storage at − 20°C). Although this is the first report of Caligus ligusticus in sharpsnout seabream, this species has been reported previously in sparid fish, including other Diplodus species, such as D. sargus (see Raibaut et al., Reference Raibaut, Combes and Benoit1998 and references therein). Other caligids are known to parasitize sparid cultures (Ragias et al., Reference Ragias, Tontis and Athanassopoulou2004; Mladineo, Reference Mladineo2006) and they should be considered as potential pathogens for fish cultures as they have often been associated with important economic losses in farmed fish (Dawson, Reference Dawson1998; Lester & Hayward, Reference Lester, Hayward and Woo2006; Costello, Reference Costello2009). Lernanthropus vorax has been often cited in wild sharpsnout seabream (Radujkovic & Raibaut, Reference Radujkovic and Raibaut1989; Raibaut et al., Reference Raibaut, Combes and Benoit1998), and no pathological effects were reported. Nevertheless the presence of L. vorax should not be undervalued since other species of the same genus, such as L. kroyeri Van Beneden, 1851, have been frequently related to outbreaks and mortalities in sea bass culture (Yardimci & Pekmezci, Reference Yardimci and Pekmezci2012). The isopod Gnathia vorax was found for the first time on sharpsnout seabream. However, its presence is not surprising since gnathiid praniza larvae are non-specific, being found previously in other Mediterranean sparids (González et al., Reference González, Sánchez, Chirivella, Carbonell, Riera and Grau2004). The species of this family are considered as potential pathogens in culture conditions, due the anaemia provoked by haematophagus feeding (Marino et al., Reference Marino, Giannetto, Paradiso, Bottari, De Vico and Macrì2004).
Parasite fauna and fish captivity
The current study provides useful information about the sharpsnout seabream parasites that can be transferred from the wild and survive in culture conditions. We observed that most parasites living in the external environment (i.e. ectoparasites and parasites of the digestive tract) disappeared under conditions of captivity, with the exception of Lamellodiscus spp. It is well known that most external parasites are highly susceptible to artificial culture conditions (Woo, Reference Woo2006). Parasites on the skin and gills are affected by water quality and fish health, and parasites in the digestive tract are also affected by the food supplied in cultures. In contrast, parasites living in the blood and tissues can survive in such internal environments. The prevalence and abundance of metacercariae of C. longicollis did not vary significantly in captivity. Moreover, this was the only digenean found in fish captive for 20 days, probably because the metacercariae were protected within the brain tissues and are normally resistant encysted stages with little metabolic activity (Paperna & Dzikowski, Reference Paperna, Dzikowski and Woo2006). A similar situation was observed for the myxozoan Ceratomyxa sp. protected within the gall bladder.
The most problematic parasites are usually the monoxenous species such as Lamellodiscus spp., which survive in cultures and are able to re-infect fish, reaching abnormally high parasite burdens. However, the importance of heteroxenous parasites should not be underestimated, because in the surroundings of the sea cages or in the nets a substantial number of crustaceans and polychaetes that could act as intermediate/alternate hosts may be present.
The information provided in this study allows us to conclude which parasites could be a risk to sharpsnout seabream culture. There are three main groups (myxozoans, monogenean microcotylids and crustaceans) that should be taken into consideration for the culture of this fish. The myxozoan Enteromyxum leei was not found in the present study, although it has been reported in numerous cases, both in the wild and in culture (Le Breton & Marques, Reference Le Breton and Marques1995; Merella et al., Reference Merella, Cherchi, Salati and Garippa2005; Álvarez-Pellitero et al., Reference Álvarez-Pellitero, Palenzuela and Sitja-Bobadilla2008), even in cultures in the same location (San Pedro del Pinatar) in Mar Menor (Montero et al., Reference Montero, Cuadrado, Padrós, Crespo and Raga2007). The microcotylid S. chrysophrii is an important finding. Its presence could cause respiratory dysfunction due to the epithelial damage and anaemia (Sitjà-Bobadilla & Álvarez-Pellitero, Reference Sitjà-Bobadilla and Álvarez-Pellitero2009). More crucial is the confirmation that wild sharpsnout seabream harbour one of the most damaging parasites in cultured S. aurata and therefore can act as reservoirs. The same effect could be expected in infections with other microcotylids such as A. seminalis. In fact, Merella et al. (Reference Merella, Cherchi, Salati and Garippa2005) reported an epizootic induced by the high prevalence and intensity of the microcotylid Atrispinum salpae (Parona & Perugia, 1890) on cultured sharpsnout seabream. The third important group to take into consideration are the crustaceans. Although their numbers were quite low and they disappeared after 20 days of captivity, species of families such as caligids can provoke severe pathological problems. In fact, the main problem for salmon mariculture is the parasitosis caused by the caligid Lepeophteirus salmonis (see Johnson & Fast, Reference Johnson and Fast2004). Moreover, in Mediterranean cultures crustaceans have been reported to provoke severe damage of different fish species (Costello, Reference Costello2009; Yardimci & Pekmezci, Reference Yardimci and Pekmezci2012).
In view of the significant economic value of this fish, a detailed risk assessment study of sharpsnout seabream would be necessary to relaunch the culture of this species, minimizing possible future parasite problems. It should also be noted that most parasites of sharpsnout seabream recorded in present study are shared by the main fish species in Mediterranean aquaculture, such as the gilthead seabream. The proximity of cages containing this fish species could clearly result in cross-infections.
Acknowledgements
We are indebted to the SCSIE of the University of Valencia. We are also grateful to Benjamín García and Jesús Cerezo from the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario of the Region of Murcia. Thanks are also given to F. Padrós, from the Autonomous University of Barcelona, and to D. Gómez, MSc in Creative and Humanistic Translation, for their comments.
Financial support
N.S.-G. benefited from a ‘V Segles’ PhD student grant from the University of Valencia. This study was supported by projects PROMETEO 2011-040 and ISIC/2012/003 of the Valencian Government; AGL2005-01 221 and AGL 2012-20 892 of the Spanish Ministry of Science and Innovation.
Conflict of interest
None.