Right-to-left shunt caused by patent foramen ovale is an intriguing field for interventionalists in particular following results of the most recent trials 1 – 2 about patent foramen ovale transcatheter closure. To precisely detail the anatomy of the interatrial septum may be important in order to dictate indications to closure and device selection strategy, as demonstrated by the increasing use of trans-oesophageal echocardiogram, intracardiac echocardiogram, and magnetic resonance imaging in the process of assessing the anatomy of the patent foramen ovale.Reference Schwinger, Gindea and Freedberg 3 – Reference Weber and Higgins 6 However, detailed anatomic variants of the interatrial septum in patients with right-to-left shunt have not been classified yet, at least for that which concerns intracardiac echocardiogram. Relationships of different anatomical subtypes and association of anatomical characteristics of the interatrial septum with risk of recurrent paradoxical embolism and shunt grade have been studied only partially for single characteristics.
We present a comprehensive classification of anatomical variants and associations of anatomical characteristics and their relationships with risk of recurrent paradoxical embolism.
Methods
We retrospectively reviewed the medical and instrumental data of 520 consecutive patients (mean age 44±15. 5 years, 355 women) who had been referred to our centre over a 10-year period (February, 2003 to February, 2013) for right-to-left shunt catheter-based closure. In line with our institutional protocol, all patients were screened with trans-oesophageal echocardiogram before the intervention. Inclusion criteria for percutaneous closure of patent foramen ovale included the following: 7 (1) a concurrent permanent shower or curtain shunt pattern on transcranial Doppler with Valsalva manoeuvre; (2) positive – single or multiple ischaemic foci – cerebral magnetic resonance imaging, (3) previous neurologically confirmed stroke or transient ischaemic attack; and (4) medium or large patent foramen ovale on trans-oesophageal echocardiogram. TTE and transcranial Doppler were used to evaluate all patients with patent foramen ovale, respectively, before trans-oesophageal echocardiogram. The Hospital Ethics Board approved the study. Written informed consent was obtained from all patients enrolled in the study (Table 1).
Table 1 Demographic and clinical data of the 520 patients.

