Vitamin A (retinol) is essential for a variety of physiological processes, including vision, immune functions, reproduction, embryonic development as well as cellular growth and differentiation (Stephensen, Reference Stephensen2001; Blomhoff & Blomhoff, Reference Blomhoff and Blomhoff2006).
Retinol represents the most abundant retinoid in bovine blood (Van Merris et al. Reference Van Merris, Meyer, Duchateau, Blum and Burvenich2004) and its levels in steers are maintained for at least 145 d even if the animals are fed a diet with no supplemental vitamin A (Gorocica-Buenfil et al. Reference Gorocica-Buenfil, Fluharty and Loerch2008). However, plasma retinol and β-carotene levels in heifers and cows decrease at the end of gestation, reach their lowest values around parturition and increase again during the first week of lactation (Johnston & Chew, Reference Johnston and Chew1984; Goff & Stabel, Reference Goff and Stabel1990; Lindberg et al. Reference Lindberg, Sinkkonen, Poso, Tesfa and Schroder1999; Kumagai et al. Reference Kumagai, Chaipan and Mitani2000; Kumagai et al. Reference Kumagai, Chaipan and Mitani2001; Nonnecke et al. Reference Nonnecke, Roberts, Godkin, Horst, Hammell and Franklin2001; Goff et al. Reference Goff, Kimura and Horst2002; Van Merris et al. Reference Van Merris, Meyer, Duchateau, Blum and Burvenich2004; Debier et al. Reference Debier, Potter, Goffe and Larondell2005; Rezamand et al. Reference Rezamand, Hoagland, Moyes, Silbart and Andrew2007). The decrease in plasma retinol concentration is probably due to the transfer of large amounts of retinol and its derivatives into colostrum (Goff et al. Reference Goff, Kimura and Horst2002), resulting in the accumulation of retinoids in colostrum rather than in milk (Debier et al. Reference Debier, Potter, Goffe and Larondell2005). In addition, plasma retinol concentrations decrease immediately after intramammary injection of Escherichia coli (Van Merris et al. Reference Van Merris, Meyer, Duchateau, Blum and Burvenich2004) suggesting that vitamin A metabolism is also affected by the acute phase reactions that occur during coliform mastitis. Indeed, the plasma retinol levels in post-partum cows with mastitis are significantly lower than those of cows without mastitis (Johnston & Chew, Reference Johnston and Chew1984), and there is a significant delay in the recovery of the plasma β-carotene concentration in post-partum cows with a subclinical intramammary infection (Rezamand et al. Reference Rezamand, Hoagland, Moyes, Silbart and Andrew2007).
In newborn calves, plasma retinol levels at birth are low and increase markedly after colostrum intake (Blum et al. Reference Blum, Hadorn, Sallmann and Schuep1997; Kumagai et al. Reference Kumagai, Chaipan and Mitani2001; Debier et al. Reference Debier, Potter, Goffe and Larondell2005; Puvogel et al. Reference Puvogel, Baumrucker and Blum2008). As colostrum provokes drastic morphological and functional changes in the gastrointestinal tract (GIT) in neonatal calves (Blum, Reference Blum2006) and vitamin A plays a role in maintaining gut integrity (Thurnham et al. Reference Thurnham, Norhrop-Clewes, McCullough, Das and Lunn2000; Quadro et al. Reference Quadro, Gamble, Vogel, Lima, Piantedosi, Moore, Colantuoni, Gottesman, Guerrant and Blaner2000; Carroll & Forsberg, Reference Carroll and Forsberg2007) the retinol in colostrum is likely to contribute to the maturation of the GIT together with non-nutrient factors such as IGF-1 (Blum, Reference Blum2006).
