INTRODUCTION
Primary progressive aphasia (PPA) refers to neurodegenerative conditions characterized by the emergence of relatively isolated and gradually worsening language impairment (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa, Ogar, Rohrer, Black, Boeve, Manes, Dronkers, Vandenberghe, Rascovsky, Patterson, Miller, Knopman, Hodges, Mesulam and Grossman2011). Each clinical variant of PPA has a prototypical pattern of language deficits, asymmetric cerebral atrophy, and underlying pathology. The logopenic variant of PPA (lvPPA) is defined by impaired sentence repetition and lexical retrieval in conversational speech (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa, Ogar, Rohrer, Black, Boeve, Manes, Dronkers, Vandenberghe, Rascovsky, Patterson, Miller, Knopman, Hodges, Mesulam and Grossman2011). In contrast, the core features of semantic variant PPA (svPPA) are progressive decline in object naming and word comprehension with grammatically correct and fluent speech (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa, Ogar, Rohrer, Black, Boeve, Manes, Dronkers, Vandenberghe, Rascovsky, Patterson, Miller, Knopman, Hodges, Mesulam and Grossman2011; Mesulam et al., Reference Mesulam, Rogalski, Wieneke, Hurley, Geula, Bigio, Thompson and Weintraub2014). Individuals with the non-fluent variant of PPA (nfvPPA) show progressive distortion in speech production as manifested by effortful speech, dysarthria, and agrammatism (Ramanan et al., Reference Ramanan, Flanagan, Leyton, Villemagne, Rowe, Hodges and Hornberger2016). Left posterior temporoparietal atrophy is characteristic of lvPPA, left anterior temporal lobe atrophy is typical in svPPA, and left posterior inferior frontal gyrus atrophy is observed in nfvPPA (Brambati et al., Reference Brambati, Amici, Racine, Neuhaus, Miller, Ogar, Dronkers, Miller, Rosen and Gorno-Tempini2015; Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa, Ogar, Rohrer, Black, Boeve, Manes, Dronkers, Vandenberghe, Rascovsky, Patterson, Miller, Knopman, Hodges, Mesulam and Grossman2011). SvPPA and nfvPPA have primary pathological features of frontotemporal lobar degeneration, which include abnormal protein aggregates of the microtubule-associated protein tau and the transactive response DNA binding protein 43 (TDP-43). In contrast, the majority of lvPPA cases have Alzheimer’s disease (AD) pathology of neurofibrillary tangles and amyloid plaques (Santos-Santos et al., Reference Santos-Santos, Rabinovici, Iaccarino, Ayakta, Tammewar, Lobach, Henry, Hubbard, Mandelli, Spinelli, Miller, Pressman, O&Neil, Ghosh, Lazaris, Meyer, Watson, Yoon, Rosen, Grinberg, Seeley, Miller, Jagust and Gorno-Tempini2018). The disparate pathological features between lvPPA, svPPA, and nfvPPA underscore the need for measures that improve diagnostic accuracy, particularly when etiology-specific treatments become available.
The correspondence between clinical PPA diagnosis and neuropathological classification is not absolute. Current diagnostic criteria do not span all presentations of PPA with as many as one third of cases deemed unclassifiable (Sajjadi, Patterson, Arnold, Watson, & Nestor, Reference Sajjadi, Patterson, Arnold, Watson and Nestor2012; Wicklund et al., Reference Wicklund, Duffy, Strand, Machulda, Whitwell and Josephs2014). The identification of lvPPA is especially challenging because of its inconsistent clinical presentation (Louwersheimer et al., Reference Louwersheimer, Keulen, Steenwijk, Wattjes, Jiskoot, Vrenken, Teunissen, van Berckel, van der Flier, Scheltens, van Swieten and Pijnenburg2016) and the difficulty distinguishing it from nfvPPA (Sajjadi et al., Reference Sajjadi, Patterson, Arnold, Watson and Nestor2012). As noted by Grossman (Reference Grossman2018), difficulties with lexical retrieval can appear in all progressive aphasias and are thus less useful as a core criterion. Moreover, lvPPA and nfvPPA patients can present with slow speech, word-finding pauses, speech sound errors, and phonological paraphasias. This overlap in linguistic profiles has spurred interest in cognitive biomarkers that can reliably differentiate lvPPA from svPPA and nfvPPA. Recent work has demonstrated that lvPPA cases show more rapid cognitive decline (Leyton, Hsieh, Mioshi, & Hodges, Reference Leyton, Hsieh, Mioshi and Hodges2013) and display greater impairment on measures of memory (Mesulam et al., Reference Mesulam, Wicklund, Johnson, Rogalski, Léger, Rademaker, Weintraub and Bigio2008), visuoconstruction (Watson et al., Reference Watson, Possin, Allen, Hubbard, Meyer, Welch, Rabinovici, Rosen, Rankin, Miller, Santos-Santos, Kramer, Miller and Gorno-Tempini2018), and calculation skills (Rohrer et al., Reference Rohrer, Ridgway, Crutch, Hailstone, Goll, Clarkson, Mead, Beck, Mummery, Ourselin, Warrington, Rossor and Warren2010) when compared to svPPA and nfvPPA patients. Poor visuoconstruction and visuospatial memory have emerged as potential cognitive biomarkers of the underlying amyloid pathology prevalent in lvPPA cases and are believed to result from atrophy in lateral/posterior temporal and medial parietal regions (Brambati et al., Reference Brambati, Amici, Racine, Neuhaus, Miller, Ogar, Dronkers, Miller, Rosen and Gorno-Tempini2015).
