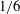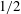1 Introduction
When a drop of a solution of a certain class of surfactants such as trisiloxane is placed on a solid substrate, it can spread at an unusually fast rate much higher than a surfactant-free solution (Hill Reference Hill1988; Zhu et al.
Reference Zhu, Miller, Scriven and Davis1994; Stoebe et al.
Reference Stoebe, Lin, Hill, Wards and Davis1996). Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) reported that this so-called superspreading can occur at a strikingly fast rate with the spreading radius growing linearly with time,
![]() $R\propto t$
– the power is 10 times greater than that of Tanner’s law (Tanner Reference Tanner1979). Although there are extensive studies (Beacham, Matar & Craster Reference Beacham, Matar and Craster2009; Karapetsas, Craster & Matar Reference Karapetsas, Craster and Matar2011; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013; Theodorakis et al.
Reference Theodorakis, Müller, Craster and Matar2015) and related investigations (Joanny Reference Joanny1989; Jensen & Grotberg Reference Jensen and Grotberg1992; Clay & Miksis Reference Clay and Miksis2004; Jensen & Naire Reference Jensen and Naire2006), the true nature of superspreading is still not fully understood. It is generally believed that the Marangoni force caused by the surface tension gradient
$R\propto t$
– the power is 10 times greater than that of Tanner’s law (Tanner Reference Tanner1979). Although there are extensive studies (Beacham, Matar & Craster Reference Beacham, Matar and Craster2009; Karapetsas, Craster & Matar Reference Karapetsas, Craster and Matar2011; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013; Theodorakis et al.
Reference Theodorakis, Müller, Craster and Matar2015) and related investigations (Joanny Reference Joanny1989; Jensen & Grotberg Reference Jensen and Grotberg1992; Clay & Miksis Reference Clay and Miksis2004; Jensen & Naire Reference Jensen and Naire2006), the true nature of superspreading is still not fully understood. It is generally believed that the Marangoni force caused by the surface tension gradient
![]() $\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
plays a major role in superspreading phenomenon (Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Nikolov & Wasan Reference Nikolov and Wasan2015). This force can drive a fluid at velocity
$\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
plays a major role in superspreading phenomenon (Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Nikolov & Wasan Reference Nikolov and Wasan2015). This force can drive a fluid at velocity
where
![]() $h$
is the drop height and
$h$
is the drop height and
![]() $\unicode[STIX]{x1D702}$
the viscosity of the wetting fluid. However, the spreading exponent is often found to be far below unity (Jensen & Grotberg Reference Jensen and Grotberg1992; Starov, de Ryck & Velarde Reference Starov, de Ryck and Velarde1997; Jensen & Naire Reference Jensen and Naire2006). In fact, it is impossible to derive the linear spreading law by having
$\unicode[STIX]{x1D702}$
the viscosity of the wetting fluid. However, the spreading exponent is often found to be far below unity (Jensen & Grotberg Reference Jensen and Grotberg1992; Starov, de Ryck & Velarde Reference Starov, de Ryck and Velarde1997; Jensen & Naire Reference Jensen and Naire2006). In fact, it is impossible to derive the linear spreading law by having
![]() $\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
across the drop spreading radius
$\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
across the drop spreading radius
![]() $R$
, especially when additional global constraints (i.e. constant drop volume and surfactant mass conservation) are imposed. This implies that the Marangoni force alone does not suffice to account for this unusual spreading caused by surfactant superspreaders. Other effects might involve surfactant assembly (Stoebe et al.
Reference Stoebe, Lin, Hill, Wards and Davis1997; Kumar et al.
Reference Kumar, Varanasi, Tilton and Garoff2003a
), surfactant adsorption on the solid surface (Kumar, Couzis & Maldarelli Reference Kumar, Couzis and Maldarelli2003b
) and surfactant transport at the contact line (Clay & Miksis Reference Clay and Miksis2004). Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) developed a lubrication model by including all the above effects and showed that the linear spreading law can be captured due to delicate interplays between these effects. In particular, whether interfacial surfactant molecules can be transferred onto the solid substrate in the vicinity of the contact line seems to play a crucial role in superspreading (Kim, Qin & Fichthorn Reference Kim, Qin and Fichthorn2006; Karapetsas et al.
Reference Karapetsas, Craster and Matar2011; Maldarelli Reference Maldarelli2011; Theodorakis et al.
Reference Theodorakis, Müller, Craster and Matar2015).
$R$
, especially when additional global constraints (i.e. constant drop volume and surfactant mass conservation) are imposed. This implies that the Marangoni force alone does not suffice to account for this unusual spreading caused by surfactant superspreaders. Other effects might involve surfactant assembly (Stoebe et al.
Reference Stoebe, Lin, Hill, Wards and Davis1997; Kumar et al.
Reference Kumar, Varanasi, Tilton and Garoff2003a
), surfactant adsorption on the solid surface (Kumar, Couzis & Maldarelli Reference Kumar, Couzis and Maldarelli2003b
) and surfactant transport at the contact line (Clay & Miksis Reference Clay and Miksis2004). Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) developed a lubrication model by including all the above effects and showed that the linear spreading law can be captured due to delicate interplays between these effects. In particular, whether interfacial surfactant molecules can be transferred onto the solid substrate in the vicinity of the contact line seems to play a crucial role in superspreading (Kim, Qin & Fichthorn Reference Kim, Qin and Fichthorn2006; Karapetsas et al.
Reference Karapetsas, Craster and Matar2011; Maldarelli Reference Maldarelli2011; Theodorakis et al.
Reference Theodorakis, Müller, Craster and Matar2015).
In fact, because
![]() $u_{M}\propto h$
$u_{M}\propto h$
![]() $\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
according to (1.1), to attain a constant spreading speed and hence the linear spreading law,
$\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
according to (1.1), to attain a constant spreading speed and hence the linear spreading law,
![]() $\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
in (1.1) needs to be established over the drop height
$\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}$
in (1.1) needs to be established over the drop height
![]() $h$
(Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Rafai & Bonn Reference Rafai and Bonn2005). If the above is true, because
$h$
(Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Rafai & Bonn Reference Rafai and Bonn2005). If the above is true, because
![]() $u_{M}$
is invariant with
$u_{M}$
is invariant with
![]() $h$
,
$h$
,
![]() $h$
in (1.1) can be taken as any local thickness of a drop that varies roughly linearly with the distance to the contact line. Taking
$h$
in (1.1) can be taken as any local thickness of a drop that varies roughly linearly with the distance to the contact line. Taking
![]() $h\rightarrow 0$
, the contact line (which is moving at speed
$h\rightarrow 0$
, the contact line (which is moving at speed
![]() $U$
) would have to be driven by a local Marangoni shearing
$U$
) would have to be driven by a local Marangoni shearing
![]() $\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}\propto 1/h$
that diverges as fast as the usual moving contact line stress singularity
$\unicode[STIX]{x1D735}_{s}\unicode[STIX]{x1D70E}\propto 1/h$
that diverges as fast as the usual moving contact line stress singularity
![]() $\unicode[STIX]{x1D702}U/h$
(Huh & Scriven Reference Huh and Scriven1971). This might explain why the spreading speed is independent of the extent of spreading. This also implies that a rational explanation of the linear spreading law can only be sought by resolving what happens near the moving contact line.
$\unicode[STIX]{x1D702}U/h$
(Huh & Scriven Reference Huh and Scriven1971). This might explain why the spreading speed is independent of the extent of spreading. This also implies that a rational explanation of the linear spreading law can only be sought by resolving what happens near the moving contact line.
Therefore, to obtain the linear spreading law, it is necessary to examine how both the flow field and surfactant transport behave in the vicinity of the contact line during a superspreading process. It is worth mentioning that in the simulation study by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011), the relationship between the dynamic contact angle and wetting spread is assumed to obey the clean-interface de Gennes–Tanner cubic law. However, it is not obvious that this has to be the case. In fact, existing theoretical studies showed that modified wetting relationships can result from a Marangoni stress induced by surfactant (Cox Reference Cox1986b ; Joanny Reference Joanny1989; Chesters & Elyousfi Reference Chesters and Elyousfi1998; Rame Reference Rame2001; Chan & Borhan Reference Chan and Borhan2006; Jensen & Naire Reference Jensen and Naire2006). So the successful capture of the linear spreading law by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) seems to imply that the classical de Gennes–Tanner law might be the correct wetting law for surfactant superspreaders, but not for regular surfactants whose spreading powers are smaller than unity. If this is the case, a completely different contact line structure must exist for the former to accommodate the de Gennes–Tanner law when a Marangoni stress is present. The resolution of this contact line structure will not only complement the simulation study by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011), but also provide more insights into how the linear spreading law results from the transport of superspreaders.
Motivated by the above, we aim at developing a dynamic contact line theory for surfactant-driven superspreading. How to construct such a theory by acquiring key ingredients of superspreading is described as follows. As mentioned earlier, the key to obtaining the linear spreading law lies in a concentrated Marangoni stress in the vicinity of the contact line. As such a Marangoni stress has to act toward the contact line, the interfacial surfactant concentration near the contact line has to be much lower than that away from the contact line. From a mass transfer point of view, a ‘sink’ or some sort of depletion mechanism must exist to draw surfactant molecules out of the air–liquid interface to sustain this local surfactant deficiency near the contact line. It has also been shown previously that the adsorption of surfactant molecules onto the substrate from the interface is one of key elements to superspreading (Karapetsas et al. Reference Karapetsas, Craster and Matar2011; Theodorakis et al. Reference Theodorakis, Müller, Craster and Matar2015). Therefore, in order to take account of such surfactant depletion or adsorption effects in a simpler way, in this work we prescribe a local surfactant leakage flux in the vicinity of the contact line in the surfactant transport equation. This will directly set-up an interfacial surfactant concentration gradient to drive the contact line with Marangoni stress. As illustrated in figure 1, such leakage can occur in two different routes, depending on whether surfactant molecules on the interface can undergo mass exchange with the bulk. For insoluble surfactant, because it cannot be desorbed back to the bulk, its leakage can only occur along the interface via its direct transfer through the contact line (see figure 1 a). In contrast, for soluble surfactant, its leakage can be through the bulk, achieved by corner diffusion from the interface to the substrate inside the drop (see figure 1 b).

Figure 1. Schematic illustration of surfactant-enhanced spreading. A Marangoni force toward the contact line can be established by an interfacial surfactant concentration gradient set-up by a local surfactant leakage. Such leakage can occur via two different routes, depending on whether surfactant molecules on the interface can undergo mass exchange with the bulk. (a) For insoluble surfactant, its leak can only occur along the interface via its direct transfer through the contact line. (b) For soluble surfactant, its leakage can be achieved through corner diffusion from the interface to the substrate inside the drop.
The above routes to the depletion of interfacial surfactant have been suggested in the modelling and simulation studies by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) and Theodorakis et al. (Reference Theodorakis, Müller, Craster and Matar2015). In their studies, the interfacial surfactant concentration gradient needed for promoting drop spreading is established by delicate interplays between surfactant molecules on the interface and those in the bulk and on the substrate. In contrast, in this work we stipulate the surfactant depletion near the contact line as the sole driving mechanism for superspreading and model it as a local surfactant leakage flux. As will be demonstrated, by incorporating this local surfactant leakage explicitly into the contact line hydrodynamics, we can theoretically show that superspreading is in effect a new class of dynamic wetting: Marangoni-enhanced capillary wetting – the joint wetting mechanism unique to superspreaders by having both surface tension and surface tension gradient forces work collaboratively.
This Marangoni–capillary wetting will not only call for a new contact line structure, but also provide a natural explanation for the linear spreading law. As will also be shown by this work, it is actually a direct consequence of the features below arising from local surfactant leakage. First, a fraction of surfactant leakage can set-up a concentrated Marangoni shearing toward the contact line with a Marangoni stress singularity diverging at a rate much faster than the usual viscous stress singularity. Second, this leak also results in a microscopic surfactant-devoid zone in the vicinity of the contact line. Because the contact line is free of surfactant, the strong Marangoni shearing does not drive the contact line directly but turns into a local capillary force, driving the contact line to advance in a purely capillary manner. Third, as a result of this local Marangoni-driven capillary wetting, the contact line is moving at a constant wetting speed governed by the de Gennes–Tanner law, thereby leading to the linear spreading law. Finally, we also determine the range of the leakage flux within which superspreading can occur without being mitigated by the contact line sweeping. This explains why superspreading occurs in a range of surfactant concentration and why only limited classes of surfactants can serve as superspreaders.
The rest of the paper derives the above results according to the following outline. In § 2 we begin with the lubrication formulation to provide the basic equations needed for modelling superspreading. Using this formulation, we first consider insoluble surfactant in § 3 and demonstrate a possible realization of superspreading. This will elicit key features of superspreading. Detailed accounts for soluble surfactant will be given in § 4, which is more relevant to superspreading. In § 5 we make connections of our findings to a variety of results seen in experiments and simulations. A criterion of superspreading will also be established. In § 6 we show that an analogy can exist between surfactant-driven spreading and thermocapillary spreading, highlighting the exclusive role of the Marangoni effect in dynamic wetting driven by non-uniform surface tension. The paper is finally concluded in § 7.
2 Formulation and basic equations
Similar to the analysis by Joanny (Reference Joanny1989), we assume that the region near the contact line is approximately wedge shaped with the macroscopic contact angle
![]() $\unicode[STIX]{x1D703}_{0}$
. We further assume that
$\unicode[STIX]{x1D703}_{0}$
. We further assume that
![]() $\unicode[STIX]{x1D703}_{0}$
is sufficiently small so that the liquid height can be approximated as
$\unicode[STIX]{x1D703}_{0}$
is sufficiently small so that the liquid height can be approximated as
![]() $h=\unicode[STIX]{x1D703}_{0}x$
. This small angle assumption allows us to apply the lubrication theory to derive the relevant equations. In the frame moving with the contact line speed
$h=\unicode[STIX]{x1D703}_{0}x$
. This small angle assumption allows us to apply the lubrication theory to derive the relevant equations. In the frame moving with the contact line speed
![]() $U$
, the horizontal velocity
$U$
, the horizontal velocity
![]() $u$
satisfies the lubrication equation
$u$
satisfies the lubrication equation
![]() $p_{x}=\unicode[STIX]{x1D702}u_{yy}$
with boundary conditions
$p_{x}=\unicode[STIX]{x1D702}u_{yy}$
with boundary conditions
![]() $u=U$
at
$u=U$
at
![]() $y=0$
and
$y=0$
and
![]() $\unicode[STIX]{x1D702}u_{y}=\unicode[STIX]{x1D70F}_{s}$
at
$\unicode[STIX]{x1D702}u_{y}=\unicode[STIX]{x1D70F}_{s}$
at
![]() $y=h$
. The solution is then given by
$y=h$
. The solution is then given by
Here the Marangoni stress
![]() $\unicode[STIX]{x1D70F}_{s}$
is written as the interfacial surfactant concentration gradient
$\unicode[STIX]{x1D70F}_{s}$
is written as the interfacial surfactant concentration gradient
![]() $\unicode[STIX]{x1D6E4}_{x}$
according to
$\unicode[STIX]{x1D6E4}_{x}$
according to
where
![]() $\unicode[STIX]{x1D6FD}\equiv -(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})$
measures the susceptibility to lowering surface tension
$\unicode[STIX]{x1D6FD}\equiv -(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})$
measures the susceptibility to lowering surface tension
![]() $\unicode[STIX]{x1D70E}$
and could vary with
$\unicode[STIX]{x1D70E}$
and could vary with
![]() $\unicode[STIX]{x1D6E4}$
, depending on the bulk surfactant concentration
$\unicode[STIX]{x1D6E4}$
, depending on the bulk surfactant concentration
![]() $C_{0}$
. While we are modelling superspreading that typically occurs at high surfactant concentrations above the critical micelle concentration (CMC), we actually will be dealing with a large change of
$C_{0}$
. While we are modelling superspreading that typically occurs at high surfactant concentrations above the critical micelle concentration (CMC), we actually will be dealing with a large change of
![]() $\unicode[STIX]{x1D6E4}$
along the interface in the vicinity of the contact line. So
$\unicode[STIX]{x1D6E4}$
along the interface in the vicinity of the contact line. So
![]() $\unicode[STIX]{x1D6FD}$
might also vary considerably. Nevertheless, we still choose a constant
$\unicode[STIX]{x1D6FD}$
might also vary considerably. Nevertheless, we still choose a constant
![]() $\unicode[STIX]{x1D6FD}$
to simplify our analysis based on the following rationales.
$\unicode[STIX]{x1D6FD}$
to simplify our analysis based on the following rationales.
First of all, we notice that it is the interfacial concentration
![]() $\unicode[STIX]{x1D6E4}$
that determines surface tension. So even at
$\unicode[STIX]{x1D6E4}$
that determines surface tension. So even at
![]() $C_{0}$
higher than the CMC, the surface tension changes are controlled by the interfacial concentration at around the CMC,
$C_{0}$
higher than the CMC, the surface tension changes are controlled by the interfacial concentration at around the CMC,
![]() $\unicode[STIX]{x1D6E4}_{CMC}$
, which does not vary with
$\unicode[STIX]{x1D6E4}_{CMC}$
, which does not vary with
![]() $C_{0}$
. Suppose that at a high
$C_{0}$
. Suppose that at a high
![]() $C_{0}$
abundant surfactant molecules are populated on the interface with the interfacial surfactant concentration
$C_{0}$
abundant surfactant molecules are populated on the interface with the interfacial surfactant concentration
![]() $\unicode[STIX]{x1D6E4}_{0}$
(at an order of
$\unicode[STIX]{x1D6E4}_{0}$
(at an order of
![]() $\unicode[STIX]{x1D6E4}_{CMC}$
) and the corresponding surface tension
$\unicode[STIX]{x1D6E4}_{CMC}$
) and the corresponding surface tension
![]() $\unicode[STIX]{x1D70E}_{0}$
. If variations of
$\unicode[STIX]{x1D70E}_{0}$
. If variations of
![]() $\unicode[STIX]{x1D6E4}$
are not large,
$\unicode[STIX]{x1D6E4}$
are not large,
![]() $\unicode[STIX]{x1D6FD}$
can be approximated as a constant value
$\unicode[STIX]{x1D6FD}$
can be approximated as a constant value
![]() $\unicode[STIX]{x1D6FD}_{0}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})_{0}$
(which is generally small) corresponding to the above state. As will be shown in §§ 3 and 4, as long as the prescribed local surfactant leakage rate is low,
$\unicode[STIX]{x1D6FD}_{0}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})_{0}$
(which is generally small) corresponding to the above state. As will be shown in §§ 3 and 4, as long as the prescribed local surfactant leakage rate is low,
![]() $\unicode[STIX]{x1D6E4}$
merely displays a little or moderate decrease from
$\unicode[STIX]{x1D6E4}$
merely displays a little or moderate decrease from
![]() $\unicode[STIX]{x1D6E4}_{0}$
far away from the contact line. So in this outer region, because the dependence of
$\unicode[STIX]{x1D6E4}_{0}$
far away from the contact line. So in this outer region, because the dependence of
![]() $\unicode[STIX]{x1D70E}$
on
$\unicode[STIX]{x1D70E}$
on
![]() $\unicode[STIX]{x1D6E4}$
is approximately linear, using
$\unicode[STIX]{x1D6E4}$
is approximately linear, using
![]() $\unicode[STIX]{x1D6FD}\approx \unicode[STIX]{x1D6FD}_{0}$
in (2.2) can still faithfully describe the Marangoni stress behaviour in the region.
