INTRODUCTION
The different species of Myxosporea are characterized as having, in their myxosporean phase, spores with a variable number of polar capsules and valves. This great diversity in myxospores does not correspond with spores of the actinosporean phase, which are defined as having a triradiate symmetry, with 3 valves and 3 polar capsules (Lom and Dyková, Reference Lom and Dyková2006). Nevertheless, it is worth noting that the majority of myxozoan life cycles are unknown, mainly for marine species.
At the present, only 4 marine life cycles have been described, all of which involve polychaetes as the invertebrate host i.e. Ellipsomyxa gobii (Køie et al. Reference Køie, Whipps and Kent2004); Gadimyxa atlantica (Køie et al. Reference Køie, Karlsbakk and Nylund2007); Ceratomyxa auerbachi (Køie et al. Reference Køie, Karlsbakk and Nylund2008) and Ellipsomyxa mugilis (=Zschokkella mugilis) (Rangel et al. Reference Rangel, Santos, Cech and Székely2009). Other marine actinospores have been reported in oligochaetes (Hallett et al. Reference Hallett, O'Donoghue and Lester1998, Reference Hallett, Erséus and Lester1999, 2001; Hallett and Lester, Reference Hallett and Lester1999), in polychaetes (Køie, Reference Køie2002, Reference Køie2005) and also in sipunculids (Ikeda, Reference Ikeda1912), for which the putative myxospore phase of their life cycle is still unknown.
With the suppression of the class Actinosporea, and the inclusion of its species as alternate phases of the Myxosporea class life cycles, it was recommended that Actinosporea genera should be considered as collective groups and new taxa should be described according to the myxosporean stages (Lom et al. Reference Lom, McGeorge, Feist, Morris and Adams1997; Kent and Lom, Reference Kent and Lom1999; Kent et al. Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Longshow, Palenzeula, Siddall and Xiao2001; Lom and Dyková, Reference Lom and Dyková2006). Of the 17 described collective groups, only 5 are known from the marine environment (Endocapsa, Sphaeractinomyxon, Tetractinomyxon, Tetraspora and Triactinomyxon) (Lom and Dyková, Reference Lom and Dyková2006).
Development of actinospores in the invertebrate host generally follows the same pattern in all studied species (see Ikeda, Reference Ikeda1912; Janiszewska, Reference Janiszewska1957; El-Matbouli and Hoffmann, Reference El-Matbouli and Hoffmann1998; Lom and Dyková, Reference Lom and Dyková2006; Meaders and Hendrickson, Reference Meaders and Hendrickson2009; Rangel et al. Reference Rangel, Santos, Cech and Székely2009; Morris, Reference Morris2010), whether this development takes place in the intestinal epithelium, in the epidermis, or in the coelomic cavity, in oligochaetes, polychaetes or sipunculids. Actinosporean development begins with a schizogony phase from which binucleated cells result. These binucleated cells produce tetranucleated cells that will form the pansporocyst stage, with 2 enveloping cells and 2 internal cells. The enveloping cells may divide further several times. During gametogony, the internal cells divide by meiosis originating 16 gametes, which, after fertilization, produce 8 zygotes. Each zygote will originate a spore (in the sporogony phase) that develops inside the pansporocyst and that, when mature, is released from the host. The only exception to this general development rule of actinosporeans is the tetraspora type, in which only 4 spores develop inside the pansporocyst (Hallett and Lester, Reference Hallett and Lester1999). Each spore develops 3 valves and 3 polar capsules.
In terms of the ecological characterization of known actinosporeans, the seasonal prevalence of infection in the invertebrate hosts has only been scarcely studied. El-Mansy et al. (1998a,b) detected a seasonal release of mature actinospores of different types from 5 species of oligochaetes, with higher prevalence in the warmer months of the year. Similar results were also obtained by Xiao and Desser (Reference Xiao and Desser1998) in 25 types of actinospores released from oligochaetes, by Yokoyama et al. (Reference Yokoyama, Ogawa and Wakabayashi1993) with 5 different species of Myxozoa in the oligochaete Branchiura sowerbyi, and also by Oumouna et al. (Reference Oumouna, Hallett, Hoffmann and El-Matbouli2003) with 12 types of actinospores released from oligochaetes. In Sphaerospora truttae, the highest prevalence of echinactinomyxon was registered in the spring (Özer and Wootten, Reference Özer and Wootten2000). In marine actinosporea, Hallett et al. (Reference Hallett, O'Donoghue and Lester1998) did not notice any seasonal variation in the prevalence of infection of Sphaeractinomyxon ersei in the oligochaete Doliodrilus diverticulatus. In contrast, E. mugilis exhibits seasonal occurrence of infection in Nereis diversicolor during winter and spring months (Rangel et al. Reference Rangel, Santos, Cech and Székely2009).