In the current study, we used meta-analytic methods to establish the neuropsychological profile of lvPPA and to determine if additional measures distinguish the primary variants of PPA. Though a meta-analysis of memory functioning in PPA was recently conducted (Eikelboom et al., Reference Eikelboom, Janssen, Jiskoot, van den Berg, Roelofs and Kessels2018), no study has comprehensively characterized the neuropsychological profile of lvPPA and compared it to svPPA and nfvPPA subtypes. We examined nine domains of neuropsychological functioning in lvPPA and the influence of demographic, disease and task characteristics on the magnitude of these study effect sizes. We then compared results obtained in lvPPA patients to results from our recent meta-analysis of neuropsychological functioning in svPPA and nfvPPA (Kamath, Chaney, DeRight, & Onyike, Reference Kamath, Chaney, DeRight and Onyikein press).
METHOD
Literature Search
We conducted a computerized literature search in PubMed limited to English-language publications with human participants indexed from 1980 through March 24, 2018. Index terms are reported in Supplementary Material Appendix A. The search included 3764 publications that were reviewed for inclusion. Additional articles were identified from reference lists and review articles.
Study Selection
Two authors (VK, GC) performed abstract screening and/or full-text evaluation of search results. Studies that met the following criteria were included in the study:
The presence of an lvPPA cohort.
A comparison group without subjective cognitive or neurologic complaints. Studies using published normative data or a disease control group were excluded. Though single case studies were excluded, case series were included if control data were provided.
The presence of relevant and sufficient neuropsychological data to generate an effect size (e.g., means and standard deviations).
In cases of sample and outcome redundancy, the publication with the largest sample size and set of outcome variables was retained. Of note, baseline task data were used in studies that collected outcomes across multiple time points.
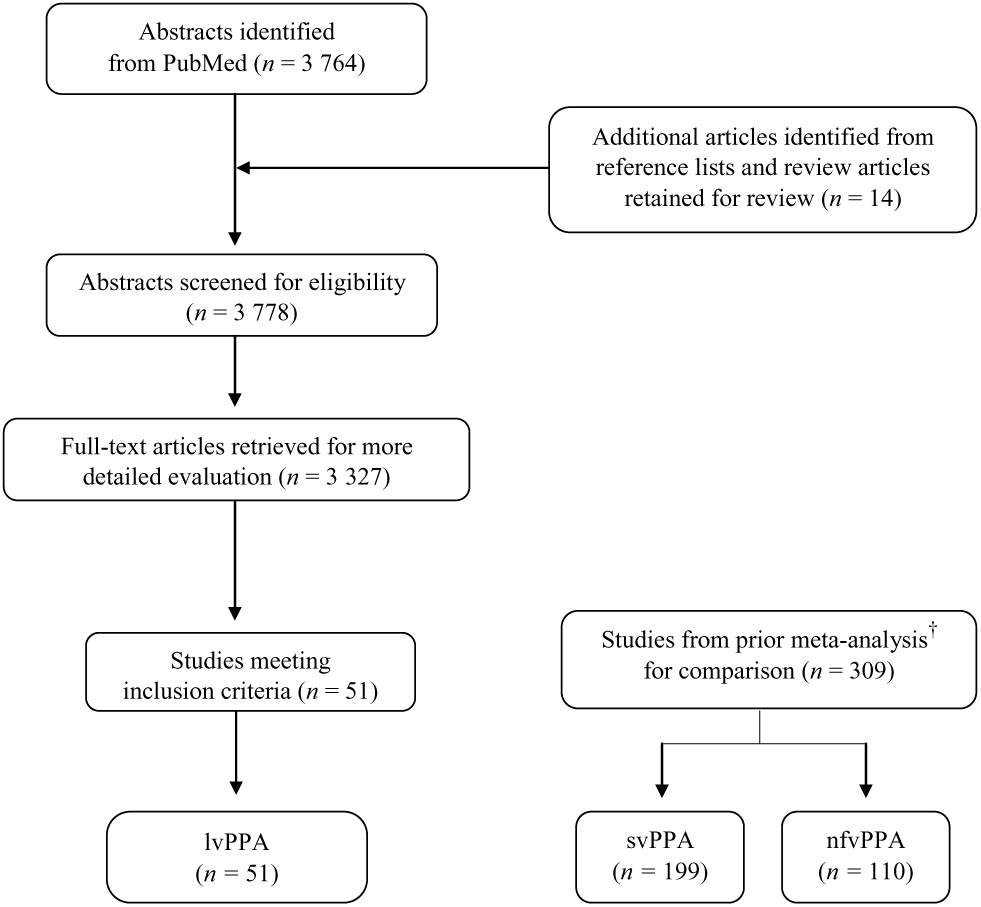
Following these criteria, 51 studies were retained for the current meta-analysis. A flowchart of the literature search and study selection is presented in Figure 1. See Supplementary Material Appendix B for a list of included studies.

Fig. 1. Flowchart of literature search and study selection. †Kamath et al. (Reference Kamath, Chaney, DeRight and Onyikein press).