Retinoids are small hydrophobic compounds and therefore associate in vivo with soluble proteins. Retinol-binding protein 4 (RBP4) is the protein responsible for plasma retinol transport and binds with retinol in a 1:1 ratio (Noy, Reference Noy2000). Recently, it has been reported that RBP4 induces insulin resistance in mice (Yang et al. Reference Yang, Graham, Mody, Preitner, Peroni, Zabolotny, Kotani, Quadro and Kahn2005) and that the serum RBP4 levels in humans correlate with the magnitude of insulin resistance in subjects with impaired glucose tolerance or type 2 diabetes (Graham et al. Reference Graham, Yang, Blüher, Hammarstedt, Ciaraldi, Henry, Wason, Oberbach, Jansson, Smith and Kahn2006). More recently, STRA6 was identified as a cell surface receptor for RBP4 (Kawaguchi et al. Reference Kawaguchi, Yu, Honda, Hu, Whitelegge, Ping, Wiita, Bok and Sun2007), indicating that RBP4 is a novel biologically active factor.
It was reported that the mean serum RBP4 and retinol concentrations in cows 4 weeks before calving were 42 μg/ml (~2 μμ) and 53 μg/dl (~1·8 μμ), respectively. RBP4 levels decreased one week before parturition and recovered to control levels at one week after calving (Lindberg et al. Reference Lindberg, Sinkkonen, Poso, Tesfa and Schroder1999) suggesting the possibility that RBP4, like retinol, is transferred from maternal stores to calves through colostrum and milk. However, a similar decrease in plasma RBP4 levels was found only in cows with subclinical intramammary infections, but not in uninfected cows (Rezamand et al. Reference Rezamand, Hoagland, Moyes, Silbart and Andrew2007), suggesting the decrease might be due to suppression of hepatic RBP4 synthesis during acute-phase reactions, as demonstrated in rats (Rosales et al. Reference Rosales, Ritter, Zolfaghari, Smith and Ross1996).
In the present study, we measured the RBP4 and retinol concentrations in the plasma, colostrum and milk of cows to assess how plasma RBP4 and retinol levels are regulated in ruminants under physiological and pathophysiological conditions. Moreover, owing to the lack of such reports in any mammalian species, the study was undertaken to describe RBP4 levels in colostrum and/or milk.
Materials and Methods
Blood sampling and collection of colostrum and milk
All experimental procedures were performed in accordance with the guidelines outlined by the Animal Care and Use Committee of Hokkaido University.
In Experiment I, five non-pregnant non-lactating Holstein cows weighing 450–550 kg were used to examine the effects of fasting on plasma RBP4 and retinol levels. They were housed in individual stalls with free access to water and trace mineral blocks and fed a mixture of forage (orchard-grass hay, alfalfa hay cubes and corn silage) and concentrates. At the start of the experiment, feed was completely withdrawn, and food deprivation was maintained for 60 h, when the metabolic effects of starvation in cows were clearly observed (Baird et al. Reference Baird, Heitzman and Hibbitt1972). Water was provided throughout the study. Blood was collected from the jugular vein at 0, 12, 24, 36, 48 and 60 h after the onset of food deprivation. Plasma was separated by centrifugation and stored at −30°C until use.
In Experiment II, six non-pregnant non-lactating Holstein cows were housed as described above and used to examine the effects of lipopolysaccharide (LPS) on plasma RBP4 and retinol levels. On the day of the experiment, the cows were divided into two groups (groups A and B, three per group). Group A received an intravenous injection of LPS (Escherichia coli 055: B5, Difco, Detroit MI, USA) solution dissolved in saline at a dose 500 ng/kg, while group B was injected with saline as a control. Blood was collected at 0, 2, 4, 6 and 8 h after the injection, and the plasma was stored at −30°C.
In Experiment III, six pregnant Holstein cows were housed as described above. Blood and milk (colostrum) samples were collected 3–6 d before calving, on the day of parturition and 1 and 2 weeks after calving. These samples were stored at −30°C.