Here we describe the morphology, the molecular data and the developmental stages (inside the marine polychaete Diopatra neapolitana) of a new type of actinospore, the Unicapsulactinomyxon. Additionally, we also report preliminary ecological data of prevalence of infection and its seasonal variation in the Aveiro estuary (Portugal) during 2 sampling years.
MATERIALS AND METHODS
The actinosporean survey included 517 specimens of Diopatra neapolitana (247 females, 214 males and 56 specimens of undetermined sex) collected monthly between January and November 2007 and 942 specimens (468 females, 372 males and 102 specimens of undetermined sex) collected monthly between January and December 2009. The worms were collected by local bait diggers in the Aveiro estuary, Portugal (40°40′N:8°45′W). The polychaetes were identified by the keys of Fauvel (Reference Fauvel1923) and Fauchauld (Reference Fauchald1977) and using the work of Dağli et al. (Reference Dağli, Ergen and Çinar2005) and Rodrigues et al. (Reference Rodrigues, Pires, Mendo and Quintino2009). The polychaetes were sexed by examination of their gametes under a microscope and then their tissues searched for myxozoan actinospores and their developmental stages. Coelomic fluid was obtained either with the help of a hypodermic needle and syringe (according to the procedure of Rangel et al. Reference Rangel, Santos, Cech and Székely2009) or by a simple incision in a parapodium, then examined under a microscope (at least 250× magnification). Samples from the intestine, muscles and tegument were also examined by compression of the tissues between 2 slides and viewing under the microscope.
Prevalence of infection was determined as the percentage of infected hosts in the total number of examined hosts, for each season and year, in 2007 and 2009 (Bush et al. Reference Bush, Lafferty, Loft and Shostak1997). Smears containing coelomic fluid with spores were air dried, and some were fixed with Davidson's seawater fixative. They were then either stained with Giemsa or with May-Grunwald and Giemsa, and finally mounted in Entellan. Tissue samples of infected polychaetes were fixed with Davidson's seawater fixative for 24 h and preserved in 70% ethanol, after which they were processed for histology and stained with haematoxylin and eosin (H&E) or Giemsa.
Potential spore production was estimated using the same methodology and formula as Meaders and Hendrickson (Reference Meaders and Hendrickson2009). The spores were counted in 5 μm histological cross-sections of D. neapolitana-infected segments. For this estimation of spore production, the median±standard deviation (s.d.) number of spores per infected segment was used.
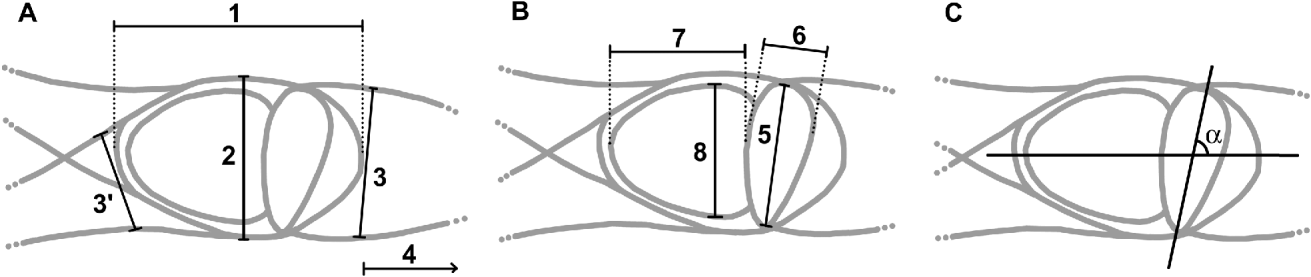
The myxozoan spores and their developmental stages were examined and photographed using a Zeiss Axiophot microscope (Grupo Taper, Sintra, Portugal), equipped with a Zeiss Axiocam Icc3 digital camera. Axiovision 4.6 software (Grupo Taper) was used for the image analysis. Morphological descriptions and measurements of actinospores were performed according to Lom et al. (Reference Lom, McGeorge, Feist, Morris and Adams1997) from fresh material, as indicated in Fig. 1 (spore total length, spore body length and width, polar capsule length and width, sporoplasm length and width, processes length and width, and polar capsule length angle in relation to the spore body axis). All measurements included mean values,±s.d. and range and number of measurements (n), and were from spores and developmental stages of 2 hosts.