Data Extraction
Demographic and clinical characteristics
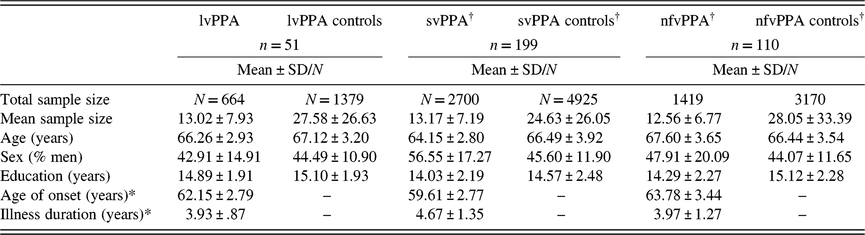
Data included in the current meta-analysis represented 664 lvPPA patients and 1379 controls. For each study, we entered available demographic and disease characteristics, including: (1) mean control age, (2) mean patient age, (3) percentage of men in patient group, (4) percentage of men in control group, (5) mean control years of education, (6) mean patient years of education, (7) mean age of illness onset, and (8) mean illness duration. Demographic and clinical information for lvPPA subgroups are presented in Table 1 alongside results obtained for svPPA and nfvPPA in a recent meta-analysis of these conditions (Kamath et al., Reference Kamath, Chaney, DeRight and Onyikein press).
Table 1. Study sample characteristics
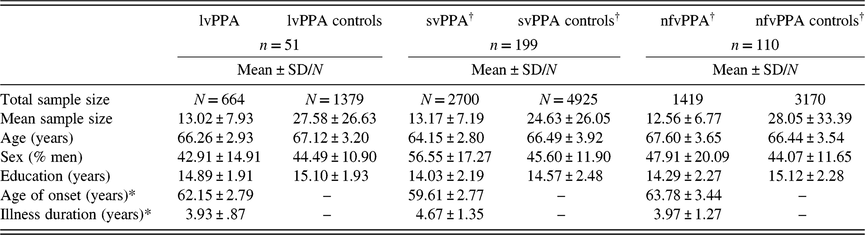
Note. lvPPA = logopenic variant primary progressive aphasia; svPPA = semantic variant of PPA; nfvPPA = non-fluent variant of PPA.
†Values for svPPA and nfvPPA are presented from a prior meta-analysis (Kamath et al., Reference Kamath, Chaney, DeRight and Onyikein press).
*n = 38.
Outcome measures
Outcome measures were extracted and imputed from 51 studies and were cross-checked and reviewed to ensure accuracy of the data entered. Using source articles and prior meta-analyses, each outcome measure was assigned to one of nine cognitive domains: global cognition, processing speed, attention, speech/language, ideational fluency, memory, visuospatial functioning, executive functioning, and social cognition. Attentional measures were further coded as simple attention or working memory. Speech/language measures were coded as speech output, repetition, naming, reading, comprehension, and semantic knowledge. Tests of ideational fluency were coded as category-guided or letter-guided verbal fluency. Memory measures were categorized as immediate recall, delayed recall, and recognition memory separately for auditory-verbal and visuospatial material. Visual set-shifting was the only subdomain within executive functioning with enough studies for further subtyping.
If multiple scores were provided from the same test stimuli but measured the same underlying cognitive construct, one representative outcome was chosen for the cognitive domain and subdomain. For example, if a study reported data for the learning, immediate recall, delayed recall, and recognition indices from the California Verbal Learning Test, we assigned the delayed recall score to the memory domain. The remaining scores were excluded from analyses involving the overall effect size and the memory domain but were assigned to their respective subdomains within memory and were included only in these analyses. If an outcome measure reported multiple scores judged to measure separable cognitive constructs, scores were pooled and assigned to the overall domain and subsequently assigned to their respective subdomain. For example, the Rey Complex Figures Test is a measure of both visuoconstruction and visuospatial memory. Therefore, the copy trial was included in the overall effect size, the visuospatial domain, and visuoconstruction subdomain, and the delayed recall trial was assigned to the overall effect size, the memory domain, and the delayed visuospatial memory subdomain. All remaining indices (e.g., immediate recall, recognition memory) were solely assigned to their respective memory subdomains. Mean outcomes were calculated so that each patient group contributed to only one effect size per analysis. If task data were divided by arbitrary subgroups in a study (e.g., with and without parkinsonism), the selected outcomes were pooled to avoid weighting a single study by the number of outcomes reported.
Statistical Analysis
Comprehensive meta-analysis, version 3.0. (CM3) was used for all analyses. A random-effects model was employed as this model accounts for within- and between-study variation in the effect size estimates and assumes greater variability between studies than sampling error alone. To control for differences in sample size during effect size computation, studies were weighted according to their inverse variance estimates. Effect sizes (Hedges’ g) provide a measure of the mean difference between the patient and control group divided by the pooled standard deviation and were calculated to standardize group differences. A negative g reflected poorer performance by the patients relative to controls. Effect size directions were inverted for tasks for which larger scores indicated greater impairment (e.g., timed tasks, rule violations). Effect sizes were categorized as small (g =−.2), medium (g = −.5), or large (g ≥ −.8).