$\unicode[STIX]{x1D6FD}\approx \unicode[STIX]{x1D6FD}_{0}$
in (2.2) can still faithfully describe the Marangoni stress behaviour in the region.
For the region near the contact line, however, because of the prescribed local surfactant leakage, there exists a small depletion region where
![]() $\unicode[STIX]{x1D6E4}$
shows a very sharp decrease to the clean-interface state. In this depletion region, especially for the close proximity to the contact line, surfactant molecules are almost emptied out by the leak. So the interface thereof is little contaminated by surfactant having an averaged interfacial concentration
$\unicode[STIX]{x1D6E4}$
shows a very sharp decrease to the clean-interface state. In this depletion region, especially for the close proximity to the contact line, surfactant molecules are almost emptied out by the leak. So the interface thereof is little contaminated by surfactant having an averaged interfacial concentration
![]() $\unicode[STIX]{x1D6E4}_{1}$
much lower than
$\unicode[STIX]{x1D6E4}_{1}$
much lower than
![]() $\unicode[STIX]{x1D6E4}_{0}({\sim}\unicode[STIX]{x1D6E4}_{CMC})$
. Hence in this inner region,
$\unicode[STIX]{x1D6E4}_{0}({\sim}\unicode[STIX]{x1D6E4}_{CMC})$
. Hence in this inner region,
![]() $\unicode[STIX]{x1D70E}$
also varies linearly with
$\unicode[STIX]{x1D70E}$
also varies linearly with
![]() $\unicode[STIX]{x1D6E4}$
under a constant susceptibility
$\unicode[STIX]{x1D6E4}$
under a constant susceptibility
![]() $\unicode[STIX]{x1D6FD}_{1}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})_{1}\approx (\unicode[STIX]{x1D70E}_{clean}-\unicode[STIX]{x1D70E}_{1})/\unicode[STIX]{x1D6E4}_{1}$
(where
$\unicode[STIX]{x1D6FD}_{1}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}\unicode[STIX]{x1D6E4})_{1}\approx (\unicode[STIX]{x1D70E}_{clean}-\unicode[STIX]{x1D70E}_{1})/\unicode[STIX]{x1D6E4}_{1}$
(where
![]() $\unicode[STIX]{x1D70E}_{clean}$
is the clean-interface surface tension and
$\unicode[STIX]{x1D70E}_{clean}$
is the clean-interface surface tension and
![]() $\unicode[STIX]{x1D70E}_{1}$
the surface tension corresponding to
$\unicode[STIX]{x1D70E}_{1}$
the surface tension corresponding to
![]() $\unicode[STIX]{x1D6E4}_{1}$
). In other words, in both outer and inner regions, because surface tension changes are small, how
$\unicode[STIX]{x1D6E4}_{1}$
). In other words, in both outer and inner regions, because surface tension changes are small, how
![]() $\unicode[STIX]{x1D70E}$
varies with
$\unicode[STIX]{x1D70E}$
varies with
![]() $\unicode[STIX]{x1D6E4}$
in each region can be approximately represented by a linear equation of state but with distinct values of
$\unicode[STIX]{x1D6E4}$
in each region can be approximately represented by a linear equation of state but with distinct values of
![]() $\unicode[STIX]{x1D6FD}$
.
$\unicode[STIX]{x1D6FD}$
.
Despite the above, since the behaviour of
![]() $\unicode[STIX]{x1D70E}$
actually deviates from two limiting surfactant states near which surface tension variations are small
$\unicode[STIX]{x1D70E}$
actually deviates from two limiting surfactant states near which surface tension variations are small
![]() $-\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
from the surfactant-crowded state and
$-\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
from the surfactant-crowded state and
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{1}$
from the clean-interface state, it might be conceivable that the characteristic surface tension changes
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{1}$
from the clean-interface state, it might be conceivable that the characteristic surface tension changes
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}=\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
and
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}=\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
and
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{1}=\unicode[STIX]{x1D6FD}_{1}\unicode[STIX]{x1D6E4}_{1}$
in these two regions are comparable in magnitude, i.e.
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{1}=\unicode[STIX]{x1D6FD}_{1}\unicode[STIX]{x1D6E4}_{1}$
in these two regions are comparable in magnitude, i.e.
![]() $\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}\sim \unicode[STIX]{x1D6FD}_{1}\unicode[STIX]{x1D6E4}_{1}$
(so
$\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}\sim \unicode[STIX]{x1D6FD}_{1}\unicode[STIX]{x1D6E4}_{1}$
(so
![]() $\unicode[STIX]{x1D6FD}_{1}\sim \unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x1D6E4}_{1}\gg \unicode[STIX]{x1D6FD}_{0}$
, which is typical for most surfactants). In this case, even using
$\unicode[STIX]{x1D6FD}_{1}\sim \unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x1D6E4}_{1}\gg \unicode[STIX]{x1D6FD}_{0}$
, which is typical for most surfactants). In this case, even using
![]() $\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
in the outer region as a scale for the surface tension change in the inner region might not cause a qualitative change to the characteristics of the inner region. Hence, throughout this work, we take
$\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
in the outer region as a scale for the surface tension change in the inner region might not cause a qualitative change to the characteristics of the inner region. Hence, throughout this work, we take
![]() $\unicode[STIX]{x1D6FD}=\unicode[STIX]{x1D6FD}_{0}$
in (2.2) and use
$\unicode[STIX]{x1D6FD}=\unicode[STIX]{x1D6FD}_{0}$
in (2.2) and use
![]() $\unicode[STIX]{x1D6E4}_{0}$
to be the characteristic interfacial concentration. In fact, as will become clear in our subsequent analysis, because it is the surface tension change near the contact line that matters and also because this change can be expressed in terms of the prescribed leakage flux, the resulting spreading speeds (3.17) and (4.14), which are our main results, will not explicitly depend on the actual value of
$\unicode[STIX]{x1D6E4}_{0}$
to be the characteristic interfacial concentration. In fact, as will become clear in our subsequent analysis, because it is the surface tension change near the contact line that matters and also because this change can be expressed in terms of the prescribed leakage flux, the resulting spreading speeds (3.17) and (4.14), which are our main results, will not explicitly depend on the actual value of
![]() $\unicode[STIX]{x1D6FD}$
.
$\unicode[STIX]{x1D6FD}$
.
To fulfil the requirement of zero flow rate across the wedge, the pressure gradient has to satisfy
The pressure here is supported by the Laplace pressure at the interface:
Again, since the surfactant concentration here is located in the regime above CMC where superspreading occurs,
![]() $\unicode[STIX]{x1D70E}$
does not vary significantly with
$\unicode[STIX]{x1D70E}$
does not vary significantly with
![]() $\unicode[STIX]{x1D6E4}$
and hence can be roughly represented by
$\unicode[STIX]{x1D6E4}$
and hence can be roughly represented by
![]() $\unicode[STIX]{x1D70E}_{0}$
. However, as will be seen in §§ 3 and 4, this approximation will break down in the close proximity of the contact line where surfactant molecules are almost emptied out by the prescribed local surfactant leakage. In this surfactant-devoid region, the clean-interface value
$\unicode[STIX]{x1D70E}_{0}$
. However, as will be seen in §§ 3 and 4, this approximation will break down in the close proximity of the contact line where surfactant molecules are almost emptied out by the prescribed local surfactant leakage. In this surfactant-devoid region, the clean-interface value
![]() $\unicode[STIX]{x1D70E}_{clean}$
will be used in
$\unicode[STIX]{x1D70E}_{clean}$
will be used in
![]() $\unicode[STIX]{x1D70E}$
instead. As for the region sufficiently away from the contact line, the interface with
$\unicode[STIX]{x1D70E}$
instead. As for the region sufficiently away from the contact line, the interface with
![]() $\unicode[STIX]{x1D70E}=\unicode[STIX]{x1D70E}_{0}$
remains virtually flat and hence
$\unicode[STIX]{x1D70E}=\unicode[STIX]{x1D70E}_{0}$
remains virtually flat and hence
![]() $p\approx 0$
. So even using
$p\approx 0$
. So even using
![]() $\unicode[STIX]{x1D70E}=\unicode[STIX]{x1D70E}_{clean}$
in (2.4) will not alter the characteristics for the region far away from the contact line. Also because (2.4) will be used to determine the actual dynamic contact angle due to the deformation of the almost clean part of the interface close to the contact line, we can approximate
$\unicode[STIX]{x1D70E}=\unicode[STIX]{x1D70E}_{clean}$
in (2.4) will not alter the characteristics for the region far away from the contact line. Also because (2.4) will be used to determine the actual dynamic contact angle due to the deformation of the almost clean part of the interface close to the contact line, we can approximate
![]() $\unicode[STIX]{x1D70E}\approx \unicode[STIX]{x1D70E}_{clean}$
in (2.4) for such an analysis, which will be implemented in §§ 3.3 and 4.2.
$\unicode[STIX]{x1D70E}\approx \unicode[STIX]{x1D70E}_{clean}$
in (2.4) for such an analysis, which will be implemented in §§ 3.3 and 4.2.
The remaining issue is to determine how
![]() $\unicode[STIX]{x1D6E4}$
distributes along the interface. Because we look at the local surfactant transport in the vicinity of the contact line and also because it occurs at a much smaller length scale than the drop’s, the associated time scale is typically much shorter than the drop spreading time scale. Hence, in the latter time scale, we can treat the surfactant transport at a quasi-steady state. This demands the net of the surface convection flux
$\unicode[STIX]{x1D6E4}$
distributes along the interface. Because we look at the local surfactant transport in the vicinity of the contact line and also because it occurs at a much smaller length scale than the drop’s, the associated time scale is typically much shorter than the drop spreading time scale. Hence, in the latter time scale, we can treat the surfactant transport at a quasi-steady state. This demands the net of the surface convection flux
![]() $u_{s}\unicode[STIX]{x1D6E4}$
to be equal to the diffusive flux
$u_{s}\unicode[STIX]{x1D6E4}$
to be equal to the diffusive flux
![]() $J_{D}$
between the interface and the bulk phase:
$J_{D}$
between the interface and the bulk phase:
where
![]() $u_{s}$
is the velocity at the interface given by
$u_{s}$
is the velocity at the interface given by
In (2.5), we assume that surface diffusion is negligible. As mentioned earlier in § 1, we model superspreading by prescribing a local surfactant leakage to set-up the required interfacial surfactant concentration gradient. So for soluble surfactant,
![]() $J_{D}$
will be specified as a local diffusive flux across the wedge to model the transfer of surfactant from the interface toward the substrate (see § 4). As for insoluble surfactant, a constant surface flux
$J_{D}$
will be specified as a local diffusive flux across the wedge to model the transfer of surfactant from the interface toward the substrate (see § 4). As for insoluble surfactant, a constant surface flux
![]() $J_{s}$
will be used to reflect the surfactant leaking effect through the contact line and the surfactant transport equation is then given by
$J_{s}$
will be used to reflect the surfactant leaking effect through the contact line and the surfactant transport equation is then given by
![]() $u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
, reduced from (2.5) with
$u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
, reduced from (2.5) with
![]() $J_{D}=0$
(see § 3).
$J_{D}=0$
(see § 3).
The main equations are (2.5) and (2.3). After substitution of (2.2), (2.4) and (2.6), we arrive at the following coupled set of equations for
![]() $h$
and
$h$
and
![]() $\unicode[STIX]{x1D6E4}$
:
$\unicode[STIX]{x1D6E4}$
:
We non-dimensionalize the above equations with
![]() $z=x/L$
,
$z=x/L$
,
![]() $H=h/\unicode[STIX]{x1D703}_{0}L$
,
$H=h/\unicode[STIX]{x1D703}_{0}L$
,
![]() $G=\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D6E4}_{0}$
,
$G=\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D6E4}_{0}$
,
![]() $j_{D}^{\prime }=J_{D}L/u_{M}\unicode[STIX]{x1D6E4}_{0}$
,
$j_{D}^{\prime }=J_{D}L/u_{M}\unicode[STIX]{x1D6E4}_{0}$
,
![]() $V=U/u_{M}$
and
$V=U/u_{M}$
and
![]() $\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}(\unicode[STIX]{x1D70E}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})$
. Here
$\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}(\unicode[STIX]{x1D70E}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})$
. Here
![]() $L$
is an appropriate length scale such that it is sufficiently long compared to the microscopic region (such as the precursor film) near the contact line, but only up to the extent that it can make connection to the macroscopic region (such as the drop) far away from the contact line. The value of
$L$
is an appropriate length scale such that it is sufficiently long compared to the microscopic region (such as the precursor film) near the contact line, but only up to the extent that it can make connection to the macroscopic region (such as the drop) far away from the contact line. The value of
![]() $u_{M}=\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}/\unicode[STIX]{x1D702}$
is the characteristic Marangoni velocity scale due to surface tension variations in the scale of
$u_{M}=\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}/\unicode[STIX]{x1D702}$
is the characteristic Marangoni velocity scale due to surface tension variations in the scale of
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}=\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
. As a result, equations (2.7) and (2.8) can be re-written in dimensionless form as
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}=\unicode[STIX]{x1D6FD}_{0}\unicode[STIX]{x1D6E4}_{0}$
. As a result, equations (2.7) and (2.8) can be re-written in dimensionless form as
Below we will use the above equations to model superspreading for both insoluble and soluble surfactants.
3 Superspreading with insoluble surfactant
3.1 Marangoni stress singularity due to weak surfactant leakage
Because superspreading generally occurs at the early stage of spreading, if the amount of surfactant exchange with the bulk is small compared to the initial amount of the surfactant on the interface, the surfactant spreader might behave like an insoluble surfactant with
![]() $j_{D}^{\prime }=0$
. Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) have shown in their simulations that superspreading can occur to insoluble surfactant through direct transfer of surfactant from the interface onto the substrate without having mass interchange with the bulk. Below we theoretically show that it is possible to realize superspreading with insoluble surfactant in principle. As will also be shown subsequently, although actual superspreaders are not insoluble, this part of the analysis is able to elicit key features of superspreading that will appear again in the more realistic situation using soluble surfactant in § 4.
$j_{D}^{\prime }=0$
. Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) have shown in their simulations that superspreading can occur to insoluble surfactant through direct transfer of surfactant from the interface onto the substrate without having mass interchange with the bulk. Below we theoretically show that it is possible to realize superspreading with insoluble surfactant in principle. As will also be shown subsequently, although actual superspreaders are not insoluble, this part of the analysis is able to elicit key features of superspreading that will appear again in the more realistic situation using soluble surfactant in § 4.
At
![]() $j_{D}^{\prime }=0$
, equation (2.9) leads to
$j_{D}^{\prime }=0$
, equation (2.9) leads to
with boundary condition
![]() $G(z\rightarrow 1)=1$
. Here
$G(z\rightarrow 1)=1$
. Here
![]() $j_{s}\equiv J_{s}/u_{M}\unicode[STIX]{x1D6E4}_{0}=J_{s}\unicode[STIX]{x1D702}/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D6E4}_{0}({>}0)$
measures the strength of surfactant leakage relative to Marangoni convection. The actual strength of the leakage flux is represented by
$j_{s}\equiv J_{s}/u_{M}\unicode[STIX]{x1D6E4}_{0}=J_{s}\unicode[STIX]{x1D702}/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D6E4}_{0}({>}0)$
measures the strength of surfactant leakage relative to Marangoni convection. The actual strength of the leakage flux is represented by
![]() $J_{s}$
, accounting for the surfactant transfer from the interface to the substrate through the contact line. This flux must be balanced by the convective surface flux along the interface:
$J_{s}$
, accounting for the surfactant transfer from the interface to the substrate through the contact line. This flux must be balanced by the convective surface flux along the interface:
![]() $u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
, the original dimensional form of (3.1) from an integration of (2.5) with
$u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
, the original dimensional form of (3.1) from an integration of (2.5) with
![]() $J_{D}=0$
. Assume that this surfactant leakage is small so that it can be sustained by a sufficiently high surfactant concentration away from the contact line. Then we can let
$J_{D}=0$
. Assume that this surfactant leakage is small so that it can be sustained by a sufficiently high surfactant concentration away from the contact line. Then we can let
![]() $-J_{s}$
be a negative constant to represent a ‘sink’ constantly drawing interfacial surfactant molecules toward the contact line. As can also be clearly seen from (3.1), to establish
$-J_{s}$
be a negative constant to represent a ‘sink’ constantly drawing interfacial surfactant molecules toward the contact line. As can also be clearly seen from (3.1), to establish
![]() $G_{z}>0$
and hence the Marangoni stress
$G_{z}>0$
and hence the Marangoni stress
![]() $\unicode[STIX]{x1D6F4}\equiv -G_{z}<0$
to aid in the contact line’s advancement, it can only be achieved by a local surfactant leak with
$\unicode[STIX]{x1D6F4}\equiv -G_{z}<0$
to aid in the contact line’s advancement, it can only be achieved by a local surfactant leak with
![]() $j_{s}>0$
(see also figure 1
a).
$j_{s}>0$
(see also figure 1
a).
However, the contact line’s advection
![]() $V$
term in (3.1) tends to sweep interfacial surfactant molecules toward the contact line. This leads to an accumulation of surfactant near the contact line and hence diminishes
$V$
term in (3.1) tends to sweep interfacial surfactant molecules toward the contact line. This leads to an accumulation of surfactant near the contact line and hence diminishes
![]() $G_{z}$
established by the
$G_{z}$
established by the
![]() $j_{s}$
term. So whether the resulting Marangoni stress can be established toward the contact line will be determined by the competition between these two terms. If the surfactant leakage is too slow
$j_{s}$
term. So whether the resulting Marangoni stress can be established toward the contact line will be determined by the competition between these two terms. If the surfactant leakage is too slow
![]() $(j_{s}\ll V/2)$
, equation (3.1) reduces to
$(j_{s}\ll V/2)$
, equation (3.1) reduces to
![]() $-G_{z}=2V/H$
, making
$-G_{z}=2V/H$
, making
![]() $\unicode[STIX]{x1D6F4}>0$
oppose the contact line motion. Obviously, it is impossible to have superspreading in this case.
$\unicode[STIX]{x1D6F4}>0$
oppose the contact line motion. Obviously, it is impossible to have superspreading in this case.