Western blot analysis of RBP4 in plasma, colostrum and milk
Bovine RBP4 was purified as previously described (Miyamoto et al. Reference Miyamoto, Katoh, Motoi, Ohashi, Nagasawa and Shimbayashi1989) and anti-sera were obtained by immunizing purified RBP4 to rabbits. Plasma was diluted 20-times with distilled water, and the whole milk and colostrum were undiluted and diluted 10-times with distilled water, respectively. These samples (7·5 μl for plasma and 24 μl for milk) and increasing concentrations of purified RBP4 were separated simultaneously by SDS-PAGE (13% gel) under reducing conditions. Thereafter, the proteins were electroblotted onto a PVDF membrane (ImmobilonTM, Millipore, Bedford MA, USA). The membrane was blocked for 1 h at room temperature in 5% (w/v) skimmed milk in a buffer [20 mm-Tris–HCl (pH 7·5), 0·15 m-NaCl and 0·1% Tween 20] and then incubated with the anti-RBP4 sera (1:2000) overnight at 4°C. The membrane was washed five times with the wash buffer and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:4000, Zymed Laboratories Inc., South San Francisco CA, USA) for 1 h at room temperature. Visualization was performed using an enhanced chemiluminescence detection system (Millipore) according to the manufacturer's instructions. The intensities of the immunoreactive bands were analysed densitometrically using the NIH Image program. RBP4 concentration was measured by comparing the intensity of the bands with those of purified RBP4. As shown in Fig. 1, the band intensity of purified bovine RBP4 was closely correlated with the protein amount, at least from 10 ng to 80 ng of RBP4. Purified RBP4, added to bovine plasma and milk, was recovered at 96·2±0·4% and 95·4±0·8%, respectively. The intra- and inter-assay variations in plasma RBP4 determination for five independent experiments were less than 5·1% and 2·4%, respectively.

Fig. 1. Quantitative analysis of RBP4 by Western blot analysis. Representative blot of the increasing amounts of purified RBP4 and a standard curve obtained by densitometric analysis of the bands are shown.
Spectrophotometric measurement of retinol in plasma, colostrum and milk
Retinol concentrations in plasma, colostrum and milk were determined by modifying the method described by Suzuki & Katoh (Reference Suzuki and Katoh1990). In brief, 50 μl of ethanol and 150 μl of hexane were added to 50 μl of plasma, colostrum, or milk, and the hexane phase was recovered after 40-min mixing and 10-min centrifugation at 6500 g. Retinol concentrations were calculated based on the absorbance of hexane extracts at 325 nm and 453 nm using the equations described (Suzuki & Katoh, Reference Suzuki and Katoh1990). Recovery of retinol added to milk was 97·8±0·4%.
Statistical analysis
Results are expressed as means±sem. Statistical analysis was performed using one-way repeated measures ANOVA for Experiments I and III and two-way repeated measures ANOVA for Experiment II, and Fisher's post hoc test, with P<0·05 being considered statistically significant.
Results
Changes in plasma RBP4 levels during fasting and after LPS challenge
Basal plasma RBP4 levels in the two experimental groups were almost the same (41·8±1·9 μg/ml in Experiment I and 46·1±4·6 μg/ml in Experiment II) and were sustained during the 60-h fasting (Fig. 2A) and after saline administration (Fig. 3A). However, RBP4 levels were significantly reduced 4 h after LPS administration and decreased for at least 8 h after the injection (Fig. 3A). Basal plasma retinol concentrations were 36·2±3·2 μg/dl (Experiment I) and 25·5±1·8 μg/dl (Experiment II). During fasting, the concentration progressively decreased to 13·1±2·7 μg/dl at 60 h (Fig. 2B), and the retinol/RBP4 molar ratio fell from 0·65±0·05 to 0·23±0·05 (Fig. 2C). Plasma retinol concentration was also significantly decreased at 2 h and had decreased by almost 50% by 8 h after the LPS injection (Fig. 3B). In contrast to the fasting experiment, the retinol/RBP4 ratio was not changed significantly after LPS injection, although there was a tendency for it to decrease in the LPS-treated cows 2–4 h after the injection (Fig. 3C).

Fig. 2. Effects of fasting on plasma RBP4 and retinol levels in cows. Cows (n=5) were housed without feeding for 60 h. Blood was collected at 0, 12, 24, 36, 48 and 60 h after the onset of food deprivation, and isolated plasma samples were stored. Plasma concentrations of RBP4 (A) and retinol (B) were quantified by Western blot analysis and a spectrophotometric method, respectively. (C) The molar ratio of retinol to RBP4 was calculated. Values are expressed as means±sem. *: P <0·05 v. 0 h.