* On trans-oesophageal echocardiography
In all patients, the attempt of transcatheter closure was preceded by the mechanical 9 Fr 9 MHz 360° scan probe (UltraICE; EP Technologies, Boston Scientific Corporation, San Jose, California, United States of America). The choice of this particular system was driven by economic issues and the need for a simple standardised protocol of study,Reference Rigatelli and Hijazi 8 which was not available for the electronic probe at the start of the study in 2003. The closure procedure was performed under the intracardiac echocardiogram guidance.
Echocardiography protocols
Trans-oesophageal echocardiography
Trans-oesophageal echocardiography was conducted using a GE Vivid 7 (General Electric Corp., Norfolk, Virgin Islands, United States of America) with contrast injection and Valsalva manoeuvre under local anaesthesia. Right-to-left shunts were defined as permanent, small, medium, and large following Homma et al,Reference Homma, Sacco and Di Tullio 9 whereas atrial septal aneurysm and prominent Eustachian valve and Chiari network were defined following standard nomenclature.Reference Lang, Bierig and Devereux 10 Patent foramen ovale diameter was calculated by measuring with an electronic caliper the maximum opening of the patent foramen ovale in the end-diastolic frames (Table 2).
Table 2 Main intracardiac echocardiography findings of the study group.
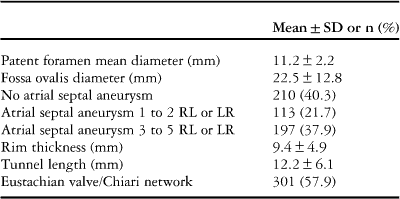
RL=right-to-left shunt; LR=left-to-right shunt
Transcranial Doppler
Transcranial Doppler was performed using an intravenous bubble study by a neurologist experienced in this examination, according to current standards,Reference Messe, Silverman and Kizer 11 using a Transcranial Doppler monitoring device (DWL MultidopX; ScanMed Medical, Moreton-in-Marsh, United Kingdom). Both middle cerebral arteries were simultaneously monitored through the temporal window by the use of 2-MHz probes. The contrast was obtained by mixing 100 cc of saline solution with 2–3 cc of Emagel and loading a 10 cc syringe with this mixture. The solution, agitated between two 10-ml syringes, and connected by a three-way stopcock, was immediately injected with a 20-G/32-mm catheter placed in the antecubital vein to obtain a bolus of air microbubbles. This procedure was performed three times during normal breathing and the same number of times during a Valsalva manoeuvre. The bolus of microbubbles was injected in 1–2 seconds when this 7-second period ended. We quantified the importance of right-to-left shunt by counting the number of signals in one mean cerebral artery within 7 seconds of the injection, as previously reported:Reference Messe, Silverman and Kizer 11 mild – <10 bubbles within three cardiac cycles, moderate – >10 bubbles within three cardiac cycles – with shower effect – many bubbles but still countable, and severe – >10 bubbles within three cardiac cycles – with curtain effect – many bubbles but not countable. A distinct pattern of shunt occurs when bubbles are identifiable before the Valsalva manoeuvre – basal or permanent shunt.
Patients who met the criteria for patent foramen ovale closure attempt were offered an intracardiac echocardiogram study using the mechanical 9 Fr 9 MHz UltraICE catheter (EP Technologies), as previously described.Reference Zanchetta, Onorato and Rigatelli 12
Intracardiac echocardiography study and measurements
Although innumerable series of planes can be displayed by intracardiac echocardiogram, only four basic transverse sections on the horizontal plane of the body and one longitudinal section in the long axis of the heart are commonly used for an exhaustive evaluation of the required anatomy from the inner confines of the right atrium and great veins during catheter-based interventional procedures.
For intracardiac echocardiogram, the UltraICE catheter was inserted via a 55° precurved 8.5 Fr long venous sheath (Convoy; EP Technologies, Boston Scientific Corporation) from the left femoral vein and advanced through the inferior vena cava into the right atrium, in order to obtain the aforementioned two orthogonal planes, and subsequently withdrawn through the body of the right atrium towards the inferior vena cava. The transducer orientation will be on the horizontal plane of the body and on the short axis of the right atrium. To appropriately display the images, they need to be electronically rotated in order to be positioned in attitudinally correct orientation, in agreement with anatomic or cardiac magnetic resonance formats. Few specific anatomic landmarks may be used to facilitate the uninitiated beginner to orientate the image in such a previously accepted manner. The first one is the central location and precise anatomy of the ascending aorta between the pulmonary trunk on its left and superior caval vein on its right. The second one is the crista terminalis, which appears as a bright and thick structure located at the junction between the posterior smooth wall and the anterolateral trabeculated portion of the right atrium. The third one is the right atrial auricle, which appears as a large Snoopy nose-like structure. The orientation of these landmarks on the screen were the ascending aorta at 2 o’clock, crista terminalis at 10 o’clock, and right atrial auricle at 12 o’clock. The left-sided structures were displayed on the viewer’s right and the right-sided structures on the viewer’s left, whereas the anterior-sided structures were at the top of the screen and the posterior-sided structures at the bottom of the screen. With the probe in this neutral position and the transducer positioned between the 6th and 7th intervertebral disk of the thoracic spine, the aortic valve plane view was obtained at the level of the fossa ovalis (Fig 1a). The four-chamber view was obtained with a 55° precurved introducer sheath advanced up to the end of the catheter and turned posterior and leftward, with the transducer orientation perpendicular relative to the long axis of the heart in order to longitudinally scan the atrial septum (Fig 1b). The intracardiac echocardiogram study was conducted in these two projections measuring diameters of the oval fossa, and the entire atrial septum length, rims, and thickness with electronic calipers edge-to-edge.