Though the use of a random-effects model can ameliorate the effects of between-study variance, we also employed sensitivity analyses to identify potential outliers in the dataset. To determine the influence that individual studies had on the mean effect size, the “one study removed’’ CM3 module was used to calculate a random-effects mean and standard error as each study is removed one at a time from the analysis (Tobias, Reference Tobias1999). Analyses were also conducted to examine the effect of publication bias. Using established methods by Begg and Mazumdar (Reference Begg and Mazumdar1994) and Egger, Davey Smith, Schneider, and Minder (Reference Egger, Davey Smith, Schneider and Minder1997), a funnel plot was generated for graphical representation as were adjusted rank-correlation tests. Effect size homogeneity was assessed using the Cochran’s Q statistic for the overall effect size and for neuropsychological domains and subdomains. We then evaluated several demographic and clinical characteristics as potential moderators that could explain heterogeneity among effect sizes. Meta-regression analyses were employed to assess the influence of continuous moderator variables, including education, age, and age of onset. To test differences in effect sizes between categories of a given moderator, we used established procedures by Borenstein, Hedges, Higgins, and Rothstein (Reference Borenstein, Hedges, Higgins and Rothstein2009). Contrast analyses that involved domains with fewer than five studies were considered preliminary but conducted for descriptive purposes (Turner, Bird, & Higgins, Reference Turner, Bird and Higgins2013). The I 2-statistic was calculated, which describes the proportion of observed dispersion reflecting differences in true scores rather than sampling error. These values are presented in Supplementary Material Appendix D.
RESULTS
Publication Bias
The “one study removed” sensitivity analysis revealed that the smallest effect size of −2.31 (95% CI: −2.61 < δ ≤ 2.01) and the largest effect size of −2.43 (95% CI: −2.75 < δ ≤ 2.12) fell within the confidence interval of the mean effect size reported, indicating minimal influence of an individual study. Assessment of publication bias revealed significant rank-correlation tests and Egger tests (p’s < .001). The classic fail-safe N indicated that 4936 studies reporting a zero effect would be required to reduce the significance of the observed effect to a p-value greater than .05. As such, publication bias imposed minimal influence on the results.
Moderator Analysis in lvPPA
Demographic and clinical characteristics
We evaluated potential moderators within the lvPPA sample. Age of onset was a significant predictor of study effect size; an earlier age of onset was associated with greater deficits across all tasks (Z =1.96, p =.05). This effect remained significant for the attention (Z =2.24, p =.03) and language (Z =2.06, p =.04) domains only. Within the attention domain, the association remained statistically significant for both simple attention (Z =2.30, p =.02) and working memory deficits (Z =2.08, p =.04). Within the language domain, this association was only significant for comprehension deficits (Z =2.35, p =.02). Illness duration (Z = −1.22, p = .22), patient sex composition (Z =−0.02, p =.98), control sex composition (Z =0.56, p =.57), patient age (Z =1.68, p =.09), control age (Z = 1.68, p = .09), patient education (Z = 1.06, p = .29), and control education (Z = 0.46, p = .65) were not significant moderators.
Neuropsychological domain comparisons in lvPPA
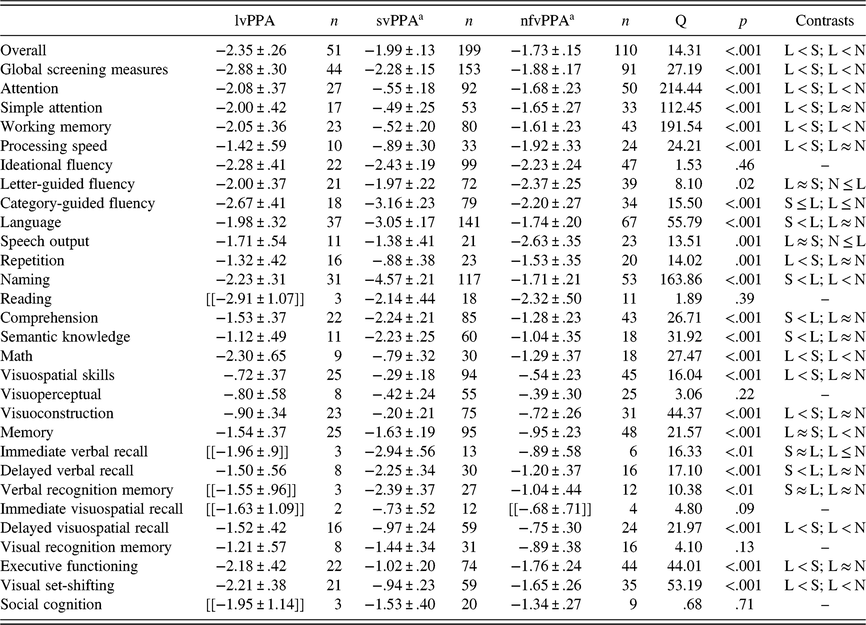
The greatest deficits in lvPPA were observed on global cognition measures (n =44; g =−2.88), mathematic ability (n =9; g =−2.30), and ideational fluency (n =22; g =−2.28). Relatively smaller deficits were observed for visuospatial functioning (n =25; g =−.72) and processing speed (n =10; g =−1.42). See Table 2 for effect sizes for cognitive domains and Supplementary Material Appendix C for Cochran’s Q statistic and p-values for contrasts between cognitive domains.