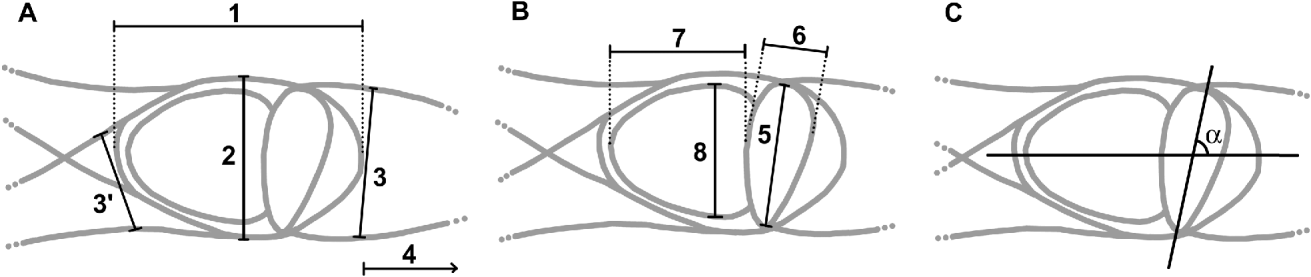
Fig. 1. New actinosporean type. Unicapsulactinomyxon: (A) Measurements of the actinospore. 1, spore body length; 2, spore body width; 3 and 3′, process width; 4, process length. (B) 5, polar capsule length; 6, polar capsule width; 7, sporoplasm length; 8, sporoplasm width. (C) Measurement of the angle of the polar capsule length in relation to the spore body axis.
For DNA extractions, a sample (collected in October 2007) preserved in ethanol was centrifuged at 8000 g for 10 min to pellet the actinospores; then the ethanol was removed. The DNA was extracted using a QIAGEN DNeasyTM tissue kit (animal tissue protocol, QIAGEN) and eluted in 75 μl of buffer AE.
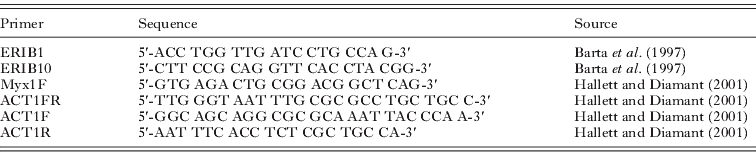
The 18S rDNA was amplified using the primers ERIB1 and ERIB10 (Table 1) in a 20 μl reaction mixture, which comprised 1 μl of extracted genomic DNA, 5 μl of 1 mm deoxy-ribonucleotide triphosphates (dNTPs, MBI Fermentas), 15 pmol of each primer, 2·5 μl 10× Taq buffer (MBI Fermentas), 1·25 μl of 25 mm MgCl2, 0·1 μl of Taq polymerase (2 U) (MBI Fermentas) and 12 μl of distilled water. The PCR cycle consisted of an initial denaturation step of 94°C for 4 min, followed by 35 cycles of 94°C for 50 s, 56°C for 50 s, 72°C for 80 s and finished with terminal extension at 72°C for 7 min and rested at 4°C. This was followed by a second round of PCR with the MYX1F-ERIB10 primer pair (Table 1). The total volume of the nested PCR reactions was 50 μl, which contained 1 μl of amplified DNA, 10 μl of 1 mm deoxy-ribonucleotide triphosphates (dNTPs, MBI Fermentas), 30 pmol of each primer, 5 μl of 10× Taq buffer (MBI Fermentas), 2·5 μl of 25 mm MgCl2, 0·2 μl of Taq polymerase (2 U) (MBI Fermentas) and 28·5 μl of water. Amplification conditions in the second round were: 94°C for 50 s, 56°C for 50 s, 72°C for 60 s for 35 cycles, and the cycle was terminated with an extension period at 72°C for 10 min and rested at 4°C. Both PCR cycles were performed in a PTC-200 thermocycler (MJ Research). The PCR products were electrophoresed in 1·0% agarose gels in Tris-Acetate-EDTA (TAE) buffer gel stained with 1% ethidium bromide and then purified with the EZ-10 Spin column PCR Purification Kit (Bio Basic Inc., Canada).