Figure 1 Aortic valve plane ( a ) and four-chamber plane ( b ) as seen on intracardiac echocardiogram studies. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. AO=ascending aorta; FO=oval fossa; LA=left atrium; MV=mitral valve; RA=right atrium; RUPV=right upper pulmonary vein; SVC=superior vena cava; TV=tricuspid valve.
The four main features used to analyse were: (a) diameter of the oval fossa, (b) presence and length of the channel, (c) presence and degree of atrial septal aneurysm, and (d) rim thickness.
We also analysed the presence of Eustachian valve/Chiari network. We considered eventual additional fenestrations within the fossa as a functional subtype of patent foramen ovale, despite the fact that anatomically it should be considered as secundum atrial septal defect. Different anatomic subtypes were compared for demographic, clinical, and functional characteristics.
Definitions
Small diameter of fossa ovalis was defined as fossa ovalis <20 mm in the four-chamber, normal fossa ovalis diameter was defined as a diameter between 20 and 25 mm, whereas >20 mm was considered large fossa ovalis. The presence and severity of atrial septal aneurysm were classified in accordance with Olivares et al.Reference Olivares-Reyes, Chan and Lazar 13 Hypertrophic rims were defined as having a thickness ≥8 mm, whereas lipomatosis was defined as thickness of ≥15 mm, on intracardiac echocardiogram study. Long tunnel-type patent foramen ovale was defined as length ≥10 mm by intracardiac echocardiogram. Prominent Eustachian valve was defined as a valve with thickness >1 mm that protrudes for at least 10 mm within the right atrium from the border of the inferior vena cava. A large Chiari network was defined as Eustachian valve with thickness <1 mm and filamentous appearance.Reference Lang, Bierig and Devereux 10 All the intracardiac echocardiogram studies were reviewed and analysed by two independent observers with extensive experience in intracardiac echocardiogram with an inter-observer variability of 99.8% (Table 3)
Table 3 Anatomical subgroup distribution as number and percentage and their morphological characteristics.

ASA=atrial septal aneurysm
* ASA degree following Olivares et al
.
Statistical methods
χ2 and the Student t-tests were used to compare frequencies and continuous variables between the groups. Stepwise logistic regression analysis was used to determine independent determinants of recurrent paradoxical embolism before closure. The analysed variables were age >45, sex, anatomical subtypes, presence of Eustachian valve/Chiari network, association between anatomical subtypes and Eustachian valve/Chiari network, and shunt grade. Correlation coefficient was used to establish a correlation between anatomical variables. Statistical analysis was performed using a statistical software package (SAS for Windows, version 8.2; SAS Institute; Cary, North Carolina, United States of America). A probability value of <0.05 was considered to be statistically significant.
Results
Following the intracardiac echocardiogram study, small diameter of fossa ovalis was defined as fossa ovalis ≤20 mm – 31% of patients, normal fossa ovalis diameter as a diameter between 21 and 25 mm – 42% of patients, whereas >25 mm was considered large fossa ovalis – 27% of patients. Thus, the combinations of interatrial septum anatomical features were classified into six main anatomical subgroups (Figs 2–7).

Figure 2 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with patent foramen ovale using a mechanical 360° scan probe: Type 1. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. FO=oval fossa; LA=left atrium; RUPV=right upper pulmonary vein.

Figure 3 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with PFO using a mechanical 360° scan probe: Type 2. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. LA=left atrium; PFO=patent foramen ovale; RA=right atrium; TV=tricuspid valve.

Figure 4 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with patent foramen ovale using a mechanical 360° scan probe: Type 3. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. ASA=atrial septal aneurysm; LA=left atrium; MV=mitral valve; RA=right atrium; TV=tricuspid valve.

Figure 5 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with patent foramen ovale using a mechanical 360° scan probe: Type 4. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. ASA=atrial septal aneurysm; LA=left atrium; RA=right atrium.

Figure 6 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with patent foramen ovale using a mechanical 360° scan probe: Type 5. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. ASA=atrial septal aneurysm; LA=left atrium; MV=mitral valve; RA=right atrium; TV=tricuspid valve.

Figure 7 Intracardiac echocardiography classification of subtypes of interatrial septum anatomy associated with patent foramen ovale using a mechanical 360° scan probe: Type 6. The left-sided structures will be displayed on the viewer’s right and the right-sided structures on the viewer’s left, with the anterior-sided structures at the top of the screen and the posterior-sided structures at the bottom of the screen. ASA=atrial septal aneurysm; FO=oval fossa; GW=guidewire 1 and 2 through two different fenestrations; LA=left atrium; RA=right atrium; TV=tricuspid valve.
Fossa ovalis diameter ≤20 mm was found to be statistically correlated to the presence of tunnelised patent foramen ovale (r=0.91, p<0.001), whereas fossa ovalis diameter>25 mm was associated with the presence of atrial septal aneurysm (r=0.88, p<0.001) and a linear correlation between diameter of the fossa ovalis and atrial septal aneurysm severity. The larger the fossa, the more severe was the atrial septal aneurysm (r=0.90, p<0.001).
The six groups did not differ significantly in demographic data (Table 4) except in the case of hypertrophic rims, which were more frequent in hypertensive and elderly patients (>50 years old). On the contrary, a statistically significant difference was noticed with regard to the frequency of recurrent paradoxical embolism and multiple ischaemic foci on brain magnetic resonance imaging and frequency of high-grade right-to-left and permanent shunt before transcatheter closure procedure between type 2, 4, 6, and the others (Table 4). Type 4 anatomical subtype (OR 4.1, 1.5–8 [95% CI], p<0.001) and type 2+Eustachian valve (OR 4.3, 1.6–9 [95% CI], p<0.001) were the strongest predictors of recurrent ischaemic events before transcatheter closure.
Table 4 Distribution of demographic, clinical and functional parameters among the six anatomical subtypes (number or %).