Table 2. Hedges’ g (Mean ± 95% CI) effect sizes for neuropsychological domains and subdomains by PPA group

Note. Hedges’ g (mean ± 95% CI) in logopenic variant of primary progressive aphasia (lvPPA or L), semantic variant of PPA (svPPA or S), and non-fluent variant of PPA (nfvPPA or N).
a Values for svPPA and nfvPPA are presented from a prior meta-analysis(Kamath et al., Reference Kamath, Chaney, DeRight and Onyikein press); Cochran’s Q statistic and p-values are presented for overall comparisons; ≈ indicates that effects size differences were not statistically significant; ≤ indicates a trend-level difference between study effect sizes (p ≤ .10); Effect sizes in double brackets are presented for descriptive purposes due to the small number of studies included in the analysis (Turner et al., Reference Turner, Bird and Higgins2013).
Neuropsychological subdomain comparisons in lvPPA
We next compared effect sizes within each neuropsychological subdomain. Within the attention domain, effect sizes for simple attention and working memory were comparable in lvPPA (Q B[1] = .04, p =.85). Patients with lvPPA had significantly greater deficits on measures of category-guided fluency when compared to letter-guided fluency (QB[1] = 10.37, p < .001). Within the language domain, deficits in naming and reading were significantly greater than deficits in repetition, comprehension, and semantic knowledge. All other language contrasts were not statistically significant. Within the memory domain, effect sizes were comparable between immediate verbal and visuospatial memory (Q B[1] = .15, p =.69), delayed verbal and visuospatial memory (Q B[1] = .001, p =.97), and verbal and visuospatial recognition memory (Q B[1] = .21, p =.65). Comparison between measures of visuoconstruction and motor-free visuoperceptual tasks were not statistically significant (Q B[1] = .24, p =.62).
Comparison of Neuropsychological Domains and Subdomains in lvPPA to svPPA and nfvPPA
Overall comparison
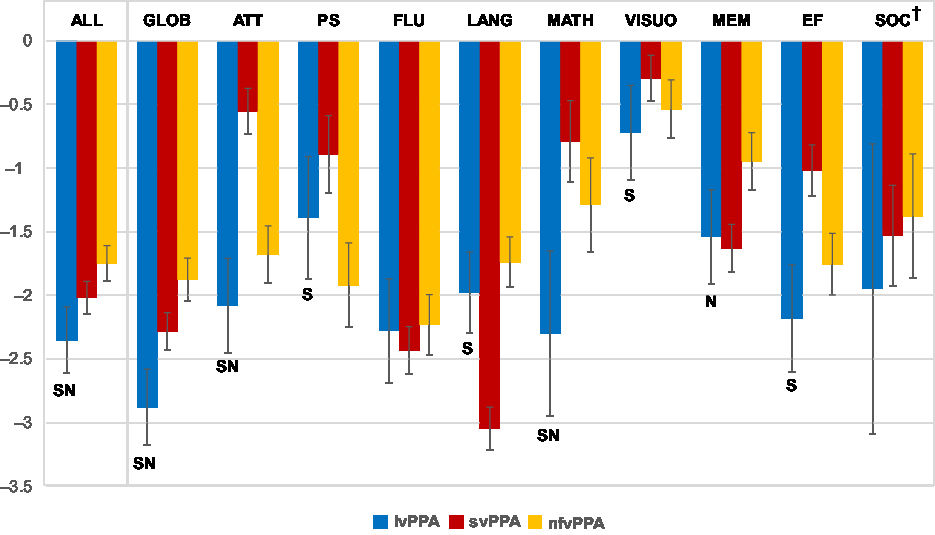
We compared effect sizes of all neuropsychological measures between the lvPPA, svPPA, and nfvPPA groups (see Table 2 and Figure 2).

Fig. 2. Effect sizes (mean Hedges’ g ± 95% CI) for neuropsychological domains by group. Note. lvPPA = logopenic variant primary progressive aphasia; svPPA = semantic variant PPA; nfvPPA = non-fluent variant PPA; GLOB = global screening measures; ATT = attention; PS = processing speed; FLU = ideational fluency; LANG = Language; MEM = memory; VISUO = visuospatial skills; EF = executive functioning; SOC = social cognition; SIndicates that effects size differences were statistically significant between the lvPPA and svPPA group; NIndicates that effects size differences were statistically significant between the lvPPA and nfvPPA group; †Differences in social cognition are presented for descriptive purposes only due to the low sample size in the lvPPA group.
The three groups differed significantly (Q B[2] = 14.31, p < .001) with lvPPA patients showing the greatest magnitude of neuropsychological impairment. As expected, these findings persisted after contributions of the language domain were excluded from analysis (Q B[2] = 22.07, p < .001).
Neuropsychological domains and subdomains
On global cognitive screening measures, lvPPA patients had greater deficits compared to svPPA and nfvPPA (all p’s ≤ .002). Attentional deficits were significantly greater in lvPPA relative to the svPPA and nfvPPA. Within the attention domain, lvPPA patients had greater deficits on simple attention tasks relative to the svPPA group and greater working memory deficits relative to svPPA and nfvPPA. In contrast, deficits in simple attention were not statistically significant between lvPPA and nfvPPA. LvPPA patients had greater processing speed deficits compared to svPPA but were not statistically different from nfvPPA patients.