Table 1. Primers used for PCR or sequencing
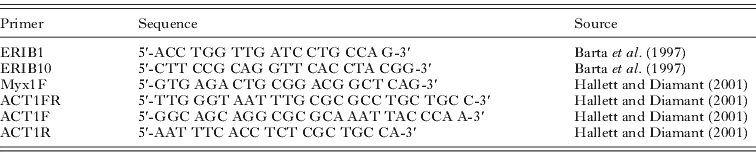
Purified PCR products were sequenced in both directions with primers as listed in Table 1 using the ABI BigDye Terminator v3.1 Cycle Sequencing Kit with an ABI 3100 Genetic Analyser.
The various forward and reverse sequence segments were aligned in BioEdit (Hall, Reference Hall1999) and ambiguous bases clarified using corresponding ABI chromatograms. Nucleotide sequences were aligned with the software CLUSTAL W (Higgins et al. Reference Higgins, Thompson, Gibson, Thompson, Higgins. and Gibson1994). The alignment was corrected manually using the alignment editor of the software MEGA 4.0 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). DNA sequence similarities were calculated with the Sequence Identity Matrix of the software BioEdit. Minimum evolution (MI) and maximum parsimony (MP) were performed and the resulting topologies were compared to each other.
MI calculations were performed with MEGA 4.0. using the Tamura-Nei model. Gaps were treated via pairwise-deletion. Pattern among lineages was handled as homogeneous. In total, 1000 bootstrap replicates were applied.
MP analyses were performed in Mega 4.0 by close-neighbour-interchange (CNI) search with random taxa addition (10 replications). All characters were treated as unordered, Ts/Tv ratio was set to 1:2, and gaps were treated as missing data. Branch supports were obtained by 100 bootstrap replicates with random sequence additions. NJ and MP tree were visualized using the tree explorer of Mega 4.0.
H&E and Giemsa-stained slides of histological sections of infected D. neapolitana and air-dried spores of Unicapsulactinomyxon are lodged at the Natural History Museum, London (Catalogue numbers: NHMUK 2011.1 and NHMUK 2011.2, respectively).
RESULTS
Survey of actinospores
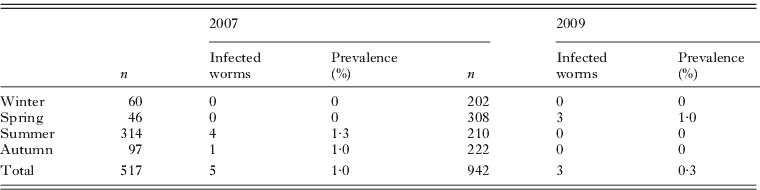
From the sample of D. neapolitana analysed in 2007 (n=517), 5 specimens (1·0%) were infected with a new type of actinospore, and from the sample of 2009 (n=942), 3 specimens (0·3%) were infected with the same type. All infected specimens were males, having a prevalence of 2·3% (5 out of 214) and 0·8% (3 out of 372), in 2007 and 2009 respectively, considering only the male worms. No other type of actinospores or species of Myxozoa was found during this survey. The infection was only present (Table 2) in the summer (July–September) and autumn (October–November) with a prevalence of 1·3% and 1·0%, respectively, in 2007; and only in the spring (April–June) with a prevalence of 1·0%, in 2009.
Table 2. Diopatra neapolitana sampling and prevalence of infection for Unicapsulactinomyxon

The actinosporean spores and their developmental stages were only observed in the polychaete's posterior (non-branchiae) segments, in the coelomic cavity, in coexistence with the worms’ male gametes (Fig. 2). No particular gross pathology was visible in the infected hosts, but there was a major reduction in the amount of gametes in the coelomic fluid in comparison with uninfected worms. The estimated potential spore production was 147 266±53 174·7 spores per infected segment, considering that D. neapolitana posterior segments have a median length of 1·3 mm in the histological longitudinal sections.

Fig. 2. Histological section of Diopatra neapolitana infected with Unicapsulactinomyxon parasites and stained with H&E. The coelomic cavity is filled with actinosporean developmental stages. Inset: amplification of the content of the coelom showing binucleated cells and pansporocysts with developing spores inside. Abbreviations: CC, coelom cavity; IE, intestinal epithelium; IL, intestinal lumen; MB, muscle bundles.