Coagulation abnormalities: deficiency of anti-thrombin III, C, Sor factor V Leiden or homozygotic mutation MTHFR or antiphospholipid or anticardiolipin antibodies
Discussion
Our study suggested that the anatomy of interatrial septum associated with right-to-left shunt was more complex than commonly thought. Moreover, some specific patterns seemed to be more frequently associated with permanent shunt and higher risk of paradoxical embolism than others. As we underlined in this study, the anatomy of the interatrial septum showed in at least 40–55% of patients a variety of different combination of anatomical features.
The combination of the varieties of such anatomical components identifying six main anatomic subtypes enhanced the clinical significance of each specific anatomical structure and might help in better clarifying the pathophysiology of paradoxical embolism, which was unlikely to be dominated by one single factor, such as atrial septal aneurysm, patent foramen ovale tunnel, or fenestrations themselves. Intriguingly, our data suggested that the fossa ovalis diameter played a role in determining the presence of both tunnelised patent foramen ovale, when the oval fossa was small (<20 mm), and the presence of aneurysm, when the fossa was large (≥20 mm). Moreover, diameters of the fossa ovalis were directly correlated with an increase in atrial septal aneurysm severity. Type 2, type 4, and type 6 anatomical patterns were associated with increased risk of ischaemic recurrences and shunt grade, and a relationship between fossa ovalis diameter, tunnelised patent foramen ovale, and atrial septal aneurysm appeared clinically significant.
Among the findings of our report, atrial septal aneurysm was confirmed to be a strong determinant in the pathophysiology of paradoxical embolism. Atrial septal aneurysm had a prevalence in the general population of between 0.22% and 4%,Reference Marelli, Mackie, Ionescu-Ittu, Rahme and Pilote 14 , Reference Rigatelli 15 but it increased to 8–15% in patients with stroke.Reference Agmon, Khandheria and Meissner 16 , Reference Pearson, Nagelhout and Castello 17 Massa and HommaReference Homma, Sacco and Di Tullio 9 , Reference Mas, Arquizan and Lamy 18 were the first to suggest that concurrence of atrial septal aneurysm and patent foramen ovale identified a high-risk cohort of patients. More recently, some studies suggested that patent foramen ovale and atrial septal aneurysm patients had multiple lesions on magnetic resonance imaging more frequently than patent foramen ovale patients. Santamarina et alReference Santamarina, Gonzalez-Alujas and Munoz 19 observed that an embolic pattern was more frequent in patent foramen/atrial septal aneurysm patients (44%) as compared with patent foramen ovale patients (26.2%).
The coexistence of patent foramen ovale and atrial septal aneurysm was strongly associated with cerebral ischaemic events through a supposed paradoxical embolism mechanism, but another new hypothesis was under investigation as the one proposed by our study group, consistent with an “atrial fibrillation like” mechanism based on left atrial dysfunction.Reference Rigatelli, Aggio and Cardaioli 20
In the current study, we found that a tunnelised patent foramen could be associated with increased risk of recurrences and higher shunt grade in particular when associated with presence of Eustachian valve/Chiari network. Not infrequently, patent foramen ovale appears as a tunnel-like opening between the right and left atrium, and this feature could be associated with a variety of other anatomical variants, such as hypertrophy of the rims, or different degrees of atrial septal aneurysm. Recently, this kind of anatomy has been correlated with an increased risk of paradoxical embolismReference Goel, Tuzcu and Shishehbor 21 for its potential role in causing thrombosis in situ within the channel, in particular if long >10–12 mm. From a technical point of view, trans-oesophageal echocardiogram and in particular intracardiac echocardiogram could be useful in determining the length of the tunnel and in selecting the proper device, which should possibly have the capability of an asymmetrical opening, which could adapt to this anatomy.