A similar competition between surfactant depletion and contact line sweeping has been shown previously by Joanny (Reference Joanny1989) and Karapetsas et al. (Reference Karapetsas, Craster and Matar2011). In particular, Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) showed in their simulations that the drop spreading rate can be promoted at a sufficiently high
![]() $Pe$
but not too high (see their figure 5), where
$Pe$
but not too high (see their figure 5), where
![]() $Pe$
is the Péclet number measuring the strength of surface convection relative to surface diffusion in the interfacial surfactant transport. Requiring a large enough
$Pe$
is the Péclet number measuring the strength of surface convection relative to surface diffusion in the interfacial surfactant transport. Requiring a large enough
![]() $Pe$
to promote spreading by Marangoni stress is simply because a too small
$Pe$
to promote spreading by Marangoni stress is simply because a too small
![]() $Pe$
will smooth out the surfactant concentration gradient by diffusion. On the other hand, if
$Pe$
will smooth out the surfactant concentration gradient by diffusion. On the other hand, if
![]() $Pe$
gets too high, strong contact line sweeping will make surfactant accumulate at the contact line, which will slow down the spreading unless surfactant depletion is fast enough to reverse the trend.
$Pe$
gets too high, strong contact line sweeping will make surfactant accumulate at the contact line, which will slow down the spreading unless surfactant depletion is fast enough to reverse the trend.
In fact, to achieve superspreading,
![]() $j_{s}>V/2$
is necessary for preventing surfactant accumulation at the contact line. If the surfactant leakage is fast (
$j_{s}>V/2$
is necessary for preventing surfactant accumulation at the contact line. If the surfactant leakage is fast (
![]() $j_{s}\gg V/2$
), equation (3.1) reduces to
$j_{s}\gg V/2$
), equation (3.1) reduces to
To determine
![]() $G$
from (3.2), we use Voinov’s approximation (Voniov Reference Voniov1976; Snoeijer Reference Snoeijer2006) by assuming the wedge shape
$G$
from (3.2), we use Voinov’s approximation (Voniov Reference Voniov1976; Snoeijer Reference Snoeijer2006) by assuming the wedge shape
![]() $H$
to be a slowly varying function with respect to the linear one:
$H$
to be a slowly varying function with respect to the linear one:
where
![]() $\unicode[STIX]{x1D6E9}\equiv \unicode[STIX]{x1D703}/\unicode[STIX]{x1D703}_{0}$
measures the departure of the point slope
$\unicode[STIX]{x1D6E9}\equiv \unicode[STIX]{x1D703}/\unicode[STIX]{x1D703}_{0}$
measures the departure of the point slope
![]() $\unicode[STIX]{x1D703}=h/x$
from
$\unicode[STIX]{x1D703}=h/x$
from
![]() $\unicode[STIX]{x1D703}_{0}$
and
$\unicode[STIX]{x1D703}_{0}$
and
![]() $|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
. Because
$|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
. Because
![]() $\unicode[STIX]{x1D6E9}$
varies slowly with
$\unicode[STIX]{x1D6E9}$
varies slowly with
![]() $z$
,
$z$
,
![]() $G$
can be determined by a straightforward integration of (3.2):
$G$
can be determined by a straightforward integration of (3.2):
This indicates that
![]() $G$
increases with the distance
$G$
increases with the distance
![]() $z$
to the contact line until it reaches the far-field value at
$z$
to the contact line until it reaches the far-field value at
![]() $z=1$
(i.e. reaching the scale of the macroscopic length
$z=1$
(i.e. reaching the scale of the macroscopic length
![]() $L$
where the interfacial surfactant concentration is nearly constant
$L$
where the interfacial surfactant concentration is nearly constant
![]() $\unicode[STIX]{x1D6E4}_{0}$
). Since surfactant molecules are constantly leaking out through the contact line due to the leakage flux
$\unicode[STIX]{x1D6E4}_{0}$
). Since surfactant molecules are constantly leaking out through the contact line due to the leakage flux
![]() $j_{s}$
,
$j_{s}$
,
![]() $G$
has to decrease on approaching the contact line (i.e. decreasing
$G$
has to decrease on approaching the contact line (i.e. decreasing
![]() $z$
). It is clear that the larger
$z$
). It is clear that the larger
![]() $j_{s}$
is, the stronger the leakage and hence the greater the reduction of
$j_{s}$
is, the stronger the leakage and hence the greater the reduction of
![]() $G$
in the direction toward the contact line. As also indicated by (3.4), since
$G$
in the direction toward the contact line. As also indicated by (3.4), since
![]() $G$
starts from a constant level away from the contact line, as its decline continues toward the contact line, at some point
$G$
starts from a constant level away from the contact line, as its decline continues toward the contact line, at some point
![]() $G$
must eventually vanish. We call this point the complete depletion point at which
$G$
must eventually vanish. We call this point the complete depletion point at which
![]() $G(z^{\ast })=0$
:
$G(z^{\ast })=0$
:
with
![]() $\unicode[STIX]{x1D6E9}^{\ast }\equiv \unicode[STIX]{x1D6E9}(z^{\ast })$
. Equation (3.5) indicates that
$\unicode[STIX]{x1D6E9}^{\ast }\equiv \unicode[STIX]{x1D6E9}(z^{\ast })$
. Equation (3.5) indicates that
![]() $z^{\ast }\rightarrow 0$
as
$z^{\ast }\rightarrow 0$
as
![]() $j_{s}\rightarrow 0$
, meaning that for a small value of
$j_{s}\rightarrow 0$
, meaning that for a small value of
![]() $j_{s}$
there must exist a small surfactant-free zone of size
$j_{s}$
there must exist a small surfactant-free zone of size
![]() $z^{\ast }$
.
$z^{\ast }$
.
Equation (3.4) yields the Marangoni stress as
As a result,
![]() $\unicode[STIX]{x1D6F4}$
will be acting toward the contact line and thereby promote the contact line advancement. Note that both (3.4) and (3.6) only hold up to the critical point
$\unicode[STIX]{x1D6F4}$
will be acting toward the contact line and thereby promote the contact line advancement. Note that both (3.4) and (3.6) only hold up to the critical point
![]() $z^{\ast }$
given by (3.5). Below this point, surfactant molecules are completely emptied out by the leak and hence the Marangoni stress vanishes. As also indicated by (3.5), the surfactant-free zone of size
$z^{\ast }$
given by (3.5). Below this point, surfactant molecules are completely emptied out by the leak and hence the Marangoni stress vanishes. As also indicated by (3.5), the surfactant-free zone of size
![]() $z^{\ast }$
will shrink very rapidly on lowering
$z^{\ast }$
will shrink very rapidly on lowering
![]() $j_{s}$
. In other words,
$j_{s}$
. In other words,
![]() $G$
with small
$G$
with small
![]() $j_{s}$
will exhibit a sharp decline near the contact line.
$j_{s}$
will exhibit a sharp decline near the contact line.
Now we inspect the behaviour of
![]() $G$
and
$G$
and
![]() $\unicode[STIX]{x1D6F4}$
given by (3.4)–(3.6). If the surfactant leakage in (3.1) is not too fast such that
$\unicode[STIX]{x1D6F4}$
given by (3.4)–(3.6). If the surfactant leakage in (3.1) is not too fast such that
the surfactant-free zone will be small, i.e.
![]() $z^{\ast }\ll 1$
. For
$z^{\ast }\ll 1$
. For
![]() $z$
sufficiently far from
$z$
sufficiently far from
![]() $z^{\ast }$
, the surfactant concentration varies slowly with
$z^{\ast }$
, the surfactant concentration varies slowly with
![]() $z$
as
$z$
as
![]() $G\approx 1+4j_{s}\times \ln (z)$
(with
$G\approx 1+4j_{s}\times \ln (z)$
(with
![]() $\unicode[STIX]{x1D6E9}\approx 1$
in (3.4)), and hence the corresponding Marangoni stress behaves as
$\unicode[STIX]{x1D6E9}\approx 1$
in (3.4)), and hence the corresponding Marangoni stress behaves as
![]() $\unicode[STIX]{x1D6F4}\approx -4j_{s}\times 1/z$
. However, for
$\unicode[STIX]{x1D6F4}\approx -4j_{s}\times 1/z$
. However, for
![]() $z$
close to
$z$
close to
![]() $z^{\ast }$
,
$z^{\ast }$
,
![]() $G\approx (1-\ln (z)/\ln (z^{\ast }))^{1/2}$
(with
$G\approx (1-\ln (z)/\ln (z^{\ast }))^{1/2}$
(with
![]() $\unicode[STIX]{x1D6E9}\approx \unicode[STIX]{x1D6E9}^{\ast }$
in (3.4) together with (3.5)). This yields
$\unicode[STIX]{x1D6E9}\approx \unicode[STIX]{x1D6E9}^{\ast }$
in (3.4) together with (3.5)). This yields
![]() $\unicode[STIX]{x1D6F4}\approx (1/2\ln (z^{\ast }))\times 1/z\times (1-\ln (z)/\ln (z^{\ast }))^{-1/2}$
, diverging much faster than the usual viscous stress singularity
$\unicode[STIX]{x1D6F4}\approx (1/2\ln (z^{\ast }))\times 1/z\times (1-\ln (z)/\ln (z^{\ast }))^{-1/2}$
, diverging much faster than the usual viscous stress singularity
![]() $1/z$
(Huh & Scriven Reference Huh and Scriven1971). As will be shown later, such Marangoni stress singularity will call for a new contact line structure that can account for the linear spreading law.
$1/z$
(Huh & Scriven Reference Huh and Scriven1971). As will be shown later, such Marangoni stress singularity will call for a new contact line structure that can account for the linear spreading law.
To confirm the features discussed above, we also solve (3.1) to determine the actual profiles of
![]() $G$
and
$G$
and
![]() $\unicode[STIX]{x1D6F4}$
. To better illuminate the singular nature for both
$\unicode[STIX]{x1D6F4}$
. To better illuminate the singular nature for both
![]() $G$
and
$G$
and
![]() $\unicode[STIX]{x1D6F4}$
, we look at the outer region by taking
$\unicode[STIX]{x1D6F4}$
, we look at the outer region by taking
![]() $H=z$
. This admits an analytical solution:
$H=z$
. This admits an analytical solution:
Figure 2 plots both
![]() $G$
and
$G$
and
![]() $\unicode[STIX]{x1D6F4}$
profiles calculated from (3.8) at a fixed value of
$\unicode[STIX]{x1D6F4}$
profiles calculated from (3.8) at a fixed value of
![]() $V$
with various values of
$V$
with various values of
![]() $j_{s}$
under the constraint (3.7). We also plot the asymptotic results (3.4) and (3.6) (with
$j_{s}$
under the constraint (3.7). We also plot the asymptotic results (3.4) and (3.6) (with
![]() $\unicode[STIX]{x1D6E9}=1$
), showing an excellent agreement with those given by (3.8). As shown in figure 2(a), for a given value of
$\unicode[STIX]{x1D6E9}=1$
), showing an excellent agreement with those given by (3.8). As shown in figure 2(a), for a given value of
![]() $j_{s}$
,
$j_{s}$
,
![]() $G$
declines as the distance
$G$
declines as the distance
![]() $z$
to the contact line decreases. Notice that
$z$
to the contact line decreases. Notice that
![]() $G$
vanishes at some particular point, which exactly corresponds to the complete depletion point
$G$
vanishes at some particular point, which exactly corresponds to the complete depletion point
![]() $z^{\ast }$
– the lower
$z^{\ast }$
– the lower
![]() $j_{s}$
the smaller
$j_{s}$
the smaller
![]() $z^{\ast }$
in accordance with (3.5). On approaching
$z^{\ast }$
in accordance with (3.5). On approaching
![]() $z^{\ast }$
, such a decline in
$z^{\ast }$
, such a decline in
![]() $G$
gets sharper. For a very little surfactant leakage having
$G$
gets sharper. For a very little surfactant leakage having
![]() $j_{s}=$
0.01 or lower,
$j_{s}=$
0.01 or lower,
![]() $G$
shows merely a small decrease from the equilibrium value (i.e.
$G$
shows merely a small decrease from the equilibrium value (i.e.
![]() $G=1$
) away from the contact line. But near the contact line, because the influence of the leak becomes perceivable, a small depletion region has to form in response to the leak, showing a very sharp decrease in
$G=1$
) away from the contact line. But near the contact line, because the influence of the leak becomes perceivable, a small depletion region has to form in response to the leak, showing a very sharp decrease in
![]() $G$
. Such a boundary-layer-like surfactant concentration profile also resembles the simulation result obtained by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) for an insoluble surfactant (see their figure 3a). As for
$G$
. Such a boundary-layer-like surfactant concentration profile also resembles the simulation result obtained by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) for an insoluble surfactant (see their figure 3a). As for
![]() $\unicode[STIX]{x1D6F4}$
, its magnitude is increased as
$\unicode[STIX]{x1D6F4}$
, its magnitude is increased as
![]() $z$
is decreased, and rises more sharply on approaching
$z$
is decreased, and rises more sharply on approaching
![]() $z^{\ast }$
, as displayed in figure 2(b). The value of
$z^{\ast }$
, as displayed in figure 2(b). The value of
![]() $\unicode[STIX]{x1D6F4}$
blows up as
$\unicode[STIX]{x1D6F4}$
blows up as
![]() $z\rightarrow z^{\ast }$
, signifying a Marangoni stress singularity. All these features confirm those given by (3.4)–(3.6).
$z\rightarrow z^{\ast }$
, signifying a Marangoni stress singularity. All these features confirm those given by (3.4)–(3.6).

Figure 2. (a) Calculated surfactant distribution
![]() $G$
for insoluble superspreader with various values of surfactant leakage flux
$G$
for insoluble superspreader with various values of surfactant leakage flux
![]() $j_{s}$
at
$j_{s}$
at
![]() $V=0.001$
. Lines: exact solution equation (3.8). Symbols: approximate solution equation (3.4). (b) Shows the corresponding Marangoni stress
$V=0.001$
. Lines: exact solution equation (3.8). Symbols: approximate solution equation (3.4). (b) Shows the corresponding Marangoni stress
![]() $\unicode[STIX]{x1D6F4}=-G_{z}$
whose exact and approximate values are evaluated using (3.1) and (3.6) respectively. All the results are calculated at
$\unicode[STIX]{x1D6F4}=-G_{z}$
whose exact and approximate values are evaluated using (3.1) and (3.6) respectively. All the results are calculated at
![]() $H=z$
.
$H=z$
.
![]() $G$
vanishes at the depletion point
$G$
vanishes at the depletion point
![]() $z^{\ast }$
near which rapid decline in
$z^{\ast }$
near which rapid decline in
![]() $G$
and divergence in
$G$
and divergence in
![]() $\!\unicode[STIX]{x1D6F4}$
are evident.
$\!\unicode[STIX]{x1D6F4}$
are evident.

Figure 3. Schematic illustration of the interface profile and flow field near the contact line. Owing to surfactant leakage near the contact line, there exists a complete depletion point
![]() $z^{\ast }$
below which there is no surfactant on the interface. The Marangoni shearing toward the contact line can lead to a pressure build-up, bending the interface into a microscopic capillary nose with dynamic contact angle
$z^{\ast }$
below which there is no surfactant on the interface. The Marangoni shearing toward the contact line can lead to a pressure build-up, bending the interface into a microscopic capillary nose with dynamic contact angle
![]() $\unicode[STIX]{x1D703}^{\ast }$
greater than the apparent dynamic contact angle
$\unicode[STIX]{x1D703}^{\ast }$
greater than the apparent dynamic contact angle
![]() $\unicode[STIX]{x1D703}_{0}$
.
$\unicode[STIX]{x1D703}_{0}$
.
3.2 Emergence of a new contact line structure
As indicated by (3.6), for small
![]() $j_{s}$
, a Marangoni stress singularity can exist on approaching the contact line. Because it diverges at a much faster rate than the usual viscous stress singularity
$j_{s}$
, a Marangoni stress singularity can exist on approaching the contact line. Because it diverges at a much faster rate than the usual viscous stress singularity
![]() $1/z$
, there is no way to dissipate it by the viscous force exerted by the substrate through the movement of the contact line (as it is derived under
$1/z$
, there is no way to dissipate it by the viscous force exerted by the substrate through the movement of the contact line (as it is derived under
![]() $j_{s}\gg V/2$
due to (3.7)). It thus follows that the dissipation of the Marangoni forcing has to be sought at the microscopic level at the scale comparable to or below
$j_{s}\gg V/2$
due to (3.7)). It thus follows that the dissipation of the Marangoni forcing has to be sought at the microscopic level at the scale comparable to or below
![]() $z^{\ast }$
, implying that a new contact line structure must emerge, which is explained as follows.
$z^{\ast }$
, implying that a new contact line structure must emerge, which is explained as follows.
According to (3.6), an intensified Marangoni stress
![]() $\unicode[STIX]{x1D6F4}=-G_{z}<0$
is shearing the interface in the direction toward the contact line. From (2.10), this shearing will lead pressure
$\unicode[STIX]{x1D6F4}=-G_{z}<0$
is shearing the interface in the direction toward the contact line. From (2.10), this shearing will lead pressure
![]() $P=-\unicode[STIX]{x1D6FE}H_{zz}$
to build up in the direction toward the contact line, i.e.
$P=-\unicode[STIX]{x1D6FE}H_{zz}$
to build up in the direction toward the contact line, i.e.
![]() $P_{z}>0$
. The mounting pressure near the contact line will in turn bend the air–liquid interface into a capillary nose (see figure 3), making the actual contact angle
$P_{z}>0$
. The mounting pressure near the contact line will in turn bend the air–liquid interface into a capillary nose (see figure 3), making the actual contact angle
![]() $\unicode[STIX]{x1D703}_{actual}$
greater than
$\unicode[STIX]{x1D703}_{actual}$
greater than
![]() $\unicode[STIX]{x1D703}_{0}$
. Such bending of the interface with an increased dynamic contact angle is consistent with the small ridge at the moving front observed in the simulation study by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) (see their figure 3a). Since it is the surfactant leakage responsible for
$\unicode[STIX]{x1D703}_{0}$
. Such bending of the interface with an increased dynamic contact angle is consistent with the small ridge at the moving front observed in the simulation study by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) (see their figure 3a). Since it is the surfactant leakage responsible for
![]() $\unicode[STIX]{x1D6F4}<0$
that bends the interface,
$\unicode[STIX]{x1D6F4}<0$
that bends the interface,
![]() $\unicode[STIX]{x1D703}_{actual}$
should mainly vary with
$\unicode[STIX]{x1D703}_{actual}$
should mainly vary with
![]() $j_{s}$
, regardless of the contact line speed
$j_{s}$
, regardless of the contact line speed
![]() $U$
as long as (3.7) is satisfied. Apparently, the behaviour of
$U$
as long as (3.7) is satisfied. Apparently, the behaviour of
![]() $\unicode[STIX]{x1D703}_{actual}$
cannot be described by the classical clean-interface de Gennes–Cox–Voinov law that links the apparent dynamic contact angle to
$\unicode[STIX]{x1D703}_{actual}$
cannot be described by the classical clean-interface de Gennes–Cox–Voinov law that links the apparent dynamic contact angle to
![]() $U$
(Voniov Reference Voniov1976; de Gennes Reference de Gennes1985; Cox Reference Cox1986a
). Instead, a new wetting law must emerge to govern how
$U$
(Voniov Reference Voniov1976; de Gennes Reference de Gennes1985; Cox Reference Cox1986a
). Instead, a new wetting law must emerge to govern how
![]() $\unicode[STIX]{x1D703}_{actual}$
varies with
$\unicode[STIX]{x1D703}_{actual}$
varies with
![]() $j_{s}$
. Because of surfactant leakage and Marangoni stress, the physics involved in determining this new law will be very different from those in deriving the de Gennes–Cox–Voinov law. So we anticipate that the corresponding contact line structure will also be distinct from the latter’s.