Fig. 3. Effect of LPS administration on plasma RBP4 and retinol levels in cows. Cows (n=6) were injected intravenously with either LPS (500 ng/kg, n=3) or saline (n=3) as a control. Blood samples were collected at 0, 2, 4, 6 and 8 h after the injection. Plasma concentrations of RBP4 (A) and retinol (B) and their molar ratio (C) are shown. Values are expressed as means±sem. *: P <0·05 v. control.
Changes in plasma RBP4 and retinol levels in cows before and after parturition
Plasma RBP4 levels at 3–6 d before parturition were 50·7±5·3 μg/ml, which were comparable to those of non-pregnant, non-lactating cows in Experiments I and II. However, on the day of parturition, RBP4 levels were significantly decreased (29·8±5·4 μg/ml) but they had returned to basal levels by 2 weeks after parturition (52·1±2·1 μg/ml) (Fig. 4A). Plasma retinol levels before parturition were 26·6±3·3 μg/dl, which were comparable to those of non-lactating cows. Similarly to RBP4, the retinol levels were significantly decreased on the day of parturition (18·5±2·7 μg/dl) and had returned to basal levels by 15 d after parturition (33·9±4·3 μg/dl) (Fig. 4B). There was no significant difference among the retinol/RBP4 ratios obtained before and after parturition.
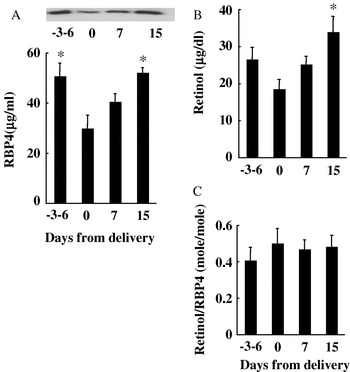
Fig. 4. Changes in plasma RBP4 and retinol levels in cows before and after parturition. Blood was collected from 6 cows before and after parturition. Plasma concentrations of RBP4 (A) and retinol (B) and their molar ratio (C) are shown. Values are expressed as means±.sem. *: P <0·05 v. day 0.
Changes in RBP4 and retinol levels in colostrum and milk from cows before and after parturition
In the two milk samples obtained 3–6 d before parturition, RBP4 was hardly detected. However, in colostrum, RBP4 was clearly present at a concentration of 16·4±5·6 μg/ml (n=6, Fig. 5A). RBP4 had almost disappeared from the milk of the cows at 7 d and 15 d after parturition. Retinol concentrations in milk (43·5±6·9 μg/dl at 7 d after delivery) were comparable to plasma retinol concentrations, but those in colostrum were markedly higher (295·3±5·9 μg/dl). The molar ratio of retinol to RBP4 in colostrum was almost 20:1.

Fig. 5. Changes in RBP4 and retinol levels in colostrum and milk. Milk was collected from cows before (n=2) and after (n=6) parturition. Concentrations of RBP4 (A) and retinol (B) in colostrum and milk were measured by Western blot analysis and a spectrophotometric method, respectively. (C) The molar ratio of retinol to RBP4 is also shown. Values are expressed as means±sem. *: P <0·05 v. day 7 and day 15.
Discussion
In the present study, we have demonstrated for the first time that considerable amounts of RBP4 are present in colostrum, but that it is scarce in milk. The increase in RBP4 levels in colostrum was accompanied by a comparable decrease in plasma RBP4 on the day of calving. The lack of alteration in plasma RBP4 levels during the 60-h fasting experiment suggests that its decrease on the day of calving may not be due to food availability or negative energy status during and shortly after parturition. The mild inflammation induced during the process of parturition may acutely decrease in plasma RBP4 as evidenced by the experiment with LPS. However, in bovine colostrum, a variety of non-nutrient bioactive compounds such as immunoglobulin G (IgG), growth hormone, prolactin, insulin and IGF-I are present, many of which are derived from blood (Barrington et al. Reference Barrington, McFadden, Huyler and Besser2001; Van de Perre, Reference Van de Perre2003; Taylor et al. Reference Taylor, Cheng, Pushpakumara, Beever and Wathes2004; Blum, Reference Blum2006). In addition, since RBP4 was substantially lacking in milk, it is unlikely that RBP4 is synthesized in the mammary gland. Therefore, it is most likely that RBP4 is transferred from the blood to colostrum, resulting in a decrease its plasma levels.