Hypertrophy of the rims of the fossa ovalis has been taken into account in our report, because of the association with classical risk factors for stroke, such as hypertension, and also because of the challenge that hypertrophic rims posed when selecting the proper occluder device. Interatrial septum thickness in the general population is about 6 mm and usually it increases to about 7 mm in the aged population.Reference Agmon, Meissner and Tajik 22 Interatrial septum hypertrophy and lipomatosis have been defined when thickness is >8 and >15 mm, respectively. Interatrial septum hypertrophy is common in elderly people and is related with hypertension and smoking but not with vascular disease.Reference Christoph, Thomas and Stefan 23 Lipomatosis of the interatrial septum is a benign tumoral process characterised by fat accumulation in the interatrial septum.Reference Marshall and Lock 24 Both conditions may have a deep impact on transcatheter closure because in cases of both patent foramen ovale and atrial septal aneurysm a stiff device such as those of the Amplatzer family should be contraindicated, owing to the inability of such device to stretch the waist zone more than 7–8 mm.Reference Rigatelli, Dell’Avvocata and Ronco 25
Eustachian valve/Chiari network have been excluded as anatomical landmarks in our classification. We believe that these anatomic structures were not essential features of the interatrial septum, but we found that their presence might increase the risk of recurrences or the severity of the shunt grade in each of the six different anatomic subtypes. From an embryological point of view, the Eustachian valve is a derivative of the right sinus venosus valve, has a semicircular shape, and is facing the anterior–inferior aspect of the inferior vena cava. Chiari network represents a very huge multi-perforated Eustachian valve with a network-like appearance and it has been found in 1.3–4% of autopsies. The Eustachian valve and Chiari network guarding the anterior–inferior aspect of the inferior vena cava have a crucial role in deflecting the blood flow through the foramen ovale during the foetal life predisposing to paradoxical embolism.Reference Schuchlenz, Saurer and Weihs 26 Large patent foramen ovale and prominent Eustachian valve or right atrial filamentous strands were found more frequently in patients with septal aneurysm as compared with those without septal aneurysm (37.7% versus 10.9%, p<0.001 and 59.4% versus 43.1%, p=0.02).Reference Schneider, Hofmann and Justen 27 In previous reports of trans-oesophageal echocardiogram studies, an Eustachian valve is present in 48% of patients with cryptogenic stroke and a large Chiari network is associated with patent foramen in 83% of cases. Our report confirmed recent studiesReference Schuchlenz, Saurer, Weihs and Rehak 28 in which both entities were predictors of large shunt and recurrent paradoxical embolism.
Finally, our findings seemed to confirm previous studies,Reference Giardini, Donti and Formigari 29 , Reference Rigatelli, Dell’Avvocata and Cardaioli 30 suggesting that particular anatomic patterns were associated with an increased severity of the shunt grade and even with permanent right-to-left shunting, accounting also for an increased recurrences risk.
Limitations
Among the number of limitations of this study, the strongest is probably the use of this particular type of intracardiac echocardiogram probe, not available in the United States of America or in northern Europe. The less high imaging resolution, the different code of imaging interpretation, and the lack of Doppler capability compared with the modern electronic equipment are the main drawbacks of mechanical probe technology. However, this kind of intracardiac echocardiogram is highly reproducible by different operators and allows for depicting the entire interatrial septum in two perfect orthogonal planes with minimal manipulation of the catheter and two only views. Second, the classification proposed in our study may be replicated with any type of electronical probe and can also be used in the trans-oesophageal echocardiogram setting, as we found in our extensive experience with other world centres. Finally, the arbitrary selection of the main anatomical characteristics to be considered in the classification should be taken in account; we selected the features that most impact in our experience on anatomy interpretation and device selection. The correlation between fossa ovalis diameters, tunnelised patent foramen ovale, and atrial septal aneurysm seem to favour this choice.
Conclusion
First of all, our study showed that in a “real world” interatrial septum anatomy greatly differs among patients with right-to-left shunt. Consequently, an anatomic-driven device selection strategy rather than a fixed single device strategy might offer the best choice; future trials should in our opinion take into account this evidence. Second, the reduction of the large anatomical complexity into a comprehensive classification, although it might be reductive, can simplify the comparison of different studies about right-to-left shunting management. Finally, the identification of different anatomical patterns and the relationships between specific anatomical variants may better clarify the still “dark” pathophysiology of paradoxical embolism.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.