$j_{s}$
. Because of surfactant leakage and Marangoni stress, the physics involved in determining this new law will be very different from those in deriving the de Gennes–Cox–Voinov law. So we anticipate that the corresponding contact line structure will also be distinct from the latter’s.
In fact, because the interface becomes clean below
![]() $x^{\ast }(=z^{\ast }L\ll L)$
, the strong Marangoni shearing will transform into an enormous capillary pressure and this pressure will act as an additional wetting force to drive the contact line. The situation is quite distinct from the usual de Gennes–Tanner capillary wetting that occurs in a somewhat macroscopic sense over the outer length scale
$x^{\ast }(=z^{\ast }L\ll L)$
, the strong Marangoni shearing will transform into an enormous capillary pressure and this pressure will act as an additional wetting force to drive the contact line. The situation is quite distinct from the usual de Gennes–Tanner capillary wetting that occurs in a somewhat macroscopic sense over the outer length scale
![]() $L$
(de Gennes Reference de Gennes1985). In contrast, such capillary wetting takes place in a microscopic sense at the length scale
$L$
(de Gennes Reference de Gennes1985). In contrast, such capillary wetting takes place in a microscopic sense at the length scale
![]() $x^{\ast }$
. More importantly, it is not purely driven by surface tension but by the Marangoni shearing set-up by the surfactant leakage near the contact line. It is this local Marangoni-driven capillary wetting responsible for constant wetting speed and hence the linear spreading law seen in superspreading.
$x^{\ast }$
. More importantly, it is not purely driven by surface tension but by the Marangoni shearing set-up by the surfactant leakage near the contact line. It is this local Marangoni-driven capillary wetting responsible for constant wetting speed and hence the linear spreading law seen in superspreading.
We should stress that the peculiar features mentioned above are the propositions that closely follow the two analytical results derived from our theory under the ‘little but fast’ leak condition (3.7): (i) the complete depletion point
![]() $z^{\ast }$
given by (3.5), and (ii) the Marangoni stress singularity at
$z^{\ast }$
given by (3.5), and (ii) the Marangoni stress singularity at
![]() $z^{\ast }$
in (3.6). Although somewhat similar views can be drawn from the simulations by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011), here we will provide a rigorous proof for these propositions, which is given next.
$z^{\ast }$
in (3.6). Although somewhat similar views can be drawn from the simulations by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011), here we will provide a rigorous proof for these propositions, which is given next.
3.3 Marangoni-driven capillary wetting: a new dynamic contact angle relationship
To determine the actual contact line speed caused by the Marangoni-driven capillary wetting described above, we need to establish the corresponding dynamic contact angle relationship. Hence we consider (2.10) by neglecting the
![]() $V$
term under (3.7). Further combining (3.2), we arrive at
$V$
term under (3.7). Further combining (3.2), we arrive at
Note here that the surface tension parameter
![]() $\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
is taken to correspond to the clean interface’s tension
$\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
is taken to correspond to the clean interface’s tension
![]() $\unicode[STIX]{x1D70E}_{clean}$
. This is because we want to determine the microscopic contact angle as
$\unicode[STIX]{x1D70E}_{clean}$
. This is because we want to determine the microscopic contact angle as
![]() $z$
approaches the complete depletion point
$z$
approaches the complete depletion point
![]() $z^{\ast }$
, the pressure has to match that at
$z^{\ast }$
, the pressure has to match that at
![]() $z^{\ast }$
. Substituting (3.4) into (3.9) and writing
$z^{\ast }$
. Substituting (3.4) into (3.9) and writing
![]() $H$
in terms of
$H$
in terms of
![]() $\unicode[STIX]{x1D6E9}$
using (3.3), (3.9) becomes
$\unicode[STIX]{x1D6E9}$
using (3.3), (3.9) becomes
For the left-hand side of (3.10), we have used Voinov’s approximation
![]() $H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
from
$H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
from
![]() $H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
.
$H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
.
Equation (3.10) will be used to determine the actual contact angle
![]() $\unicode[STIX]{x1D703}_{actual}$
. Because
$\unicode[STIX]{x1D703}_{actual}$
. Because
![]() $z^{\ast }$
is small,
$z^{\ast }$
is small,
![]() $\unicode[STIX]{x1D703}_{actual}$
can be roughly represented by
$\unicode[STIX]{x1D703}_{actual}$
can be roughly represented by
![]() $\unicode[STIX]{x1D703}(z^{\ast })$
. Therefore, the purpose of solving (3.10) is to find
$\unicode[STIX]{x1D703}(z^{\ast })$
. Therefore, the purpose of solving (3.10) is to find
![]() $\unicode[STIX]{x1D6E9}(z^{\ast })$
. Following the approach to solving the Bretherton equation for deriving the de Gennes–Cox–Voinov wetting law (see appendix A, § A.1), equation (3.10) can be solved approximately using Voinov’s method (see appendix A, § A.2). Integrating both the left- and right-hand sides of (3.10) respectively and making use of the fact that
$\unicode[STIX]{x1D6E9}(z^{\ast })$
. Following the approach to solving the Bretherton equation for deriving the de Gennes–Cox–Voinov wetting law (see appendix A, § A.1), equation (3.10) can be solved approximately using Voinov’s method (see appendix A, § A.2). Integrating both the left- and right-hand sides of (3.10) respectively and making use of the fact that
![]() $\int _{1}^{z}\unicode[STIX]{x1D6E9}^{2}\unicode[STIX]{x1D6E9}_{zz}\,\text{d}z\approx \unicode[STIX]{x1D6E9}^{2}\unicode[STIX]{x1D6E9}_{z}$
(see (A 3) in appendix A), equation (3.10) becomes
$\int _{1}^{z}\unicode[STIX]{x1D6E9}^{2}\unicode[STIX]{x1D6E9}_{zz}\,\text{d}z\approx \unicode[STIX]{x1D6E9}^{2}\unicode[STIX]{x1D6E9}_{z}$
(see (A 3) in appendix A), equation (3.10) becomes
where
![]() $\unicode[STIX]{x1D719}(z)$
is the integral below (obtained by (A 11) in appendix A):
$\unicode[STIX]{x1D719}(z)$
is the integral below (obtained by (A 11) in appendix A):
Integrating (3.11) from
![]() $z=1$
from
$z=1$
from
![]() $z=z^{\ast }$
and recognizing
$z=z^{\ast }$
and recognizing
![]() $\unicode[STIX]{x1D6E9}(z=1)=1$
and
$\unicode[STIX]{x1D6E9}(z=1)=1$
and
![]() $\int _{1}^{z^{\ast }}\ln (z)\,\text{d}z\approx 1$
for small
$\int _{1}^{z^{\ast }}\ln (z)\,\text{d}z\approx 1$
for small
![]() $z^{\ast }$
, we obtain
$z^{\ast }$
, we obtain
With
![]() $\unicode[STIX]{x1D6E9}(z^{\ast })=\unicode[STIX]{x1D703}^{\ast }/\unicode[STIX]{x1D703}_{0}$
,
$\unicode[STIX]{x1D6E9}(z^{\ast })=\unicode[STIX]{x1D703}^{\ast }/\unicode[STIX]{x1D703}_{0}$
,
![]() $\unicode[STIX]{x1D6FE}=(\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})\unicode[STIX]{x1D703}_{0}^{2}$
and
$\unicode[STIX]{x1D6FE}=(\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})\unicode[STIX]{x1D703}_{0}^{2}$
and
![]() $j_{s}=J_{s}\unicode[STIX]{x1D702}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0}$
, the slope
$j_{s}=J_{s}\unicode[STIX]{x1D702}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0}$
, the slope
![]() $\unicode[STIX]{x1D703}^{\ast }$
at
$\unicode[STIX]{x1D703}^{\ast }$
at
![]() $z^{\ast }(\ll 1)$
, which can represent the actual contact angle
$z^{\ast }(\ll 1)$
, which can represent the actual contact angle
![]() $\unicode[STIX]{x1D703}_{actual}$
, can be determined as
$\unicode[STIX]{x1D703}_{actual}$
, can be determined as
Here, instead of the usual capillary number
![]() $Ca=\unicode[STIX]{x1D702}U/\unicode[STIX]{x1D70E}_{clean}$
, equation (3.14) is characterized by the modified capillary number based on the speed of surfactant leakage
$Ca=\unicode[STIX]{x1D702}U/\unicode[STIX]{x1D70E}_{clean}$
, equation (3.14) is characterized by the modified capillary number based on the speed of surfactant leakage
![]() $J_{s}/\unicode[STIX]{x1D6E4}_{0}$
,
$J_{s}/\unicode[STIX]{x1D6E4}_{0}$
,
Somewhat surprisingly, equation (3.14) looks quite similar to the well-known de Gennes–Cox–Voinov law (see (A 9) in appendix A). As
![]() $\unicode[STIX]{x1D703}^{\ast }$
in fact measures the slope of the ‘capillary nose’ which is surfactant free, it should match the clean-interface contact angle described by the de Gennes–Cox–Voinov law (see (A 9) in appendix A):
$\unicode[STIX]{x1D703}^{\ast }$
in fact measures the slope of the ‘capillary nose’ which is surfactant free, it should match the clean-interface contact angle described by the de Gennes–Cox–Voinov law (see (A 9) in appendix A):
Here the length scale ratio in the logarithmic term is taken as the length
![]() $z^{\ast }L$
of the surfactant-free zone (with
$z^{\ast }L$
of the surfactant-free zone (with
![]() $z^{\ast }$
given by (3.5)) to a microscopic cutoff length
$z^{\ast }$
given by (3.5)) to a microscopic cutoff length
![]() $\ell (\ll z^{\ast }L)$
or an inner length scale (due, for instance, to the precursor film ahead of the capillary nose or to wall slip). The associated microscopic contact angle
$\ell (\ll z^{\ast }L)$
or an inner length scale (due, for instance, to the precursor film ahead of the capillary nose or to wall slip). The associated microscopic contact angle
![]() $\unicode[STIX]{x1D703}(\ell )$
is assumed negligible here. Matching
$\unicode[STIX]{x1D703}(\ell )$
is assumed negligible here. Matching
![]() $\unicode[STIX]{x1D703}^{\ast }$
given by (3.14) to
$\unicode[STIX]{x1D703}^{\ast }$
given by (3.14) to
![]() $\unicode[STIX]{x1D703}_{clean}$
given by (3.16), we can determine the contact line speed as
$\unicode[STIX]{x1D703}_{clean}$
given by (3.16), we can determine the contact line speed as
wherein
![]() $\ln (z^{\ast })=-(1+18\unicode[STIX]{x1D6FE}^{-1}j_{s})^{1/3}/8j_{s}$
is a result of combining (3.5) and (3.13). For
$\ln (z^{\ast })=-(1+18\unicode[STIX]{x1D6FE}^{-1}j_{s})^{1/3}/8j_{s}$
is a result of combining (3.5) and (3.13). For
![]() $18\unicode[STIX]{x1D6FE}^{-1}j_{s}\sim O(1)$
or smaller (i.e.
$18\unicode[STIX]{x1D6FE}^{-1}j_{s}\sim O(1)$
or smaller (i.e.
![]() $18Ca_{1}\sim O(\unicode[STIX]{x1D703}_{0}^{3})$
or smaller),
$18Ca_{1}\sim O(\unicode[STIX]{x1D703}_{0}^{3})$
or smaller),
![]() $U$
scales as the capillary velocity
$U$
scales as the capillary velocity
![]() $\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}$
, just like that of capillary wetting. Notice that during the spreading
$\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}$
, just like that of capillary wetting. Notice that during the spreading
![]() $\unicode[STIX]{x1D703}_{0}$
is gradually decreasing whereas the surfactant leakage contribution
$\unicode[STIX]{x1D703}_{0}$
is gradually decreasing whereas the surfactant leakage contribution
![]() $18Ca_{1}$
in (3.14) is kept constant. Because the ratio of the latter to
$18Ca_{1}$
in (3.14) is kept constant. Because the ratio of the latter to
![]() $\unicode[STIX]{x1D703}_{0}^{3}$
in (3.14) varies as
$\unicode[STIX]{x1D703}_{0}^{3}$
in (3.14) varies as
![]() $\unicode[STIX]{x1D703}_{0}^{-3}$
, the contribution from surfactant leakage
$\unicode[STIX]{x1D703}_{0}^{-3}$
, the contribution from surfactant leakage
![]() $18Ca_{1}$
to
$18Ca_{1}$
to
![]() $\unicode[STIX]{x1D703}^{\ast }$
would become increasingly important during the spreading. In other words, as long as
$\unicode[STIX]{x1D703}^{\ast }$
would become increasingly important during the spreading. In other words, as long as
![]() $18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
is not too small compared to unity,
$18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
is not too small compared to unity,
![]() $\unicode[STIX]{x1D703}^{\ast }$
might still be dominated by
$\unicode[STIX]{x1D703}^{\ast }$
might still be dominated by
![]() $18Ca_{1}$
from the surfactant leakage and hence might not change significantly during spreading. In fact, if
$18Ca_{1}$
from the surfactant leakage and hence might not change significantly during spreading. In fact, if
![]() $18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
is too small, the situation will reduce to the usual capillary wetting and there will be no superspreading at all, making (3.17) no longer applicable.
$18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
is too small, the situation will reduce to the usual capillary wetting and there will be no superspreading at all, making (3.17) no longer applicable.
On the other hand, if surfactant leakage is sufficiently strong such that
![]() $18\unicode[STIX]{x1D6FE}^{-1}j_{s}\gg 1$
(i.e.
$18\unicode[STIX]{x1D6FE}^{-1}j_{s}\gg 1$
(i.e.
![]() $18Ca_{1}\gg \unicode[STIX]{x1D703}_{0}^{3}$
), not only does the contact angle
$18Ca_{1}\gg \unicode[STIX]{x1D703}_{0}^{3}$
), not only does the contact angle
![]() $\unicode[STIX]{x1D703}^{\ast }\approx (8Ca_{1})^{1/3}$
become independent of
$\unicode[STIX]{x1D703}^{\ast }\approx (8Ca_{1})^{1/3}$
become independent of
![]() $\unicode[STIX]{x1D703}_{0}$
in (3.14), but also the contact line speed (3.17) reduces to
$\unicode[STIX]{x1D703}_{0}$
in (3.14), but also the contact line speed (3.17) reduces to
It is essentially the speed of surfactant leakage
![]() $J_{s}/\unicode[STIX]{x1D6E4}_{0}$
which is constant and independent of
$J_{s}/\unicode[STIX]{x1D6E4}_{0}$
which is constant and independent of
![]() $\unicode[STIX]{x1D703}_{0}$
. This speed comes from the surface flow set-up by the surfactant leakage near the contact line through
$\unicode[STIX]{x1D703}_{0}$
. This speed comes from the surface flow set-up by the surfactant leakage near the contact line through
![]() $u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
which gives
$u_{s}\unicode[STIX]{x1D6E4}=-J_{s}$
which gives
![]() $u_{s}\sim -J_{s}/\unicode[STIX]{x1D6E4}_{0}$
. This surface flow is sustained by the corresponding Marangoni shearing working with surface tension to push the contact line forward, making the contact line advance at a constant speed given by (3.18). Together with the fact that
$u_{s}\sim -J_{s}/\unicode[STIX]{x1D6E4}_{0}$
. This surface flow is sustained by the corresponding Marangoni shearing working with surface tension to push the contact line forward, making the contact line advance at a constant speed given by (3.18). Together with the fact that
![]() $U$
with not too small
$U$
with not too small
![]() $18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
also does not change significantly during spreading,
$18\unicode[STIX]{x1D6FE}^{-1}j_{s}$
also does not change significantly during spreading,
![]() $U$
given by (3.17) can be deemed as a constant, thereby explaining the linear spreading law seen in superspreading.
$U$
given by (3.17) can be deemed as a constant, thereby explaining the linear spreading law seen in superspreading.
4 Superspreading with soluble surfactant
4.1 Surfactant leakage due to corner diffusion and corresponding Marangoni shearing
Having analysed superspreading with an insoluble surfactant, we now move on to soluble surfactant which is more relevant to superspreading. A similar contact line structure can also form due to surfactant leakage around the contact line. But unlike direct surfactant transfer from the interface onto the substrate for insoluble surfactant, the surfactant transport (2.5) is driven by a bulk surfactant concentration gradient resulting from desorption of surfactant from the interface to the substrate (see figure 1
b). This process establishes a corner diffusion flux
![]() $J_{D}={\mathcal{D}}(C_{1}-C_{2})/h$
from the surfactant-rich interface at concentration
$J_{D}={\mathcal{D}}(C_{1}-C_{2})/h$
from the surfactant-rich interface at concentration
![]() $C_{1}$
toward the surfactant-poor substrate at concentration
$C_{1}$
toward the surfactant-poor substrate at concentration
![]() $C_{2}({<}C_{1})$
, where
$C_{2}({<}C_{1})$
, where
![]() ${\mathcal{D}}$
is the bulk diffusivity of surfactant. In the dimensionless form, this flux can be re-written as
${\mathcal{D}}$
is the bulk diffusivity of surfactant. In the dimensionless form, this flux can be re-written as
![]() $j_{D}^{\prime }=j_{D}/H$
, where
$j_{D}^{\prime }=j_{D}/H$
, where
![]() $j_{D}=({\mathcal{D}}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0})/u_{M}=\unicode[STIX]{x1D702}{\mathcal{D}}(\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D703}_{0}^{2}$
measures the strength of the corner diffusion flux relative to Marangoni convection and
$j_{D}=({\mathcal{D}}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0})/u_{M}=\unicode[STIX]{x1D702}{\mathcal{D}}(\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\unicode[STIX]{x1D703}_{0}^{2}$
measures the strength of the corner diffusion flux relative to Marangoni convection and
![]() $\unicode[STIX]{x0394}C=C_{1}$
–
$\unicode[STIX]{x0394}C=C_{1}$
–
![]() $C_{2}$
. Hence (2.9) becomes
$C_{2}$
. Hence (2.9) becomes
We again focus on the little but fast leak scenario:
where Marangoni shearing toward the contact line can be established by a weak surfactant leakage without being mitigated by the contact line’s sweeping. Under this condition, equation (4.1) reduces to
Using Voinov’s approximation (3.3):
![]() $H=z\unicode[STIX]{x1D6E9}$
modulated by a slowly varying function
$H=z\unicode[STIX]{x1D6E9}$
modulated by a slowly varying function
![]() $\unicode[STIX]{x1D6E9}(z)$
,
$\unicode[STIX]{x1D6E9}(z)$
,
![]() $G$
can be determined below by integrating (4.3) with
$G$
can be determined below by integrating (4.3) with
![]() $G(z\rightarrow 1)=1$
:
$G(z\rightarrow 1)=1$
:
with the corresponding compete depletion point
The Marangoni stress is then
For
![]() $z$
close to
$z$
close to
![]() $z^{\ast }$
, since
$z^{\ast }$
, since
![]() $\unicode[STIX]{x1D6E9}\approx \unicode[STIX]{x1D6E9}^{\ast }$
, equation (4.4) behaves as
$\unicode[STIX]{x1D6E9}\approx \unicode[STIX]{x1D6E9}^{\ast }$
, equation (4.4) behaves as
![]() $G\approx [1-(\ln (z)/\ln (z^{\ast }))^{2}]^{1/2}$
, exhibiting a very sharp decay near
$G\approx [1-(\ln (z)/\ln (z^{\ast }))^{2}]^{1/2}$
, exhibiting a very sharp decay near
![]() $z^{\ast }$
. The corresponding Marangoni stress is
$z^{\ast }$
. The corresponding Marangoni stress is
![]() $\unicode[STIX]{x1D6F4}\approx \ln (z^{\ast })\times (\ln (z)/z)\times (1-(\ln (z)/\ln (z^{\ast }))^{2})^{-1/2}$
, again diverging much faster than the viscous stress singularity
$\unicode[STIX]{x1D6F4}\approx \ln (z^{\ast })\times (\ln (z)/z)\times (1-(\ln (z)/\ln (z^{\ast }))^{2})^{-1/2}$
, again diverging much faster than the viscous stress singularity
![]() $1/z$
.