Effect sizes for ideational fluency did not differ significantly between PPA groups. Consistent with expectation, the svPPA group had greater language impairment relative to the lvPPA group; lvPPA and nfvPPA were not statistically different. On language subdomains, deficits in repetition were not significantly different between lvPPA and nfvPPA patients. SvPPA patients had poor naming and semantic knowledge relative to lvPPA; nfvPPA patients had greater speech output deficits compared to lvPPA cases. Compared to svPPA and nfvPPA, the lvPPA group had the largest math deficits.
LvPPA and svPPA patients had comparable memory deficits, whereas lvPPA patients had greater memory deficits than nfvPPA patients. SvPPA patients had significantly greater material-specific memory impairment for auditory-verbal information compared to lvPPA patients. In particular, svPPA had greater delayed verbal memory deficits than lvPPA cases, whereas differences between lvPPA and nfvPPA were not statistically significant. In contrast, lvPPA has significantly greater delayed visuospatial memory deficits compared to the svPPA and nfvPPA groups. Additional memory subdomains are presented in Table 2 for descriptive purposes due to the limited data available for the lvPPA group.
Across all visuospatial tasks, lvPPA had greater impairment compared to svPPA and nfvPPA. These findings remained true for visuoconstruction tasks but not motor-free visuoperceptual tests, as groups were not statistically different on measures of visuoperception. Finally, effect sizes for social cognition were not statistically different between groups; however, these findings are preliminary due to the small sample size of the lvPPA group.
DISCUSSION
The results of the current meta-analysis confirm and extend prior work documenting the neuropsychological profile of lvPPA and its similarities to and differences from svPPA and nfvPPA. We found that lvPPA patients display a broad range of impairment across neuropsychological measures compared with healthy controls. Demographic factors, including patient/control age, sex, and educational attainment, were not significant moderators of effect size magnitude. Previous studies have shown more widespread cortical atrophy in PPA cases with Alzheimer’s pathology (Rohrer et al., Reference Rohrer, Ridgway, Crutch, Hailstone, Goll, Clarkson, Mead, Beck, Mummery, Ourselin, Warrington, Rossor and Warren2010) and greater rates of whole-brain atrophy in longitudinal analyses (Rohrer et al., Reference Rohrer, Caso, Mahoney, Henry, Rosen, Rabinovici, Rossor, Miller, Warren, Fox, Ridgway and Gorno-Tempini2013). In addition, disease progression is associated with worsening language performances and memory deficits in lvPPA (Leyton et al., Reference Leyton, Hsieh, Mioshi and Hodges2013) and longitudinal cognitive decline has been reported in autopsy-confirmed tau-negative and tau-positive cases (Grossman et al., Reference Grossman, Xie, Libon, Wang, Massimo, Moore, Vesely, Berkowitz, Chatterjee, Coslett, Hurtig, Forman, Lee and Trojanowski2008). Given these prior findings, we hypothesized that duration of illness would significantly moderate the magnitude of neuropsychological deficits in lvPPA. Contrary to expectation, illness duration was not a significant moderator of overall effect size. It is possible that the following factors made it difficult to detect associations with illness duration using meta-analytic methods: (1) the restricted range of illness duration across lvPPA cohorts, (2) the curvilinear nature of cognitive decline in lvPPA, and (3) combining populations with differing rates of disease progression. We also questioned whether examining associations across all cognitive domains may have obscured potential relationships with individual cognitive domains. Therefore, we conducted exploratory moderator analyses of illness duration for each cognitive domain separately and found a trend-level relationship between longer illness duration and increased language deficits in lvPPA (p = .08). No other relationships were statistically significant. In contrast, we found that earlier age of illness onset in lvPPA was associated with greater comprehension deficits and attentional impairment. Studies examining the association between age of onset and cognitive performance in lvPPA are lacking though at least one study found that early-onset AD was associated with greater attentional difficulties compared to late-onset AD (Smits et al., Reference Smits, Pijnenburg, Koedam, van der Vlies, Reuling, Koene, Scheltens and van der Flier2012). In general, more longitudinal autopsy-confirmed studies are needed on the relationship between disease characteristics and cognitive performance are needed cross the PPA variants.
Across the nine cognitive domains analyzed, lvPPA patients showed the greatest deficits on cognitive screening (e.g., mini-mental state examination (MMSE)), mathematic and verbal fluency tasks. Surprisingly, language was not the domain most affected in lvPPA as deficits in attention, executive functioning, and math were as prominent in lvPPA as language difficulties. Within the language domain, patients with lvPPA showed significantly greater deficits in naming when compared to their performance on repetition measures. This finding was unexpected as repetition difficulties are a central feature of lvPPA, as reflected in the current diagnosis criteria (Gorno-Tempini et al., Reference Gorno-Tempini, Hillis, Weintraub, Kertesz, Mendez, Cappa, Ogar, Rohrer, Black, Boeve, Manes, Dronkers, Vandenberghe, Rascovsky, Patterson, Miller, Knopman, Hodges, Mesulam and Grossman2011). As such, we anticipated that repetition deficits would be more prominent than naming difficulties. Recent work has demonstrated that lvPPA patients show poorer performance on repetition tasks across different length conditions, with greater deficits observed for repetition of long phrases and non-meaningful phrases (Lukic et al., Reference Lukic, Mandelli, Welch, Jordan, Shwe, Neuhaus, Miller, Hubbard, Henry, Miller, Dronkers and Gorno-Tempini2019). Based on this work, we generated an effect size for repetition deficits in lvPPA that excluded performance on simple and mixed repetition tasks. As expected, the magnitude of deficits increased and was more comparable to the severity of naming deficits in lvPPA (g = −2.12). A sizeable number of studies administered and reported the total score of the widely used Repetition subtest of the Western Aphasia Battery, which combines repetition of words, phrases, and sentences. Lukic et al. (Reference Lukic, Mandelli, Welch, Jordan, Shwe, Neuhaus, Miller, Hubbard, Henry, Miller, Dronkers and Gorno-Tempini2019) and others have noted that the final six sentences are the most difficult items and are sensitive to the repetition difficulties characteristics of lvPPA. However, since studies typically combine measures of varying complexity, future PPA studies that separate repetition scores by difficulty level and meaning will be important for improving the clinical utility of repetition measures in the differential diagnosis of PPA.