Spore morphology and developmental stages
The development of this parasite is asynchronous, as several gametogony and sporogony development stages could be simultaneously observed in the coelomic fluid. Within gametogonic pansporocysts, the development of the gametes is asynchronous. In the sporogonic pansporocysts, the development of the spores is initially asynchronous, whereas the later stages are synchronous.
The earliest identifiable stages are round binucleated cells, 13·6±1·7 μm (9·4–16·0; n=20; Figs 3A and 4A–B) in diameter. The binucleated cells originate a tetranucleated cell (Fig. 4C,D). These cells then divide, by plasmotomy, into 4 new cells (Fig. 3B–D), which originate the early form of the pansporocyst stage. This measured 15·8±1·2×14·0±0·9 μm (13·2–18·0×12·7–16·0; n=17; Fig. 3E) and is formed by 2 outer enveloping cells plus 2 inner cells (α and β cells). The two internal cells continue to divide originating a pansporocyst with 4 internal cells that measured 17·0±0·8×15·0±0·8 μm (16·1–18·4×14·1–16·1; n=12; Figs 3F and 4E). After this stage, the pansporocyst develops into 6 internal cells (4 α cells smaller than the other 2 β cells) that measured 17·3±1·0×16·1±0·8 μm (15·4–18·8×14·7–17·5; n=16). The next observed stage was the pansporocyst, with 10 internal cells (8 α cells smaller than the other 2 β cells), and measuring 20·4±1·9×19·3±1·7 μm (18·3–23·7×17·4–22·7; n=10; Figs 3G and 4F–H). This stage is then followed by pansporocysts of 12 inner cells (8 smaller α cells plus 4 cells originated from the 2 larger β cells of the previous stage) (Figs 3H and 4J,K) and, finally, 16 equally sized inner cells, measuring 27·7±4·7×25·6±3·9 μm (21·4–37·9×20·5–33·3; n=20; Figs 3I and 4L). This last stage marks the end of the gametogony phase, which is then followed by the fusion of the 16 gametes (Fig. 4N). Fig. 4M depicts this process. There, 10 gametes had already formed 5 zygotes, while 6 gametes are still visible. In Fig. 4M–N, the 2 nuclei of each fused gamete (α and β) are still visible inside the zygotes. In all these stages, the pansporocysts were usually rounded or slightly oval in shape.

Fig. 3. Developmental stages of the Unicapsulactinomyxon (actinosporean) parasite in the coelomic fluid of Diopatra neapolitana, from fresh material. (A) Initial binucleated round cells. (B–E) Pansporocyst formation. (F) Pansporocyst with 4 inner cells. (G) Pansporocyst with 10 inner cells. (H) Pansporocyst with 12 inner cells. (I) Pansporocyst with 16 inner cells or gametes. (J–O) Pansporocysts with 8 developing spores.

Fig. 4. Gametogony developmental stages of the Unicapsulactinomyxon (actinosporean) parasite in the coelomic fluid of Diopatra neapolitana, from smears stained with Giemsa. (A–B) Initial binucleated cells. (C) A binucleated cell with its 2 nuclei in division. (D) A tetranucleated cell stage. (E) Pansporocyst with 4 inner cells. (F) Pansporocyst with 10 inner cells (2 β cells and 8 α smaller cells). (G) Pansporocyst in the 10 cells stage with 8 α cells undergoing meiosis. (H) Pansporocyst in the 10 cells stage with 8 α cells undergoing meiosis and the 2 β cells undergoing their second mitotic division. (I) The same as H, in which same α cells are in the process of expulsion of their polar bodies. (J) Pansporocyst in the 12 cells stage, with 8 α gametes, 4 β cells and 8 polar bodies. (K) Same as J, but meiosis had not occurred yet. (L) Pansporocyst with 16 gametes and 16 polar bodies. (M) Pansporocyst where copulation was occurring, showing 5 zygotes and 6 gametes. (N) Pansporocyst showing 8 zygotes in which 2 nuclei are still visible inside 5 zygotes.