$1/z$
.
Figure 3 plots both
![]() $G$
and
$G$
and
![]() $\unicode[STIX]{x1D6F4}$
profiles by numerically solving (4.1) with
$\unicode[STIX]{x1D6F4}$
profiles by numerically solving (4.1) with
![]() $H=z$
under (4.2). Similar to figure 2 for insoluble surfactant, the larger
$H=z$
under (4.2). Similar to figure 2 for insoluble surfactant, the larger
![]() $j_{D}$
is the sharper the decrease for
$j_{D}$
is the sharper the decrease for
![]() $G$
and hence the greater build-up of
$G$
and hence the greater build-up of
![]() $\unicode[STIX]{x1D6F4}$
. The results also show an excellent agreement with the approximation solution (4.4) and (4.6) with
$\unicode[STIX]{x1D6F4}$
. The results also show an excellent agreement with the approximation solution (4.4) and (4.6) with
![]() $\unicode[STIX]{x1D6E9}=1$
, confirming the sharp decline in
$\unicode[STIX]{x1D6E9}=1$
, confirming the sharp decline in
![]() $G$
and the divergence in
$G$
and the divergence in
![]() $\unicode[STIX]{x1D6F4}$
near
$\unicode[STIX]{x1D6F4}$
near
![]() $z^{\ast }$
due to weak surfactant transfer shown above. The boundary-layer-like profile for
$z^{\ast }$
due to weak surfactant transfer shown above. The boundary-layer-like profile for
![]() $G$
also resembles the simulation result reported by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) for soluble surfactant (see their figure 9a).
$G$
also resembles the simulation result reported by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) for soluble surfactant (see their figure 9a).
4.2 New contact line structure and dynamic contact angle relationship
To determine the dynamic contact angle relationship based on (4.6), we need (2.10) by neglecting the
![]() $V$
term under (4.2):
$V$
term under (4.2):
Substituting (4.6) into (4.7) and re-writing it in terms of
![]() $\unicode[STIX]{x1D6E9}$
using
$\unicode[STIX]{x1D6E9}$
using
![]() $H=z\unicode[STIX]{x1D6E9}$
and
$H=z\unicode[STIX]{x1D6E9}$
and
![]() $H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
, we arrive at
$H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
, we arrive at
Following the procedures given by appendix A (see § A.3), to solve (4.8) we first integrate its left- and right-hand sides to yield
where
![]() $\unicode[STIX]{x1D713}(z)$
is the integral below (obtained by (A 13) in appendix A):
$\unicode[STIX]{x1D713}(z)$
is the integral below (obtained by (A 13) in appendix A):
Integrating (4.9) from
![]() $z=1$
to
$z=1$
to
![]() $z=z^{\ast }$
and knowing
$z=z^{\ast }$
and knowing
![]() $\unicode[STIX]{x1D6E9}(z=1)=1$
and
$\unicode[STIX]{x1D6E9}(z=1)=1$
and
![]() $\int _{1}^{z^{\ast }}[\ln (z)]^{2}\,\text{d}z\approx -2$
for small
$\int _{1}^{z^{\ast }}[\ln (z)]^{2}\,\text{d}z\approx -2$
for small
![]() $z^{\ast }$
, we arrive at
$z^{\ast }$
, we arrive at
Compared to (3.13) for an insoluble superspreader, the last term due to surfactant leakage has the same dependence on surface tension and surfactant leakage flux. However, the power of
![]() $\unicode[STIX]{x1D6E9}$
is different. The additional power comes from the corner diffusion term
$\unicode[STIX]{x1D6E9}$
is different. The additional power comes from the corner diffusion term
![]() $-4j_{D}/H$
in (4.3). Re-writing (4.11) in terms of actual physical quantities, we obtain the following dynamic contact angle relationship for a soluble superspreader:
$-4j_{D}/H$
in (4.3). Re-writing (4.11) in terms of actual physical quantities, we obtain the following dynamic contact angle relationship for a soluble superspreader:
characterized by the modified capillary number
So the velocity here
![]() $D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0}$
can be interpreted as the velocity of the surface flow induced by the corner diffusion flux, as indicated by (4.3). Matching
$D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0}$
can be interpreted as the velocity of the surface flow induced by the corner diffusion flux, as indicated by (4.3). Matching
![]() $\unicode[STIX]{x1D703}^{\ast }$
given by (4.12) to
$\unicode[STIX]{x1D703}^{\ast }$
given by (4.12) to
![]() $\unicode[STIX]{x1D703}_{clean}$
with (3.16), the contact line speed is found to be
$\unicode[STIX]{x1D703}_{clean}$
with (3.16), the contact line speed is found to be
wherein
![]() $\ln (z^{\ast })=-(1+24\unicode[STIX]{x1D6FE}^{-1}\text{ }j_{D})^{1/4}/2j_{D}^{1/2}$
is a result of combining (4.5) and (4.11). Similar to (3.17) for an insoluble superspreader,
$\ln (z^{\ast })=-(1+24\unicode[STIX]{x1D6FE}^{-1}\text{ }j_{D})^{1/4}/2j_{D}^{1/2}$
is a result of combining (4.5) and (4.11). Similar to (3.17) for an insoluble superspreader,
![]() $U$
again scales as the capillary wetting velocity
$U$
again scales as the capillary wetting velocity
![]() $\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}$
. Impacts of surfactant leakage are mainly reflected in
$\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}$
. Impacts of surfactant leakage are mainly reflected in
![]() $(1+24\unicode[STIX]{x1D6FE}^{-1}j_{D})^{3/4}=(1+(D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})\unicode[STIX]{x1D702}/\unicode[STIX]{x1D70E}_{clean}\unicode[STIX]{x1D703}_{0}^{4})^{3/4}$
. So for
$(1+24\unicode[STIX]{x1D6FE}^{-1}j_{D})^{3/4}=(1+(D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})\unicode[STIX]{x1D702}/\unicode[STIX]{x1D70E}_{clean}\unicode[STIX]{x1D703}_{0}^{4})^{3/4}$
. So for
![]() $24\unicode[STIX]{x1D6FE}^{-1}j_{D}\gg 1$
, equation (4.14) is reduced to
$24\unicode[STIX]{x1D6FE}^{-1}j_{D}\gg 1$
, equation (4.14) is reduced to
Again,
![]() $U$
is independent of
$U$
is independent of
![]() $\unicode[STIX]{x1D703}_{0}$
. But
$\unicode[STIX]{x1D703}_{0}$
. But
![]() $U$
will vary as the
$U$
will vary as the
![]() $3/4$
power of the driving surface flow
$3/4$
power of the driving surface flow
![]() $D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0}$
, in contrast to the insoluble surfactant result (3.18) in which
$D\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0}$
, in contrast to the insoluble surfactant result (3.18) in which
![]() $U$
is linearly proportional to
$U$
is linearly proportional to
![]() $J_{s}/\unicode[STIX]{x1D6E4}_{0}$
. The reason for this difference is that the power of
$J_{s}/\unicode[STIX]{x1D6E4}_{0}$
. The reason for this difference is that the power of
![]() $\unicode[STIX]{x1D703}^{\ast }$
in (4.12) does not match that of
$\unicode[STIX]{x1D703}^{\ast }$
in (4.12) does not match that of
![]() $\unicode[STIX]{x1D703}_{clean}$
in (3.16) because of the additional corner diffusion term
$\unicode[STIX]{x1D703}_{clean}$
in (3.16) because of the additional corner diffusion term
![]() $-4j_{D}/H$
in (4.1).
$-4j_{D}/H$
in (4.1).
A remark is worth making below concerning the spreading speed given by (4.14). Recall that the concentrated Marangoni stress near the contact line is a result of a tiny surfactant leakage imposed by our model to account for the effects of the surfactant transfer from the interface to the substrate. This feature also agrees with what Karapetsas et al. (Reference Karapetsas, Craster and Matar2011) observed in their simulation study. And yet, because of the leak, the contact line is actually surfactant free. So this Marangoni stress does not drive the contact line directly. Instead, it transforms into an enormous Laplace pressure, which in turn drives the contact line in a clean-interface manner. It is this Marangoni-driven capillary wetting that drives superspreading, leading to a constant spreading speed given by (4.14) and why the resulting speed scales as the capillary velocity. It is also this reason why the spreading speed
![]() $U$
does not directly depend on the Marangoni stress but on the leakage flux
$U$
does not directly depend on the Marangoni stress but on the leakage flux
![]() $j_{D}$
.
$j_{D}$
.
4.3 Criterion of superspreading
Because only a certain class of surfactants can exhibit superspreading, this implies that, to be a superspreader, certain requirements have to be met. While the present analysis is made under the constraint (4.2), a superspreader must at least satisfy
where
![]() $V=U/u_{M}$
and
$V=U/u_{M}$
and
![]() $j_{D}=({\mathcal{D}}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0})/u_{M}$
with
$j_{D}=({\mathcal{D}}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x1D6E4}_{0})/u_{M}$
with
![]() $u_{M}=\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}/\unicode[STIX]{x1D702}$
. The criterion
$u_{M}=\unicode[STIX]{x1D703}_{0}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}/\unicode[STIX]{x1D702}$
. The criterion
![]() $4j_{D}<1$
to ensure that surfactant leakage is weak so that the surfactant depletion zone can be confined in a small region near the contact line. On the other hand, the leak has to be sufficiently strong to establish the surfactant concentration gradient needed to push the contact line without being mitigated by the contact line’s sweeping. This demands
$4j_{D}<1$
to ensure that surfactant leakage is weak so that the surfactant depletion zone can be confined in a small region near the contact line. On the other hand, the leak has to be sufficiently strong to establish the surfactant concentration gradient needed to push the contact line without being mitigated by the contact line’s sweeping. This demands
![]() $2V<4j_{D}$
.
$2V<4j_{D}$
.
In addition to (4.16), we also need the following condition from (4.11) to ensure that the Marangoni flow is strong enough to bend the interface by making
![]() $\unicode[STIX]{x1D703}^{\ast }$
significantly deviated from
$\unicode[STIX]{x1D703}^{\ast }$
significantly deviated from
![]() $\unicode[STIX]{x1D703}_{0}$
according to (4.12):
$\unicode[STIX]{x1D703}_{0}$
according to (4.12):
with
![]() $\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}(\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})$
. Hence, to satisfy both (4.16) and (4.17),
$\unicode[STIX]{x1D6FE}=\unicode[STIX]{x1D703}_{0}^{2}(\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0})$
. Hence, to satisfy both (4.16) and (4.17),
![]() $j_{D}$
has to be within the following range under which superspreading might occur:
$j_{D}$
has to be within the following range under which superspreading might occur:
It is worth pointing out that to make (4.18) hold it is necessary to have
![]() $\unicode[STIX]{x1D6FE}/24>V/2$
so that
$\unicode[STIX]{x1D6FE}/24>V/2$
so that
![]() $V/2<j_{D}$
in (4.16) can be replaced by
$V/2<j_{D}$
in (4.16) can be replaced by
![]() $\unicode[STIX]{x1D6FE}/24<j_{D}$
. In fact,
$\unicode[STIX]{x1D6FE}/24<j_{D}$
. In fact,
![]() $\unicode[STIX]{x1D6FE}/24>V/2$
is equivalent to
$\unicode[STIX]{x1D6FE}/24>V/2$
is equivalent to
![]() $V/\unicode[STIX]{x1D6FE}=Ca_{clean}\unicode[STIX]{x1D703}_{0}^{-3}<1/12$
, meaning that surface tension force has to be sufficiently strong compared to the viscous force. We further notice that
$V/\unicode[STIX]{x1D6FE}=Ca_{clean}\unicode[STIX]{x1D703}_{0}^{-3}<1/12$
, meaning that surface tension force has to be sufficiently strong compared to the viscous force. We further notice that
![]() $j_{D}=({\mathcal{D}}\unicode[STIX]{x1D702}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})/\unicode[STIX]{x1D703}_{0}^{2}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
is approximately proportional to the surfactant concentration
$j_{D}=({\mathcal{D}}\unicode[STIX]{x1D702}\unicode[STIX]{x0394}C/\unicode[STIX]{x1D6E4}_{0})/\unicode[STIX]{x1D703}_{0}^{2}\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}$
is approximately proportional to the surfactant concentration
![]() $C_{0}$
because superspreading generally occurs in the high concentration regime above the CMC where the surface coverage
$C_{0}$
because superspreading generally occurs in the high concentration regime above the CMC where the surface coverage
![]() $\unicode[STIX]{x1D6E4}_{0}$
is nearly constant. As will be illustrated later in § 5, the actual value of
$\unicode[STIX]{x1D6E4}_{0}$
is nearly constant. As will be illustrated later in § 5, the actual value of
![]() $C_{0}$
is close to the lower bound from
$C_{0}$
is close to the lower bound from
![]() $\unicode[STIX]{x1D6FE}/24<j_{D}$
in (4.18). This underpins the need for a sufficiently strong surfactant leakage in driving the contact line with Marangoni shearing. Also because
$\unicode[STIX]{x1D6FE}/24<j_{D}$
in (4.18). This underpins the need for a sufficiently strong surfactant leakage in driving the contact line with Marangoni shearing. Also because
![]() $j_{D}>\unicode[STIX]{x1D6FE}/24>V/2$
here, not only
$j_{D}>\unicode[STIX]{x1D6FE}/24>V/2$
here, not only
![]() $j_{D}>V/2$
guarantees that the as-established surfactant concentration gradient is not diminished by the contact line’s sweeping, but also
$j_{D}>V/2$
guarantees that the as-established surfactant concentration gradient is not diminished by the contact line’s sweeping, but also
![]() $\unicode[STIX]{x1D6FE}/24>V/2$
ensures that the Marangoni shearing can turn into a capillary wetting force to drive the contact line.
$\unicode[STIX]{x1D6FE}/24>V/2$
ensures that the Marangoni shearing can turn into a capillary wetting force to drive the contact line.
On the other hand, if the surfactant leakage is too strong, Marangoni shearing would no longer be concentrated near the contact line to make the Marangoni–capillary mechanism work. In fact, in this case the entire interface would be short of surfactant, which reduces to the standard capillary wetting and hence does not lead to superspreading. This demands
![]() $1/4>j_{D}$
to give the upper bound of
$1/4>j_{D}$
to give the upper bound of
![]() $C_{0}$
. All these requirements result in a range for
$C_{0}$
. All these requirements result in a range for
![]() $C_{0}$
, restricting the surfactant concentration and properties for seeing superspreading.
$C_{0}$
, restricting the surfactant concentration and properties for seeing superspreading.
To better see how the condition for seeing superspreading is determined by surfactant concentration, we re-write
![]() $j_{D}$
in terms of the adsorption length
$j_{D}$
in terms of the adsorption length
![]() $\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C$
. This allows us to transform (4.18) to the following range for the dimensionless adsorption length
$\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C$
. This allows us to transform (4.18) to the following range for the dimensionless adsorption length
![]() $\unicode[STIX]{x1D6EC}\equiv \unicode[STIX]{x1D706}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}{\mathcal{D}}$
:
$\unicode[STIX]{x1D6EC}\equiv \unicode[STIX]{x1D706}\unicode[STIX]{x1D70E}_{clean}/\unicode[STIX]{x1D702}{\mathcal{D}}$
:
In (4.19), the lower bound comes from
![]() $j_{D}<1/4$
and the upper bound corresponds to
$j_{D}<1/4$
and the upper bound corresponds to
![]() $\unicode[STIX]{x1D6FE}/24<j_{D}$
in (4.18). In
$\unicode[STIX]{x1D6FE}/24<j_{D}$
in (4.18). In
![]() $\unicode[STIX]{x1D6EC}$
, the diffusivity of surfactant molecule
$\unicode[STIX]{x1D6EC}$
, the diffusivity of surfactant molecule
![]() ${\mathcal{D}}$
can be evaluated using the Stokes–Einstein equation
${\mathcal{D}}$
can be evaluated using the Stokes–Einstein equation
![]() ${\mathcal{D}}=k_{B}T/6\unicode[STIX]{x03C0}\unicode[STIX]{x1D702}a$
with
${\mathcal{D}}=k_{B}T/6\unicode[STIX]{x03C0}\unicode[STIX]{x1D702}a$
with
![]() $k_{B}T$
being the thermal energy and
$k_{B}T$
being the thermal energy and
![]() $a$
the size of surfactant molecule. Hence
$a$
the size of surfactant molecule. Hence
![]() $\unicode[STIX]{x1D6EC}$
can be re-written as
$\unicode[STIX]{x1D6EC}$
can be re-written as
![]() $\unicode[STIX]{x1D6EC}=6\unicode[STIX]{x03C0}\unicode[STIX]{x1D706}a\unicode[STIX]{x1D70E}_{clean}/k_{B}T$
, measuring the amount of the work needed to spread a surfactant monolayer of thickness
$\unicode[STIX]{x1D6EC}=6\unicode[STIX]{x03C0}\unicode[STIX]{x1D706}a\unicode[STIX]{x1D70E}_{clean}/k_{B}T$
, measuring the amount of the work needed to spread a surfactant monolayer of thickness
![]() $a$
over distance
$a$
over distance
![]() $\unicode[STIX]{x1D706}$
under the actions of surface tension
$\unicode[STIX]{x1D706}$
under the actions of surface tension
![]() $\unicode[STIX]{x1D70E}_{clean}$
.