Though the three PPA groups showed highly similar effect sizes for fluency measures, measures of mathematic ability were helpful in distinguishing lvPPA from svPPA and nfvPPA. Patients with lvPPA performed almost a standard deviation below the nfvPPA group (−2.30 vs. −1.29), a finding consistent with the dyscalculia observed in a biomarker-confirmed sample of lvPPA (Rohrer et al., Reference Rohrer, Ridgway, Crutch, Hailstone, Goll, Clarkson, Mead, Beck, Mummery, Ourselin, Warrington, Rossor and Warren2010) and reflective of the parietal lobe deficits frequently observed in this condition. On visuospatial measures, lvPPA patients performed poorly relative to svPPA patients; however, their deficits were comparable in magnitude to nfvPPA patients. Within the visuospatial domain, motor-free visuoperceptual tests did not distinguish PPA groups, whereas visuoconstruction deficits distinguished nfvPPA and lvPPA from the small effect sizes observed in svPPA (g = −.20). These findings are consistent with a longitudinal study of visuoconstruction ability in PPA variants in which svPPA patients performed remarkably well on measures of visuoconstruction, whereas nfvPPA and lvPPA patients showed declining ability to reproduce complex figures over time (Watson et al., Reference Watson, Possin, Allen, Hubbard, Meyer, Welch, Rabinovici, Rosen, Rankin, Miller, Santos-Santos, Kramer, Miller and Gorno-Tempini2018). Of note, visual set-shifting measures, which involve heightened visuospatial search demands and cognitive flexibility, were helpful in distinguishing lvPPA from both svPPA and nfvPPA. Findings on the neuroanatomical correlates of visual set-shifting are mixed. Studies of patients with focal brain lesions have implicated non-specific left-lateralized prefrontal, insular, temporal, and parietal areas and right-lateralized frontal regions (for a review, see Varjacic, Mantini, Demeyere, & Gillebert, Reference Varjacic, Mantini, Demeyere and Gillebert2018). As greater widespread cortical atrophy and greater rates of whole-brain atrophy have been reported in PPA cases with Alzheimer’s pathology (Rohrer et al., Reference Rohrer, Ridgway, Crutch, Hailstone, Goll, Clarkson, Mead, Beck, Mummery, Ourselin, Warrington, Rossor and Warren2010, Reference Rohrer, Caso, Mahoney, Henry, Rosen, Rabinovici, Rossor, Miller, Warren, Fox, Ridgway and Gorno-Tempini2013), it is possible that visual set-shifting tasks are a sensitive markers of the cerebral dysfunction observed in lvPPA.
In lvPPA, the magnitude of delayed visuospatial and verbal memory deficits was comparable. In contrast, svPPA and nfvPPA patients demonstrated a greater degree of material-specific memory impairment as delayed auditory-verbal recall was poorer compared to recall of visuospatial information. Moreover, lvPPA patients had greater deficits on delayed visuospatial recall measures when compared to svPPA and nfvPPA cases. These findings correspond with recent work indicating that lvPPA patients have greater cognitive impairment, particularly for visuospatial memory, when compared to the svPPA and nfvPPA variants (Watson et al., Reference Watson, Possin, Allen, Hubbard, Meyer, Welch, Rabinovici, Rosen, Rankin, Miller, Santos-Santos, Kramer, Miller and Gorno-Tempini2018). Using a biomarker-confirmed lvPPA sample, Ramanan et al., (Reference Ramanan, Flanagan, Leyton, Villemagne, Rowe, Hodges and Hornberger2016) found that visuospatial memory impairment had utility in discriminating lvPPA from nfvPPA and was predictive of amyloid pathology assessed via amyloid-PET imaging. Flanagan, Tu, Ahmed, Hodges, & Hornberger (Reference Flanagan, Tu, Ahmed, Hodges and Hornberger2014) similarly found that lvPPA patients had greater difficulties on measures of memory and orientation when compared to nfvPPA patients and had comparable scores to individuals with AD. Thus, poor visuospatial memory may serve as a potential cognitive biomarker of the underlying amyloid pathology highly prevalent in AD and lvPPA cases (Santos-Santos et al., Reference Santos-Santos, Rabinovici, Iaccarino, Ayakta, Tammewar, Lobach, Henry, Hubbard, Mandelli, Spinelli, Miller, Pressman, O&Neil, Ghosh, Lazaris, Meyer, Watson, Yoon, Rosen, Grinberg, Seeley, Miller, Jagust and Gorno-Tempini2018) and its inclusion in neuropsychological evaluations could aid in the differentiation of PPA subtypes. Our findings contrast those of a recent meta-analysis of memory in PPA, which reported comparable effect sizes for verbal and visuospatial memory in nfvPPA (Eikelboom et al., Reference Eikelboom, Janssen, Jiskoot, van den Berg, Roelofs and Kessels2018). One potential reason for this discrepancy could be the greater number of studies available for inclusion in the current analysis. Our prior work demonstrates that reporting effect sizes for memory indices separately is particularly relevant for nfvPPA, as this group has material-specific memory differences for delayed recall tasks but not immediate recall or recognition memory tasks (Kamath et al., Reference Kamath, Chaney, DeRight and Onyikein press). Taken together, our memory findings in lvPPA support the utility of delayed visuospatial memory in differentiating lvPPA from svPPA and nfvPPA. Recent efforts to develop new spatial memory and topographical orientation measures will also be helpful (Bird et al., Reference Bird, Chan, Hartley, Pijnenburg, Rossor and Burgess2009; Tu et al., Reference Tu, Wong, Hodges, Irish, Piguet and Hornberger2015), as these tasks probe the posterior cingulate regions that are affected early in lvPPA but are relatively spared in svPPA and nfvPPA.