In milk, β-lactoglobulin acts as a retinol carrier and thereby enhances intestinal retinol uptake in calves (Said et al. Reference Said, Ong and Shingleton1989; Perez & Calvo, Reference Perez and Calvo1995). As one molecule of RBP4 binds to one molecule of retinol (Noy, Reference Noy2000) the presence of almost a 20-fold excess of retinol over RBP4 in colostrum suggests that β-lactoglobulin is the primary retinol carrier in colostrum as well as in milk. Thus, the physiological relevance of colostrum RBP4 as a retinol carrier is currently unknown, but it is plausible that RBP4 contributes to calf development as a metabolic regulator, as described in rodents (Yang et al. Reference Yang, Graham, Mody, Preitner, Peroni, Zabolotny, Kotani, Quadro and Kahn2005). It is, however, unlikely that maternal RBP4 contributes to the circulating RBP4 level in calves, although some macromolecules in colostrum such as IgG are transported across the intestinal epithelium into the neonatal circulation (Barrington et al. Reference Barrington, McFadden, Huyler and Besser2001; Van de Perre, Reference Van de Perre2003; Blum, Reference Blum2006). This is because RBP4 is detected in the plasma of calves shortly after birth (before being fed colostrum) and its levels are unchanged one day after colostrum feeding, although its levels are approximately 50% of those in dairy heifers and cows (Nonnecke et al. Reference Nonnecke, Roberts, Godkin, Horst, Hammell and Franklin2001).
Nonnecke et al. (Reference Nonnecke, Roberts, Godkin, Horst, Hammell and Franklin2001) also showed a positive correlation between plasma retinol and RBP4 levels in pre-ruminant calves (from birth to 27 d of age) fed a milk replacement with different amounts of supplemental vitamin A. A similar relationship between circulating retinol and RBP4 levels is observed in children and infants (Shenai et al. Reference Shenai, Rush, Stahlman and Chytil1990; Craft, Reference Craft2001; Aeberli et al. Reference Aeberli, Biebinger, Lehmann, l'Allemand, Spinas and Zimmermann2007) suggesting that RBP4 is a surrogate marker for retinol. However, the apparent dissociation of the plasma retinol levels from the plasma RBP4 levels seen during the 60-h fasting indicates that their levels in the plasma of cows are regulated independently of each other. Moreover, there were rapid changes in both plasma retinol and RBP4 levels after LPS challenge, and both changes seemed to be independent. The decrease of plasma retinol caused by LPS challenge was confirmed by a similar finding showing a decrease in plasma retinol concentrations immediately after intramammary injection of Esch. coli (Van Merris et al. Reference Van Merris, Meyer, Duchateau, Blum and Burvenich2004) and may be attributed to an increase of vitamin A consumption in various tissues such as the lung during inflammation (Kanda et al. Reference Kanda, Yamamoto and Yoshino1990). On the other hand, the decrease of plasma RBP4 induced by LPS injection was probably due to the inhibition of hepatic RBP4 synthesis, as observed in rats (Rosales et al. Reference Rosales, Ritter, Zolfaghari, Smith and Ross1996). It is therefore suggested that plasma RBP4 levels in cows are regulated independently of retinol concentrations under these conditions.
In summary, RBP4 was found in abundance in cow colostrum, accompanied by a comparable decrease in plasma RBP4, suggesting that RBP4 is transported from the blood to colostrum. In addition, RBP4 and retinol levels are independently regulated under physiological and pathophysiological conditions.
We are grateful to Dr Masato Fukui (Nippon Zenyaku Kogyo Co., Ltd.) for providing the blood, colostrum and milk for Experiment III. This study was supported by grants from the Japan Society for the Promotion of Science (JSPS) to KK and YO-O. The study was also supported by a JSPS Research Fellowship for Young Scientists awarded to AK.