$\unicode[STIX]{x1D70E}_{clean}$
.
As indicated by (4.19), to be a superspreader, the adsorption length
![]() $\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C\sim \unicode[STIX]{x1D6E4}_{0}/C_{0}$
, has to be within a certain range. Apparently, not every surfactant can meet this criterion. As
$\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C\sim \unicode[STIX]{x1D6E4}_{0}/C_{0}$
, has to be within a certain range. Apparently, not every surfactant can meet this criterion. As
![]() $\unicode[STIX]{x1D706}$
essentially represents the adsorption isotherm of a surfactant, for a given surfactant and a given surface coverage
$\unicode[STIX]{x1D706}$
essentially represents the adsorption isotherm of a surfactant, for a given surfactant and a given surface coverage
![]() $\unicode[STIX]{x1D6E4}_{0}$
, equation (4.19) will fix the range of surfactant concentration
$\unicode[STIX]{x1D6E4}_{0}$
, equation (4.19) will fix the range of surfactant concentration
![]() $C_{0}$
in which superspreading will be observed. In addition, the range strongly depends on the apparent dynamic contact angle
$C_{0}$
in which superspreading will be observed. In addition, the range strongly depends on the apparent dynamic contact angle
![]() $\unicode[STIX]{x1D703}_{0}$
, suggesting that surfactant wettability and surface chemistry also have a part to play. As a potential candidate of superspreader has to fulfil all of the requirements listed above, this restrains the types of surfactants that become superspreaders.
$\unicode[STIX]{x1D703}_{0}$
, suggesting that surfactant wettability and surface chemistry also have a part to play. As a potential candidate of superspreader has to fulfil all of the requirements listed above, this restrains the types of surfactants that become superspreaders.
Recall that (4.19) comes from the need for a local surfactant leakage under the flux constraint (4.18). It seems that an arbitrary surfactant can produce such a leakage effect, which actually is not true. The reason is that to meet (4.18), the surfactant might require a specific affinity and a special kinetic pathway to adsorb onto the substrate, so that the surfactant transfer from the interface to the substrate can occur at the desired level. And to acquire such affinity and sorption kinetics, it is also necessary for the surfactant to possess certain chemical properties or structure that favour such a very restrictive adsorption process. In other words, the special physical and chemical properties needed for being a superspreader are actually hidden in the local surfactant leakage flux imposed by the present hydrodynamic model. As superspreading can also be influenced by other factors such as dynamic surface tension (Rafai & Bonn Reference Rafai and Bonn2005), evaporation (Semenov et al. Reference Semenov, Trybala, Agogo, Kovalchuk, Ortega, Rubio, Starov and Velarde2013), wetting and surface chemistry (Ivanova, Zhantenova & Starov Reference Ivanova, Zhantenova and Starov2012), surfactant assembly (Stoebe et al. Reference Stoebe, Lin, Hill, Wards and Davis1997; Kumar et al. Reference Kumar, Varanasi, Tilton and Garoff2003a ), humidity (Ivanova et al. Reference Ivanova, Zhantenova and Starov2012), etc., the criterion (4.18) or (4.19) should be viewed as a part of the requirements for superspreading. Hence, it is a sufficient condition for superspreading. That is, if this criterion is satisfied, superspreading might occur; but if superspreading occurs, then it must be fulfilled. Because we do not take into account all of these effects, to see superspreading, the range of surfactant concentration predicted by (4.19) would be wider than the actual concentration range occurring in experiments, which is indeed found in the case considered in the next section. Nevertheless, we at least verify that local surfactant leakage is one of the necessary elements for triggering superspreading.
5 Connections to experiments and simulations
Our analysis can capture a variety of results seen in experiments and simulations reported previously. Having established the criterion (4.19) for superspreading, we first estimate the range of surfactant concentration within which superspreading might occur experimentally. Assume
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
taken about 10 % of
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
taken about 10 % of
![]() $\unicode[STIX]{x1D70E}_{0}\approx 20~\text{mN}~\text{m}^{-1}$
(Rafai & Bonn Reference Rafai and Bonn2005) (because near the contact line the interface is only a little contaminated by surfactant). With
$\unicode[STIX]{x1D70E}_{0}\approx 20~\text{mN}~\text{m}^{-1}$
(Rafai & Bonn Reference Rafai and Bonn2005) (because near the contact line the interface is only a little contaminated by surfactant). With
![]() $\unicode[STIX]{x1D703}_{0}\sim 10^{-1}$
and
$\unicode[STIX]{x1D703}_{0}\sim 10^{-1}$
and
![]() $\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
, equation (4.19) yields
$\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
, equation (4.19) yields
![]() $3.5\times 10^{3}<\unicode[STIX]{x1D6EC}/4<2.4\times 10^{6}$
. For
$3.5\times 10^{3}<\unicode[STIX]{x1D6EC}/4<2.4\times 10^{6}$
. For
![]() $a\sim 1$
nm and
$a\sim 1$
nm and
![]() $k_{B}T\approx 4\times 10^{-21}$
J at
$k_{B}T\approx 4\times 10^{-21}$
J at
![]() $25\,^{\circ }\text{C}$
, the above range of
$25\,^{\circ }\text{C}$
, the above range of
![]() $\unicode[STIX]{x1D6EC}$
gives
$\unicode[STIX]{x1D6EC}$
gives
![]() $\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
. Since we stipulate that superspreading is driven by a local surfactant leakage through corner diffusion across the wedge (with soluble surfactant spreaders), to make this leaking process more effective, it might be more desirable to use high surfactant concentrations. In this case, the interface would likely be covered by densely packed surfactant molecules with the interfacial concentration
$\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
. Since we stipulate that superspreading is driven by a local surfactant leakage through corner diffusion across the wedge (with soluble surfactant spreaders), to make this leaking process more effective, it might be more desirable to use high surfactant concentrations. In this case, the interface would likely be covered by densely packed surfactant molecules with the interfacial concentration
![]() $\unicode[STIX]{x1D6E4}_{0}$
as high as that at the CMC,
$\unicode[STIX]{x1D6E4}_{0}$
as high as that at the CMC,
![]() $\unicode[STIX]{x1D6E4}_{CMC}$
(which is nearly the maximum surface coverage that does not change beyond CMC). Hence,
$\unicode[STIX]{x1D6E4}_{CMC}$
(which is nearly the maximum surface coverage that does not change beyond CMC). Hence,
![]() $\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C$
can be approximated as
$\unicode[STIX]{x1D706}\equiv \unicode[STIX]{x1D6E4}_{0}/\unicode[STIX]{x0394}C$
can be approximated as
![]() $\unicode[STIX]{x1D6E4}_{CMC}/C_{0}$
with
$\unicode[STIX]{x1D6E4}_{CMC}/C_{0}$
with
![]() $C_{0}$
being the surfactant concentration. With
$C_{0}$
being the surfactant concentration. With
![]() $\unicode[STIX]{x1D6E4}_{CMC}\sim 10^{-10}~\text{mol}~\text{cm}^{-2}$
and
$\unicode[STIX]{x1D6E4}_{CMC}\sim 10^{-10}~\text{mol}~\text{cm}^{-2}$
and
![]() $C_{CMC}\sim 10^{-7}~\text{mol}~\text{cm}^{-3}$
for typical trisiloxane superspreaders (Kumar et al.
Reference Kumar, Couzis and Maldarelli2003b
),
$C_{CMC}\sim 10^{-7}~\text{mol}~\text{cm}^{-3}$
for typical trisiloxane superspreaders (Kumar et al.
Reference Kumar, Couzis and Maldarelli2003b
),
![]() $\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
yields
$\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
yields
![]() $C_{0}$
that falls in the range of
$C_{0}$
that falls in the range of
![]() $10^{-8}{-}10^{-5}~\text{mol}~\text{cm}^{-3}$
. It is approximately
$10^{-8}{-}10^{-5}~\text{mol}~\text{cm}^{-3}$
. It is approximately
![]() $1/10{-}100$
times
$1/10{-}100$
times
![]() $C_{CMC}$
. In the experimental study by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002), superspreading was observed at
$C_{CMC}$
. In the experimental study by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002), superspreading was observed at
![]() $C_{0}=10{-}50C_{CMC}$
, which falls into the range estimated above.
$C_{0}=10{-}50C_{CMC}$
, which falls into the range estimated above.
As for the spreading speed
![]() $U$
, because superspreader surfactants are typically soluble,
$U$
, because superspreader surfactants are typically soluble,
![]() $U$
can be roughly estimated as the capillary wetting velocity
$U$
can be roughly estimated as the capillary wetting velocity
![]() $\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/9\unicode[STIX]{x1D702}$
according to (4.14). With
$\unicode[STIX]{x1D703}_{0}^{3}\unicode[STIX]{x1D70E}_{clean}/9\unicode[STIX]{x1D702}$
according to (4.14). With
![]() $\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
$\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
![]() $\unicode[STIX]{x1D702}\approx 1~\text{m}~\text{Pa}~\text{s}$
and
$\unicode[STIX]{x1D702}\approx 1~\text{m}~\text{Pa}~\text{s}$
and
![]() $\unicode[STIX]{x1D703}_{0}\sim O(10^{-1})$
, we find
$\unicode[STIX]{x1D703}_{0}\sim O(10^{-1})$
, we find
![]() $U\sim 1~\text{mm}~\text{s}^{-1}$
in agreement with experimental observations (Nikolov et al.
Reference Nikolov, Wasan, Chengara, Koczo, Policello and Kolossvary2002; Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013; Nikolov & Wasan Reference Nikolov and Wasan2015). Using (4.14), we can see how
$U\sim 1~\text{mm}~\text{s}^{-1}$
in agreement with experimental observations (Nikolov et al.
Reference Nikolov, Wasan, Chengara, Koczo, Policello and Kolossvary2002; Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013; Nikolov & Wasan Reference Nikolov and Wasan2015). Using (4.14), we can see how
![]() $U$
(in mm s
$U$
(in mm s
![]() $^{-1}$
) varies with
$^{-1}$
) varies with
![]() $j_{D}$
, as displayed in figure 5. With
$j_{D}$
, as displayed in figure 5. With
![]() $\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
$\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
,
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
,
![]() $\unicode[STIX]{x1D703}_{0}\sim 10^{-1}$
and
$\unicode[STIX]{x1D703}_{0}\sim 10^{-1}$
and
![]() $z^{\ast }L/\ell \sim 10^{2}$
(by taking 1 % of the typical value
$z^{\ast }L/\ell \sim 10^{2}$
(by taking 1 % of the typical value
![]() $L/\ell \sim 10^{4}$
), to have
$L/\ell \sim 10^{4}$
), to have
![]() $U$
be of the order of mm/s observed in experiments, figure 5 reveals that the strength of the surfactant leakage is
$U$
be of the order of mm/s observed in experiments, figure 5 reveals that the strength of the surfactant leakage is
![]() $0.02<j_{D}<0.2$
, which also falls within
$0.02<j_{D}<0.2$
, which also falls within
![]() $\unicode[STIX]{x1D6FE}/24<j_{D}<1/4$
given by (4.18). This also confirms that a fraction of surfactant leakage will suffice to sustain the Marangoni-driven capillary wetting mechanism needed for superspreading.
$\unicode[STIX]{x1D6FE}/24<j_{D}<1/4$
given by (4.18). This also confirms that a fraction of surfactant leakage will suffice to sustain the Marangoni-driven capillary wetting mechanism needed for superspreading.
The need for surfactant transfer from the interface to the substrate or local surfactant leakage to trigger superspreading implies that a special sorption kinetics is necessary to be a superspreader. Why such a characteristic is essential to a superspreader seems to have been partially supported by the experimental study of Rafai & Bonn (Reference Rafai and Bonn2005). These authors measured the dynamic surface tension for a trisiloxane superspreader and compared with that of a normal surfactant (dioctyl sulfosuccinate sodium salt, AOT) whose spreading does not deviate much from Tanner’s law. Both measurements were conducted near the CMC using pendant drop and maximum bubble pressure methods. Their results reveal that the differences between these two surfactants are rather striking (see their figure 4). In the normal surfactant case, the surface tension merely exhibits a slight decrease around
![]() $30~\text{mN}~\text{m}^{-1}$
and quickly reaches an equilibrium (within 1 s). In contrast, the trisiloxane’s surface tension shows a large change from the nearly clean-interface value
$30~\text{mN}~\text{m}^{-1}$
and quickly reaches an equilibrium (within 1 s). In contrast, the trisiloxane’s surface tension shows a large change from the nearly clean-interface value
![]() $70~\text{mN}~\text{m}^{-1}$
to the CMC value
$70~\text{mN}~\text{m}^{-1}$
to the CMC value
![]() $22~\text{mN}~\text{m}^{-1}$
but is slowly decreasing with time (in a period of 100 s). The much larger and slower change in the dynamic surface tension for the trisiloxane case indicates that trisiloxane surfactants would take a longer time to absorb onto the bubble/drop surface than normal surfactants. This implies that trisiloxane surfactants have less inclination to land onto an air–liquid interface compared to normal surfactants. If spreading a trisiloxane surfactant to a substrate that has some degree of affinity to the surfactant, the surfactant would be more inclined to absorb onto the substrate and hence even discourage the surfactant from adsorption onto the interface. Because the closer to the contact line the stronger the surfactant adsorption toward the substrate, the more severe surfactant deficiency would occur at the interface near the contact line, acting as if there were an additional sink to remove the surfactant. This in turn would establish a local Marangoni shearing toward the contact line to aid in its motion. But for normal surfactants, since they can be quickly absorbed onto an air–liquid interface and lack an adsorption bias to a solid substrate, they are not able to generate a local surfactant leakage to establish the Marangoni stress needed for speeding up the contact line advancement. In short, the peculiar dynamic surface tension behaviour of trisiloxane surfactant seen in the experiment by Rafai & Bonn (Reference Rafai and Bonn2005) can be linked to the notion of local surfactant leakage in our model for superspreading. Hence, the distinctions between superspreaders and normal surfactants are actually implied in the presupposition of local surfactant leakage used in our model.
$22~\text{mN}~\text{m}^{-1}$
but is slowly decreasing with time (in a period of 100 s). The much larger and slower change in the dynamic surface tension for the trisiloxane case indicates that trisiloxane surfactants would take a longer time to absorb onto the bubble/drop surface than normal surfactants. This implies that trisiloxane surfactants have less inclination to land onto an air–liquid interface compared to normal surfactants. If spreading a trisiloxane surfactant to a substrate that has some degree of affinity to the surfactant, the surfactant would be more inclined to absorb onto the substrate and hence even discourage the surfactant from adsorption onto the interface. Because the closer to the contact line the stronger the surfactant adsorption toward the substrate, the more severe surfactant deficiency would occur at the interface near the contact line, acting as if there were an additional sink to remove the surfactant. This in turn would establish a local Marangoni shearing toward the contact line to aid in its motion. But for normal surfactants, since they can be quickly absorbed onto an air–liquid interface and lack an adsorption bias to a solid substrate, they are not able to generate a local surfactant leakage to establish the Marangoni stress needed for speeding up the contact line advancement. In short, the peculiar dynamic surface tension behaviour of trisiloxane surfactant seen in the experiment by Rafai & Bonn (Reference Rafai and Bonn2005) can be linked to the notion of local surfactant leakage in our model for superspreading. Hence, the distinctions between superspreaders and normal surfactants are actually implied in the presupposition of local surfactant leakage used in our model.

Figure 4. (a) Calculated surfactant distribution
![]() $G$
for soluble superspreader with various values of corner diffusion flux
$G$
for soluble superspreader with various values of corner diffusion flux
![]() $j_{D}$
at
$j_{D}$
at
![]() $V=0.001$
. Lines: numerical solution to (4.1). Symbols: approximate solution (4.3). (b) Shows the corresponding Marangoni stress
$V=0.001$
. Lines: numerical solution to (4.1). Symbols: approximate solution (4.3). (b) Shows the corresponding Marangoni stress
![]() $\unicode[STIX]{x1D6F4}=-G_{z}$
whose exact and approximate values are evaluated using (4.1) and (4.6) respectively. All of the results are calculated at
$\unicode[STIX]{x1D6F4}=-G_{z}$
whose exact and approximate values are evaluated using (4.1) and (4.6) respectively. All of the results are calculated at
![]() $H=z$
.
$H=z$
.
![]() $G$
vanishes at the depletion point
$G$
vanishes at the depletion point
![]() $z^{\ast }$
. Similar to figure 2 for an insoluble superspreader, rapid decline in
$z^{\ast }$
. Similar to figure 2 for an insoluble superspreader, rapid decline in
![]() $G$
and divergence in
$G$
and divergence in
![]() $\unicode[STIX]{x1D6F4}$
can also occur near
$\unicode[STIX]{x1D6F4}$
can also occur near
![]() $z^{\ast }$
.
$z^{\ast }$
.

Figure 5. Dependence of actual wetting speed
![]() $U$
on the surfactant transfer rate
$U$
on the surfactant transfer rate
![]() $j_{D}$
for soluble superspreader. The result is obtained from (4.14) with
$j_{D}$
for soluble superspreader. The result is obtained from (4.14) with
![]() $\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
$\unicode[STIX]{x1D70E}_{clean}\approx 70~\text{mN}~\text{m}^{-1}$
,
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
(approximately 10 % of
$\unicode[STIX]{x0394}\unicode[STIX]{x1D70E}_{0}\approx 2~\text{mN}~\text{m}^{-1}$
(approximately 10 % of
![]() $\unicode[STIX]{x1D70E}_{0}\approx 20~\text{mN}~\text{m}^{-1}$
),
$\unicode[STIX]{x1D70E}_{0}\approx 20~\text{mN}~\text{m}^{-1}$
),
![]() $\unicode[STIX]{x1D703}_{0}\approx 10^{-1}$
and
$\unicode[STIX]{x1D703}_{0}\approx 10^{-1}$
and
![]() $L/\ell \approx 10^{4}$
.
$L/\ell \approx 10^{4}$
.
Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) conducted their experiment for
![]() $C_{0}=0.8{-}50C_{CMC}$
and found that the spreading exponent can vary between 0.16 and 1, depending on
$C_{0}=0.8{-}50C_{CMC}$
and found that the spreading exponent can vary between 0.16 and 1, depending on
![]() $C_{0}$
. At
$C_{0}$
. At
![]() $C_{0}=10{-}50C_{CMC}$
the linear spreading law can be observed (see figure 6
a). This concentration range corresponds to
$C_{0}=10{-}50C_{CMC}$
the linear spreading law can be observed (see figure 6
a). This concentration range corresponds to
![]() $\unicode[STIX]{x1D706}=200~\text{nm}{-}1~\unicode[STIX]{x03BC}\text{m}$
, falling within
$\unicode[STIX]{x1D706}=200~\text{nm}{-}1~\unicode[STIX]{x03BC}\text{m}$
, falling within
![]() $\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
estimated from (4.19). For
$\unicode[STIX]{x1D706}=45~\text{nm}{-}30~\unicode[STIX]{x03BC}\text{m}$
estimated from (4.19). For
![]() $C_{0}$
below
$C_{0}$
below
![]() $10C_{CMC}$
, however, the spreading is slowed down and the spreading exponent decreases on lowering
$10C_{CMC}$
, however, the spreading is slowed down and the spreading exponent decreases on lowering
![]() $C_{0}$
. At
$C_{0}$
. At
![]() $C_{0}=$
$C_{0}=$
![]() $0.8C_{CMC}$
, which is the lowest surfactant concentration in their experiment, in particular, the spreading is the slowest, showing that the spreading radius
$0.8C_{CMC}$
, which is the lowest surfactant concentration in their experiment, in particular, the spreading is the slowest, showing that the spreading radius
![]() $R$
is growing as a
$R$
is growing as a
![]() $1/6$
power of time (see figure 6
b).