There were several limitations of the current meta-analysis. Measures of social cognition have received widespread attention in frontotemporal lobar degeneration (FTLD), as these measures are thought to be sensitive to the early deterioration in social comportment and behavior observed in svPPA and the behavioral variant of FTD (bvFTD; Bora, Velakoulis, & Walterfang, Reference Bora, Velakoulis and Walterfang2016; Bora, Walterfang, & Velakoulis, Reference Bora, Walterfang and Velakoulis2015). Social cognitive deficits are more characteristic of bvFTD than AD (Bora et al., Reference Bora, Velakoulis and Walterfang2016; LaMarre & Kramer, Reference LaMarre, Kramer, Ravdin and Katzen2013), possibly reflecting neurodegeneration of anterior and ventral aspects of the prefrontal cortex affected early in the course of bvFTD but relatively spared in early-stage lvPPA. The limited number of studies in lvPPA precluded interpretation of social cognitive findings relative to svPPA and nfvPPA and emphasizes the need for further work in this area. Similarly, few studies have examined all facets of executive functioning in lvPPA (i.e., concept formation, response inhibition, sequential planning). As such, we were unable to compare subdomains of executive functioning within lvPPA and across PPA variants. The current meta-analysis was also limited by the low number of studies assessing math performance and the lack of information provided on math tasks in PPA variants. This limited our ability to conduct analyses separated by math task type (i.e., mental vs. written arithmetic). Different math tasks recruit different cognitive functions depending on the nature of the tasks and may variably engage frontoparietal brain regions as a result. For example, mental calculations involve working memory, whereas written calculations involve visuospatial functioning. Finally, inclusion of lvPPA was based on clinical diagnosis as opposed to a pathology-confirmed diagnosis. AD is the primary pathology of lvPPA; however, tau, TDP-43, Lewy body dementia, and Creutzfeldt–Jakob disease have been identified as etiologies for lvPPA. In certain cases of lvPPA, these pathologies can be associated with differing patterns of cerebral atrophy and cognitive performances. For example, lvPPA patients with a progranulin genetic mutation were found to have more anterior temporal lobe involvement and poorer scores on measures of naming, single-word comprehension and irregular word reading – factors most common in svPPA (Rohrer et al., Reference Rohrer, Ridgway, Crutch, Hailstone, Goll, Clarkson, Mead, Beck, Mummery, Ourselin, Warrington, Rossor and Warren2010). The increasing opportunity to recruit and characterize biomarker- and pathology-confirmed samples of PPA will be important in this regard.
LvPPA has emerged as a form of PPA with a separable pattern of linguistic, anatomical, and pathological changes. Though the non-fluent/agrammatic and semantic variants of PPA have historically received more attention, our understanding of lvPPA has increased exponentially over the past 15 years. Findings from this meta-analysis extend our understanding of the neuropsychological profile of lvPPA and its comparison to svPPA and nfvPPA by synthesizing results from available lvPPA samples. We found that tests of visuospatial memory, working memory, and math have utility in distinguishing lvPPA from svPPA and nfvPPA. It is possible these tasks serve as cognitive biomarkers of the posterior temporoparietal atrophy observed early in the course of lvPPA. Collectively, our findings support the inclusion of measures of beyond speech and language in the neuropsychological assessment of lvPPA. Tasks assessing mathematic ability, visual set-shifting, and delayed visuospatial recall may have utility in distinguishing lvPPA from the semantic and non-fluent variants in clinical assessment. Future clinicopathological and longitudinal studies of these measures in PPA cohorts will be important.
ACKNOWLEDGMENTS
The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of the US Government. The authors thank Martin D. Smith, M.A., Jonathan DeRight, Ph.D., Janae Cephas, Madalyn Myers, Erin Schnappauf, and Alana Spears for assistance with article procurement, data entry, and quality control checks of the data. VK is supported through the Johns Hopkins Clinical Research Scholars Program (grant number: KL2TR001077) and the Johns Hopkins Department of Psychiatry and Behavioral Sciences. The authors declare that they have no conflicts of interest.
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719001115