$1/6$
power of time (see figure 6
b).

Figure 6. Experimental data by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002). (a) At surfactant concentration
![]() $C_{0}=10{-}50$
CMC, linear spreading law can be observed. (b) At
$C_{0}=10{-}50$
CMC, linear spreading law can be observed. (b) At
![]() $C_{0}=0.8$
CMC however, which is the lowest surfactant concentration in the experiment, the spreading radius grows as the
$C_{0}=0.8$
CMC however, which is the lowest surfactant concentration in the experiment, the spreading radius grows as the
![]() $1/6$
power of time. The
$1/6$
power of time. The
![]() $1/6$
spreading exponent is the smallest in their experiment.
$1/6$
spreading exponent is the smallest in their experiment.
Various spreading power laws can be explained by the fact that surface tension gradients no longer occur locally near the contact line but are established over the drop radius
![]() $R$
. In other words, the spreading is driven by a global Marangoni force. Balancing this force to the viscous force from (1.1), we get
$R$
. In other words, the spreading is driven by a global Marangoni force. Balancing this force to the viscous force from (1.1), we get
![]() $U\sim \unicode[STIX]{x1D6FD}h\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D702}R\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}$
(because
$U\sim \unicode[STIX]{x1D6FD}h\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D702}R\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}$
(because
![]() $h\propto R^{-2}$
for fulfilling the constant drop volume constraint). Here
$h\propto R^{-2}$
for fulfilling the constant drop volume constraint). Here
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}$
is controlled by the amount of the surfactant over the drop surface,
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}$
is controlled by the amount of the surfactant over the drop surface,
![]() $\unicode[STIX]{x1D6E4}R^{2}\sim M_{i}-qt$
with
$\unicode[STIX]{x1D6E4}R^{2}\sim M_{i}-qt$
with
![]() $M_{i}$
being the initial amount of the interfacial surfactant and
$M_{i}$
being the initial amount of the interfacial surfactant and
![]() $q$
the surfactant leakage rate due to the surfactant transfer/leak to the bulk/substrate.
$q$
the surfactant leakage rate due to the surfactant transfer/leak to the bulk/substrate.
At low
![]() $C_{0}$
,
$C_{0}$
,
![]() $q$
is small. So the surfactant might behave like an insoluble surfactant with
$q$
is small. So the surfactant might behave like an insoluble surfactant with
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto R^{-2}$
, giving
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto R^{-2}$
, giving
![]() $U\propto$
$U\propto$
![]() $R^{-5}$
and hence
$R^{-5}$
and hence
![]() $R\propto t^{1/6}$
. This explains the smallest spreading power
$R\propto t^{1/6}$
. This explains the smallest spreading power
![]() $1/6$
observed by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002).
$1/6$
observed by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002).
At
![]() $C_{0}$
higher than the CMC, we have to consider impacts of
$C_{0}$
higher than the CMC, we have to consider impacts of
![]() $q\sim (J_{b}R^{2}+J_{s}R)$
that comprise the surfactant flux to the bulk
$q\sim (J_{b}R^{2}+J_{s}R)$
that comprise the surfactant flux to the bulk
![]() $J_{b}$
and the leakage flux
$J_{b}$
and the leakage flux
![]() $J_{s}$
at the contact line. In this case, the surface concentration varies as
$J_{s}$
at the contact line. In this case, the surface concentration varies as
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\sim qt/R^{2}$
, giving
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\sim qt/R^{2}$
, giving
![]() $U\propto qt/R^{5}$
. The spreading dynamics for this case is determined by how
$U\propto qt/R^{5}$
. The spreading dynamics for this case is determined by how
![]() $q$
varies with
$q$
varies with
![]() $R$
, depending on whether
$R$
, depending on whether
![]() $J_{b}$
is diffusion controlled or kinetics controlled.
$J_{b}$
is diffusion controlled or kinetics controlled.
When
![]() $C_{0}$
is slightly higher than the CMC,
$C_{0}$
is slightly higher than the CMC,
![]() $q$
could be dominated by
$q$
could be dominated by
![]() $J_{s}R$
or controlled by lateral diffusion with
$J_{s}R$
or controlled by lateral diffusion with
![]() $J_{b}\propto R^{-1}$
. This leads to
$J_{b}\propto R^{-1}$
. This leads to
![]() $q\propto R$
and hence
$q\propto R$
and hence
![]() $U\propto t/R^{4}$
, giving
$U\propto t/R^{4}$
, giving
![]() $R\propto t^{2/5}$
.
$R\propto t^{2/5}$
.
At
![]() $C_{0}$
much higher than the CMC,
$C_{0}$
much higher than the CMC,
![]() $q$
might be dominated by
$q$
might be dominated by
![]() $J_{b}R^{2}$
. Because
$J_{b}R^{2}$
. Because
![]() $J_{b}$
is likely kinetics controlled, it is roughly constant, especially at the late stage of spreading where plenty of surfactant molecules are absorbed onto the substrate or the interface. This leads to
$J_{b}$
is likely kinetics controlled, it is roughly constant, especially at the late stage of spreading where plenty of surfactant molecules are absorbed onto the substrate or the interface. This leads to
![]() $q\propto R^{2}$
and hence
$q\propto R^{2}$
and hence
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto$
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto$
![]() $t$
as assumed by Nikolov & Wasan (Reference Nikolov and Wasan2015). As a result,
$t$
as assumed by Nikolov & Wasan (Reference Nikolov and Wasan2015). As a result,
![]() $U\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}$
$U\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}$
![]() $\propto t/R^{3}$
, giving
$\propto t/R^{3}$
, giving
![]() $R\propto t^{1/2}$
. This
$R\propto t^{1/2}$
. This
![]() $1/2$
power law has been observed during the late-time spreading in the experiment by Nikolov & Wasan (Reference Nikolov and Wasan2015) (see figure 7).
$1/2$
power law has been observed during the late-time spreading in the experiment by Nikolov & Wasan (Reference Nikolov and Wasan2015) (see figure 7).

Figure 7. Experimental data by Nikolov & Wasan (Reference Nikolov and Wasan2015). The spreading first follows the linear law as shown in (a). At late times it then slows down to the
![]() $1/2$
law, as displayed in (b).
$1/2$
law, as displayed in (b).
It is possible that
![]() $J_{b}$
is dominated by vertical diffusion with
$J_{b}$
is dominated by vertical diffusion with
![]() $J_{b}\propto h^{-1}$
. This can happen when the transport is driven by the surfactant transfer from the interface to the substrate. Because this flux is most amplified near the contact line, this is essentially equivalent to the corner diffusion analysed in § 4. So
$J_{b}\propto h^{-1}$
. This can happen when the transport is driven by the surfactant transfer from the interface to the substrate. Because this flux is most amplified near the contact line, this is essentially equivalent to the corner diffusion analysed in § 4. So
![]() $J_{b}\propto h^{-1}\propto R^{2}$
yields
$J_{b}\propto h^{-1}\propto R^{2}$
yields
![]() $q\sim J_{b}R^{2}\propto R^{4}$
. Together with
$q\sim J_{b}R^{2}\propto R^{4}$
. Together with
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\sim qt/R^{2}$
, we arrive at
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\sim qt/R^{2}$
, we arrive at
![]() $U\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}\propto t/R$
, recovering the linear spreading law
$U\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/R^{3}\propto t/R$
, recovering the linear spreading law
![]() $R\propto t$
. Together with the fact that the initial spreading in this case is also superspreading at constant
$R\propto t$
. Together with the fact that the initial spreading in this case is also superspreading at constant
![]() $U$
, this explains why the linear spreading law somewhat persists as it emerges, as Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) observed. The linear spreading law followed by the
$U$
, this explains why the linear spreading law somewhat persists as it emerges, as Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) observed. The linear spreading law followed by the
![]() $1/2$
power law observed by Nikolov & Wasan (Reference Nikolov and Wasan2015) (see figure 7) suggests that the surfactant transport might undergo a transition from corner diffusion to kinetics controlled.
$1/2$
power law observed by Nikolov & Wasan (Reference Nikolov and Wasan2015) (see figure 7) suggests that the surfactant transport might undergo a transition from corner diffusion to kinetics controlled.
Likewise, for two-dimensional spreading with surfactant, we can also find the corresponding spreading laws in accordance with those reported previously. In this case, the drop width
![]() $W$
expands under constraints
$W$
expands under constraints
![]() $h\propto W^{-1}$
and
$h\propto W^{-1}$
and
![]() $\unicode[STIX]{x1D6E4}\sim (M_{i}-qt)/W$
with
$\unicode[STIX]{x1D6E4}\sim (M_{i}-qt)/W$
with
![]() $q\sim (J_{b}W+J_{s})$
. Also because the spreading speed now varies like
$q\sim (J_{b}W+J_{s})$
. Also because the spreading speed now varies like
![]() $U\sim \unicode[STIX]{x1D6FD}h\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D702}W\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/W^{2}$
, the resulting spreading dynamics will be different from three-dimensional cases.
$U\sim \unicode[STIX]{x1D6FD}h\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/\unicode[STIX]{x1D702}W\propto \unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}/W^{2}$
, the resulting spreading dynamics will be different from three-dimensional cases.
When
![]() $q$
is small at low
$q$
is small at low
![]() $C_{0}$
, because
$C_{0}$
, because
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto W^{-1}$
, we get
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto W^{-1}$
, we get
![]() $U$
$U$
![]() $\propto W^{-3}$
and hence
$\propto W^{-3}$
and hence
![]() $W\propto t^{1/4}$
. This can occur after the linear superspreading law
$W\propto t^{1/4}$
. This can occur after the linear superspreading law
![]() $W\propto t$
, as seen in simulations for insoluble surfactant (Karapetsas et al.
Reference Karapetsas, Craster and Matar2011). In the high
$W\propto t$
, as seen in simulations for insoluble surfactant (Karapetsas et al.
Reference Karapetsas, Craster and Matar2011). In the high
![]() $C_{0}$
regime, effects of
$C_{0}$
regime, effects of
![]() $q$
have to come into play. Because
$q$
have to come into play. Because
![]() $\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto qt/W$
, we have
$\unicode[STIX]{x0394}\unicode[STIX]{x1D6E4}\propto qt/W$
, we have
![]() $U\propto qt/W^{3}$
, giving
$U\propto qt/W^{3}$
, giving
![]() $W\propto (qt^{2})^{1/4}$
. If
$W\propto (qt^{2})^{1/4}$
. If
![]() $q$
is dominated by
$q$
is dominated by
![]() $J_{s}$
or controlled by lateral diffusion with
$J_{s}$
or controlled by lateral diffusion with
![]() $J_{b}\propto W^{-1}$
,
$J_{b}\propto W^{-1}$
,
![]() $q$
is roughly constant and we arrive at
$q$
is roughly constant and we arrive at
![]() $W\propto t^{1/2}$
. If
$W\propto t^{1/2}$
. If
![]() $q$
is dominated by corner diffusion with
$q$
is dominated by corner diffusion with
![]() $J_{b}\propto h^{-1}$
, the linear superspreading law
$J_{b}\propto h^{-1}$
, the linear superspreading law
![]() $W\propto t$
is recovered. At late times the surfactant transport could be controlled by lateral diffusion again, making the spreading slow down to
$W\propto t$
is recovered. At late times the surfactant transport could be controlled by lateral diffusion again, making the spreading slow down to
![]() $W\propto t^{1/2}$
. Such a transition from the linear law to the
$W\propto t^{1/2}$
. Such a transition from the linear law to the
![]() $1/2$
law has been observed by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011). On the other hand, if
$1/2$
law has been observed by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011). On the other hand, if
![]() $q$
is kinetics controlled and dominated by
$q$
is kinetics controlled and dominated by
![]() $J_{b}W$
(with constant
$J_{b}W$
(with constant
![]() $J_{b}$
), then
$J_{b}$
), then
![]() $W\propto t^{2/3}$
, which explains the main finding reported by Beacham et al. (Reference Beacham, Matar and Craster2009).
$W\propto t^{2/3}$
, which explains the main finding reported by Beacham et al. (Reference Beacham, Matar and Craster2009).
In short, for three-dimensional (3-D) axisymmetric drop spreading with surfactant, its spreading dynamics follows
![]() $R\propto t^{\unicode[STIX]{x1D6FC}}$
with
$R\propto t^{\unicode[STIX]{x1D6FC}}$
with
![]() $\unicode[STIX]{x1D6FC}=1/6-1$
. Similarly, for two-dimensional (2-D) spreading we find
$\unicode[STIX]{x1D6FC}=1/6-1$
. Similarly, for two-dimensional (2-D) spreading we find
![]() $W\propto t^{\unicode[STIX]{x1D6FC}}$
with
$W\propto t^{\unicode[STIX]{x1D6FC}}$
with
![]() $\unicode[STIX]{x1D6FC}$
$\unicode[STIX]{x1D6FC}$
![]() $=1/4-1$
. Table 1 summarizes the various spreading laws shown above.
$=1/4-1$
. Table 1 summarizes the various spreading laws shown above.
Table 1. Various values of spreading exponent
![]() $\unicode[STIX]{x1D6FC}$
for 3-D spreading and 2-D spreading with surfactant.
$\unicode[STIX]{x1D6FC}$
for 3-D spreading and 2-D spreading with surfactant.

6 Analogous thermocapillary spreading
The present superspreading with surfactant can be analogous to thermocapillary spreading because both are driven by surface tension gradients. A similar Marangoni enhancement can also occur to thermocapillary spreading on a cooled plate (Ehrhard & Davis Reference Ehrhard and Davis1991; Ehrhard Reference Ehrhard1993; Smith Reference Smith1995; Sui & Splet Reference Sui and Splet2015). Such thermally driven spreading can also display a linear spreading law but it is achieved by nonlinear surface tension variations arising from a temperature minimum inside the droplet (Karapetsas et al.
Reference Karapetsas, Sahu, Sefiane and Matar2014; Chaudhury & Chakraborty Reference Chaudhury and Chakraborty2015). If surface tension still varies linearly with temperature as commonly assumed, the situation would be quite similar to the present surfactant-driven spreading using a linear equation of state. In this case, we find that the same
![]() $1/6$
and
$1/6$
and
![]() $1/2$
spreading laws can emerge, depending on the cooling rate.
$1/2$
spreading laws can emerge, depending on the cooling rate.
Similar to surfactant-driven spreading, a drop in this case will spread at speed
![]() $U_{T}\sim h\unicode[STIX]{x1D6FD}_{T}K_{T}/\unicode[STIX]{x1D702}$
driven by the gradient
$U_{T}\sim h\unicode[STIX]{x1D6FD}_{T}K_{T}/\unicode[STIX]{x1D702}$
driven by the gradient
![]() $K_{T}\equiv \unicode[STIX]{x2202}_{x}T_{s}$
of the interfacial temperature
$K_{T}\equiv \unicode[STIX]{x2202}_{x}T_{s}$
of the interfacial temperature
![]() $T_{s}=T_{\infty }-(T_{\infty }-T_{w})/(1+Bh/d)$
(Ehrhard & Davis Reference Ehrhard and Davis1991). Here
$T_{s}=T_{\infty }-(T_{\infty }-T_{w})/(1+Bh/d)$
(Ehrhard & Davis Reference Ehrhard and Davis1991). Here
![]() $B=h_{air}d/k_{drop}$
is the Biot number measuring the heat flux on the air side (having heat transfer coefficient
$B=h_{air}d/k_{drop}$
is the Biot number measuring the heat flux on the air side (having heat transfer coefficient
![]() $h_{air}$
) relative to that on the fluid side (having thermal conductivity
$h_{air}$
) relative to that on the fluid side (having thermal conductivity
![]() $k_{drop}$
),
$k_{drop}$
),
![]() $d$
is the scale of the drop height,
$d$
is the scale of the drop height,
![]() $T_{w}$
the plate temperature and
$T_{w}$
the plate temperature and
![]() $T_{\infty }$
the ambient air temperature, and
$T_{\infty }$
the ambient air temperature, and
![]() $\unicode[STIX]{x1D6FD}_{T}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}T)$
.
$\unicode[STIX]{x1D6FD}_{T}=-(\unicode[STIX]{x2202}\unicode[STIX]{x1D70E}/\unicode[STIX]{x2202}T)$
.
When
![]() $B$
is small, this describes the insulated limit, resembling spreading with insoluble surfactant. In this case, because
$B$
is small, this describes the insulated limit, resembling spreading with insoluble surfactant. In this case, because
![]() $\unicode[STIX]{x0394}T_{s}\equiv T_{s}-T_{\infty }\propto h$
, we get
$\unicode[STIX]{x0394}T_{s}\equiv T_{s}-T_{\infty }\propto h$
, we get
![]() $U_{T}\propto hK_{T}\propto h\unicode[STIX]{x2202}_{x}h\propto h^{2}/R\propto R^{-5}$
and hence arrive at
$U_{T}\propto hK_{T}\propto h\unicode[STIX]{x2202}_{x}h\propto h^{2}/R\propto R^{-5}$
and hence arrive at
![]() $R\propto t^{1/6}$
, similar to the late-time spreading of the insoluble superspreader shown in figure 6(b). This
$R\propto t^{1/6}$
, similar to the late-time spreading of the insoluble superspreader shown in figure 6(b). This
![]() $1/6$
spreading law is also supported by the experimental data measured by Ehrhard (Reference Ehrhard1993), as shown in figure 8.
$1/6$
spreading law is also supported by the experimental data measured by Ehrhard (Reference Ehrhard1993), as shown in figure 8.

Figure 8. Experimental data by Ehrhard (Reference Ehrhard1993). The same
![]() $1/6$
law seen in figure 6(b) can also occur to the analogous thermocapillary on a cooled plate.
$1/6$
law seen in figure 6(b) can also occur to the analogous thermocapillary on a cooled plate.
On the contrary, large
![]() $B$
corresponds to the perfectly conducting limit, analogous to the spreading with soluble surfactant. Because this case has
$B$
corresponds to the perfectly conducting limit, analogous to the spreading with soluble surfactant. Because this case has
![]() $\unicode[STIX]{x0394}T_{s}\propto h^{-1}$
much greater than the small
$\unicode[STIX]{x0394}T_{s}\propto h^{-1}$
much greater than the small
![]() $B$
case, the gradient
$B$
case, the gradient
![]() $K_{T}\propto \unicode[STIX]{x2202}_{x}h/h^{2}\sim 1/(hR)\propto R$
increases with
$K_{T}\propto \unicode[STIX]{x2202}_{x}h/h^{2}\sim 1/(hR)\propto R$
increases with
![]() $R$
instead of decreasing with
$R$
instead of decreasing with
![]() $R$
. The spreading speed thus behaves as
$R$
. The spreading speed thus behaves as
![]() $U_{T}\propto hK_{T}\propto R^{-1}$
. This results in
$U_{T}\propto hK_{T}\propto R^{-1}$
. This results in
![]() $R\propto t^{1/2}$
, similar to the late-time spreading of soluble superspreader shown in figure 7.
$R\propto t^{1/2}$
, similar to the late-time spreading of soluble superspreader shown in figure 7.
7 Concluding remarks
In conclusion, we have demonstrated that the curious linear superspreading law observed in experiments (Rafai et al. Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Nikolov & Wasan Reference Nikolov and Wasan2015) can be explained by the new contact structure resulting from a local surfactant leakage to the substrate near the contact line. As some affinity to the substrate is necessary for a surfactant superspreader, the rate of surfactant leakage represents the ability to transfer surfactant to the substrate. We identify that a tiny rate of surfactant leakage will suffice to trigger superspreading. Specifically, a small surfactant depletion zone can form to almost remove interfacial surfactant molecules near the contact line. As a result, a concentrated Marangoni shearing can be established toward the contact line, exhibiting a Marangoni stress singularity diverging at a rate much faster than the usual viscous stress singularity (Huh & Scriven Reference Huh and Scriven1971). And more importantly, being devoid of surfactant near the contact line, this strong Marangoni shearing can turn into a local capillary force to drive the contact line in a surfactant-free manner. The actual dynamic contact angle depends only on the properties (i.e. affinity and concentration) of a surfactant spreader, giving a constant spreading speed according to the de Gennes–Cox–Voinov law and hence the linear spreading law independent of the extent of spreading. As such, we demonstrate that a rational account for superspreading can be made in a purely hydrodynamic manner without appealing to a specific surfactant structure or sorption kinetics. Our results also capture many features seen in the simulation study by Karapetsas et al. (Reference Karapetsas, Craster and Matar2011).
It actually turns out that the condition for the occurrence of superspreading is quite stringent. On the one hand, the contact line movement can advect surfactant molecules toward the contact line to diminish the surface tension gradient established by the surfactant leakage. So if the rate of surfactant leakage is too low compared to the speed of the moving contact line, the spreading will be opposed by Marangoni retardation. On the other hand, the rate of surfactant leakage cannot be too high either; otherwise the drop would become virtually clean and its spreading would be governed by Tanner’s
![]() $1/10$
law. Even if there is a surfactant concentration gradient, it would no longer be confined near the contact line but extend over the drop, making Marangoni shearing less stronger than that in the tiny surfactant leakage case. Therefore, there must exist a range of the surfactant leakage rate within which superspreading can occur. We also convert this rate range in terms of surfactant concentration (see (4.19)). This might explain the existence of a maximum spreading rate in a range of surfactant concentration (Hill Reference Hill1988; Stoebe et al.
Reference Stoebe, Lin, Hill, Wards and Davis1996, Reference Stoebe, Lin, Hill, Wards and Davis1997; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013). The estimated surfactant concentration range is found to cover concentrations much higher than the CMC, just like the condition for seeing superspreading experimentally observed by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002). This explains why only a certain class of surfactants such as trisiloxane can serve as superspreaders.
$1/10$
law. Even if there is a surfactant concentration gradient, it would no longer be confined near the contact line but extend over the drop, making Marangoni shearing less stronger than that in the tiny surfactant leakage case. Therefore, there must exist a range of the surfactant leakage rate within which superspreading can occur. We also convert this rate range in terms of surfactant concentration (see (4.19)). This might explain the existence of a maximum spreading rate in a range of surfactant concentration (Hill Reference Hill1988; Stoebe et al.
Reference Stoebe, Lin, Hill, Wards and Davis1996, Reference Stoebe, Lin, Hill, Wards and Davis1997; Wang et al.
Reference Wang, Chen, Bonaccurso and Venzmer2013). The estimated surfactant concentration range is found to cover concentrations much higher than the CMC, just like the condition for seeing superspreading experimentally observed by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002). This explains why only a certain class of surfactants such as trisiloxane can serve as superspreaders.
Experiments show that the spreading of surfactant spreaders can be slowed down to a variety of powers of time (Rafai et al.
Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002; Nikolov & Wasan Reference Nikolov and Wasan2015). We show that a variety of power laws can be attributed to distinct surfactant transport mechanisms governing at different surfactant concentration levels. In particular, the
![]() $1/6$
and the
$1/6$
and the
![]() $1/2$
power laws found respectively by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) and Nikolov & Wasan (Reference Nikolov and Wasan2015) can occur to the analogous thermocapillary spreading processes. The same
$1/2$
power laws found respectively by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) and Nikolov & Wasan (Reference Nikolov and Wasan2015) can occur to the analogous thermocapillary spreading processes. The same
![]() $1/6$
power law seen in the surfactant spreading experiment by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) can appear in the thermocapillary spreading experiment by Ehrhard (Reference Ehrhard1993). These resemblances between surfactant-driven spreading and thermocapillary spreading reverberate the ubiquitous role of the Marangoni effect in these dynamic wetting phenomena driven by non-uniform surface tension.
$1/6$
power law seen in the surfactant spreading experiment by Rafai et al. (Reference Rafai, Sarker, Bergeron, Meunier and Bonn2002) can appear in the thermocapillary spreading experiment by Ehrhard (Reference Ehrhard1993). These resemblances between surfactant-driven spreading and thermocapillary spreading reverberate the ubiquitous role of the Marangoni effect in these dynamic wetting phenomena driven by non-uniform surface tension.
Acknowledgements
This work is supported by the Ministry of Science and Technology of Taiwan. The author would like to thank T.-I. Lin and Y.-S. Liu for preparing figures.
Appendix A. Voinov’s method for solving Bretherton-like equations
The equations we are tackling can be generalized as of Bretherton type:
where
![]() $f_{1}$
is a given function and the power
$f_{1}$
is a given function and the power
![]() $n>0$
. The solution to (A 1) can be approximately determined using the approach below due to Voniov (Reference Voniov1976) and Snoeijer (Reference Snoeijer2006).
$n>0$
. The solution to (A 1) can be approximately determined using the approach below due to Voniov (Reference Voniov1976) and Snoeijer (Reference Snoeijer2006).
The essence of this method is that
![]() $H$
is assumed to vary slowly with respect to
$H$
is assumed to vary slowly with respect to
![]() $z:H=z\unicode[STIX]{x1D6E9}$
with
$z:H=z\unicode[STIX]{x1D6E9}$
with
![]() $|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
. Because
$|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
. Because
![]() $H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
,
$H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
,
![]() $H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
. Hence (A 1) becomes
$H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
. Hence (A 1) becomes
with
![]() $f(z)=f_{1}(z)/z^{n}$
.
$f(z)=f_{1}(z)/z^{n}$
.
The solution to (A 2) can be obtained by integrating (A 2) twice with the slow variation approximation for
![]() $\unicode[STIX]{x1D6E9}$
. The first step is to take respective integration for the left- and right-hand sides of (A 2). Integrating the left hand side gives
$\unicode[STIX]{x1D6E9}$
. The first step is to take respective integration for the left- and right-hand sides of (A 2). Integrating the left hand side gives
where we have used
![]() $\unicode[STIX]{x1D6E9}_{z}(z\rightarrow 1)\rightarrow 0$
(viz.,
$\unicode[STIX]{x1D6E9}_{z}(z\rightarrow 1)\rightarrow 0$
(viz.,
![]() $H_{zz}(z\rightarrow 1)\rightarrow 0$
) and neglected the second term containing
$H_{zz}(z\rightarrow 1)\rightarrow 0$
) and neglected the second term containing
![]() $\unicode[STIX]{x1D6E9}_{z}$
which is assumed small here. Let the result of integrating the right-hand side of (A 2) be
$\unicode[STIX]{x1D6E9}_{z}$
which is assumed small here. Let the result of integrating the right-hand side of (A 2) be
![]() $\int _{1}^{z}fdz\equiv F(z)$
. Then (A 2) reduces to
$\int _{1}^{z}fdz\equiv F(z)$
. Then (A 2) reduces to
So the solution can be readily found by integrating both the left- and right-hand sides of (A 4).
A.1 Derivation of the de Gennes–Cox–Voinov law for capillary wetting
The approach described above can be used to solve (3.9) and (4.7) in the presence of surfactant. Prior to solving these equations, we validate this approach by first demonstrating its use for solving the Bretherton equation for complete capillary wetting:
![]() $h^{2}h_{xxx}=-3Ca$
or
$h^{2}h_{xxx}=-3Ca$
or
by letting
![]() $h=Ca^{1/3}LH(z)$
with
$h=Ca^{1/3}LH(z)$
with
![]() $z=x/L$
(Bonn et al.
Reference Bonn, Eggers, Indekeu, Meunier and Rolly2009). Using
$z=x/L$
(Bonn et al.
Reference Bonn, Eggers, Indekeu, Meunier and Rolly2009). Using
![]() $H=z\unicode[STIX]{x1D6E9}$
with
$H=z\unicode[STIX]{x1D6E9}$
with
![]() $|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
and
$|\unicode[STIX]{x1D6E9}_{z}|\ll 1$
and
![]() $H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
(from
$H_{zzz}\approx \unicode[STIX]{x1D6E9}_{zz}$
(from
![]() $H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
), equation (A 5) becomes
$H_{z}=\unicode[STIX]{x1D6E9}+z\unicode[STIX]{x1D6E9}_{z}\approx \unicode[STIX]{x1D6E9}$
), equation (A 5) becomes
Note that
![]() $\unicode[STIX]{x1D6E9}$
in this case can be thought of as a rescaled slope in the sense that
$\unicode[STIX]{x1D6E9}$
in this case can be thought of as a rescaled slope in the sense that
![]() $h_{x}\approx Ca^{1/3}\unicode[STIX]{x1D6E9}$
. Following the derivation from (A 2) to (A 4), equation (A 6) can be reduced to
$h_{x}\approx Ca^{1/3}\unicode[STIX]{x1D6E9}$
. Following the derivation from (A 2) to (A 4), equation (A 6) can be reduced to
which yields the solution
Taking
![]() $z_{1}=\ell /L$
associated with the inner length scale
$z_{1}=\ell /L$
associated with the inner length scale
![]() $\ell$
and replacing
$\ell$
and replacing
![]() $\unicode[STIX]{x1D6E9}$
by
$\unicode[STIX]{x1D6E9}$
by
![]() $\unicode[STIX]{x1D6E9}\approx Ca^{-1/3}h_{x}$
, equation (A 8) recovers the well-known de Gennes–Cox–Voinov law for the dynamic contact angle
$\unicode[STIX]{x1D6E9}\approx Ca^{-1/3}h_{x}$
, equation (A 8) recovers the well-known de Gennes–Cox–Voinov law for the dynamic contact angle
![]() $\unicode[STIX]{x1D703}\approx h_{x}$
(Voniov Reference Voniov1976; de Gennes Reference de Gennes1985; Cox Reference Cox1986a
):
$\unicode[STIX]{x1D703}\approx h_{x}$
(Voniov Reference Voniov1976; de Gennes Reference de Gennes1985; Cox Reference Cox1986a
):
Having validated the use of Voinov’s method in solving (A 6) for the standard capillary wetting problem, we next proceed to use it to solve the new equations (3.9) and (4.7) for modelling superspreading.
A.2 Superspreading with insoluble surfactant
For superspreading with insoluble surfactant, the equation is (A 2) by taking
![]() $n=2$
and
$n=2$
and
![]() $f=Bz^{-2}[1+A\ln (z)]^{-1/2}$
with
$f=Bz^{-2}[1+A\ln (z)]^{-1/2}$
with
![]() $A=8j_{s}/\unicode[STIX]{x1D6E9}(\ll 1)$
and
$A=8j_{s}/\unicode[STIX]{x1D6E9}(\ll 1)$
and
![]() $B=6\unicode[STIX]{x1D6FE}^{-1}j_{s}^{}$
, as in (3.10). So an integration of
$B=6\unicode[STIX]{x1D6FE}^{-1}j_{s}^{}$
, as in (3.10). So an integration of
![]() $f/B$
in (A 4) is
$f/B$
in (A 4) is
![]() $\unicode[STIX]{x1D719}(z)$
in (3.11):
$\unicode[STIX]{x1D719}(z)$
in (3.11):
Let
![]() $\unicode[STIX]{x1D70C}^{2}=1+A\ln (z)$
. Equation (A 10) changes to
$\unicode[STIX]{x1D70C}^{2}=1+A\ln (z)$
. Equation (A 10) changes to
 $$\begin{eqnarray}\displaystyle \unicode[STIX]{x1D719}(z) & = & \displaystyle (2/A)e^{1/A}\int _{1}^{\unicode[STIX]{x1D70C}(z)}\exp (-\unicode[STIX]{x1D70C}^{2}/A)\,\text{d}\unicode[STIX]{x1D70C}\nonumber\\ \displaystyle & = & \displaystyle (\unicode[STIX]{x03C0}/A)^{1/2}e^{1/A}(\text{erf}(\unicode[STIX]{x1D70C}(z)/\sqrt{A})-\text{erf}(1/\sqrt{A}))\approx \ln (z).\end{eqnarray}$$
$$\begin{eqnarray}\displaystyle \unicode[STIX]{x1D719}(z) & = & \displaystyle (2/A)e^{1/A}\int _{1}^{\unicode[STIX]{x1D70C}(z)}\exp (-\unicode[STIX]{x1D70C}^{2}/A)\,\text{d}\unicode[STIX]{x1D70C}\nonumber\\ \displaystyle & = & \displaystyle (\unicode[STIX]{x03C0}/A)^{1/2}e^{1/A}(\text{erf}(\unicode[STIX]{x1D70C}(z)/\sqrt{A})-\text{erf}(1/\sqrt{A}))\approx \ln (z).\end{eqnarray}$$
The final result
![]() $\ln (z)$
comes from a Taylor expansion of the integral at
$\ln (z)$
comes from a Taylor expansion of the integral at
![]() $\unicode[STIX]{x1D70C}=1$
. Integrating (3.11) from
$\unicode[STIX]{x1D70C}=1$
. Integrating (3.11) from
![]() $z=1$
to
$z=1$
to
![]() $z=z^{\ast }$
and recognizing that
$z=z^{\ast }$
and recognizing that
![]() $\int _{1}^{z^{\ast }}\ln (z)\,\text{d}z\approx 1$
for small
$\int _{1}^{z^{\ast }}\ln (z)\,\text{d}z\approx 1$
for small
![]() $z^{\ast }$
, the solution can be readily obtained, as given by (3.13).
$z^{\ast }$
, the solution can be readily obtained, as given by (3.13).
A.3 Superspreading with soluble surfactant
For superspreading with soluble surfactant, we need to solve (4.7) or (A 2) with
![]() $n=3$
and
$n=3$
and
![]() $f=B(\ln (z)/z^{2})\times [1-A(\ln (z))^{2}]^{-1/2}$
where
$f=B(\ln (z)/z^{2})\times [1-A(\ln (z))^{2}]^{-1/2}$
where
![]() $A=4j_{D}/\unicode[STIX]{x1D6E9}^{2}(\ll 1)$
and
$A=4j_{D}/\unicode[STIX]{x1D6E9}^{2}(\ll 1)$
and
![]() $B=-6\unicode[STIX]{x1D6FE}^{-1}j_{D}$
. After integrating
$B=-6\unicode[STIX]{x1D6FE}^{-1}j_{D}$
. After integrating
![]() $f$
in (A 4), we have to evaluate the following integral which is
$f$
in (A 4), we have to evaluate the following integral which is
![]() $\unicode[STIX]{x1D713}(z)$
in (4.9):
$\unicode[STIX]{x1D713}(z)$
in (4.9):
Let
![]() $\unicode[STIX]{x1D70C}^{2}=1-A(\ln (z))^{2}$
. Equation (A 12) can be changed to the following integral which can be evaluated approximately by taking a Taylor expansion at
$\unicode[STIX]{x1D70C}^{2}=1-A(\ln (z))^{2}$
. Equation (A 12) can be changed to the following integral which can be evaluated approximately by taking a Taylor expansion at
![]() $\unicode[STIX]{x1D70C}=1$
:
$\unicode[STIX]{x1D70C}=1$
:
 $$\begin{eqnarray}\displaystyle \unicode[STIX]{x1D713}(z) & = & \displaystyle -A^{-1}\int _{1}^{(1-A(\ln (z))^{2})^{1/2}}\exp (-A^{-1/2}(1-\unicode[STIX]{x1D70C}^{2})^{1/2})\,\text{d}\unicode[STIX]{x1D70C}\nonumber\\ \displaystyle & {\approx} & \displaystyle (-A^{-1})\times \exp (-A^{-1/2}(1-\unicode[STIX]{x1D70C}^{2})^{1/2})|_{\unicode[STIX]{x1D70C}=1}\times (-A/2)\times (1-A(\ln (z))^{2})^{-1/2}|_{z=1}\nonumber\\ \displaystyle & & \displaystyle \times \,(\ln (z))^{2}\nonumber\\ \displaystyle & {\approx} & \displaystyle (\ln (z))^{2}/2.\end{eqnarray}$$
$$\begin{eqnarray}\displaystyle \unicode[STIX]{x1D713}(z) & = & \displaystyle -A^{-1}\int _{1}^{(1-A(\ln (z))^{2})^{1/2}}\exp (-A^{-1/2}(1-\unicode[STIX]{x1D70C}^{2})^{1/2})\,\text{d}\unicode[STIX]{x1D70C}\nonumber\\ \displaystyle & {\approx} & \displaystyle (-A^{-1})\times \exp (-A^{-1/2}(1-\unicode[STIX]{x1D70C}^{2})^{1/2})|_{\unicode[STIX]{x1D70C}=1}\times (-A/2)\times (1-A(\ln (z))^{2})^{-1/2}|_{z=1}\nonumber\\ \displaystyle & & \displaystyle \times \,(\ln (z))^{2}\nonumber\\ \displaystyle & {\approx} & \displaystyle (\ln (z))^{2}/2.\end{eqnarray}$$
With (A 13), we can obtain the solution (4.11) by integrating (4.9) from
![]() $z=1$
to
$z=1$
to
![]() $z=z^{\ast }$
with
$z=z^{\ast }$
with
![]() $\int _{1}^{z^{\ast }}[\ln (z)]^{2}\,\text{d}z\approx -2$
for small
$\int _{1}^{z^{\ast }}[\ln (z)]^{2}\,\text{d}z\approx -2$
for small
![]() $z^{\ast }$
.
$z^{\ast }$
.