During gametogenesis, the process of meiosis was only observed starting at the stage of pansporocysts with 10 internal cells (8 α smaller cells and 2 β larger cells; Fig. 4G). The first free polar bodies were only observed when the 2 β cells were finishing their second mitotic division (Fig. 4I). Fig. 4I shows several α cells expulsing their polar bodies. In some pansporocysts, the α cells’ meioses started before the 2 β cells initiated their second mitotic division (Fig. 4G). In other pansporocysts, the α cells’ meiosis started when the 2 β cells were already in their second mitotic division (Fig. 4H), whereas, in some others, the pansporocysts were already in the stage of 12 internal cells (8 α and 4 β), while the 8 α cells had not yet initiated meiosis (Fig. 4K). In fact, we could observe pansporocysts in the 12 internal cells stage, without any polar bodies inside (Fig. 4K), and equivalent stages with 8 free polar bodies inside the pansporocyts, resulting from the meiosis of the 8 α cells (Fig. 4J). The process of meiosis only happened after the internal cells (α or β) had completed their third mitotic division. For this reason, the α cells were the first to undergo meiosis, and the β cells only started meiosis after their third mitotic division, at the stage of pansporocysts with 16 internal cells. The polar bodies could be seen until the initial stages of sporogony (when the spores started the development of their processes) (Fig. 5F, G).

Fig. 5. Sporogony of developmental stages of the Unicapsulactinomyxon (actinosporean) parasite in the coelomic fluid of Diopatra neapolitana, from smears stained with Giemsa. (A) Pansporocyst with 8 zygotes and 16 polar bodies. (B–C) Pansporocysts showing 8 zygotes undergoing their first mitotic division. (D) Pansporocyst with 8 early spores formed by 2 cells each. (E) Pansporocyst with early spores constituted by 3 or 4 cells, some of which are in division. (F) Early spores constituted by 4 or 5 cells. Polar bodies are still visible in spite of the rupture of the pansporocyst wall. (G) Pansporocyst with spores already developing their long valve processes. An isolated spore within this stage can be seen in M. (H) Pansporocyst with more mature spores. An isolated spore within this stage can be seen in Q. (I) Mature pansporocyst with mature spores. (J) An isolated early spore in the stage of 5 cells. (K) Same as J, but one cell is in division (maybe producing the binucleated sporoplasm) or is in a process of second meiosis; see the text for more details. (L) An isolated early spore showing 3 valvogenic cells (vc), 1 binucleated sporoplasm (sc) and 1 capsulogenic cell (cc), occupying more or less their definitive positions. (M) An isolated spore already with the valves extending in 3 processes, showing the same cells as in L. (N) A young spore showing the development of a capsular primordium (cp) extended by an external tube (et) inside the capsulogenic cell. (O) Same as N, but the external tube seems smaller in length (growing or reducing?). (P) An isolated spore with its polar capsule without the external tube, and with a precocious polar filament inside. The valvogenic cell nuclei were very slender, and the sporoplasm has started to take up stain more intensively. (Q) An almost mature spore. In figures O and P, some artefacts (*) are visible which resulted from the mounting medium.
Within the sporogony phase, we observed pansporocysts with 8 zygotes (Fig. 5A–C). Pansporocysts in the initial phases of sporogony measured 28·7±1·7×27·1±1·5 μm (26·8–33·7×22·8–29·1; n=15; Figs 3J–L and 5D–F). The spores continued their development and the valve processes started to form (Figs 3M and 5G). In the last stages, mature pansporocysts could be observed, usually with a very irregular form and measuring 38·5±5·0×28·1±2·7 μm (30·2–46·8×22·1–32·6; n=20; Figs 3O and 5I), with 8 spores inside. In some pansporocysts we could observe the spores with their valve processes aligned against the pansporocyst wall, giving the misleading impression of very thick walls (Fig. 3N). During the entire development, the pansporocysts were composed of only 2 enveloping cells.
Spore development initiated with division of the zygotes (Fig. 5B, C). Each zygote underwent several divisions (Fig. 5D–F, J, K), giving rise to spores with 5 cells (Fig. 5J, K). These 5 cells (1 of which was binucleated) positioned themselves in such a way that they occupied more or less their definitive positions (Fig. 5L), and the function of each cell has therefore become clearer. The binucleated cell – with 2 rounded nuclei – was the sporoplasm (Fig. 5L, sc), the 3 peripheral cells – with smaller and more elongated nuclei – were the valvogenic cells (Fig. 5L, vc), and the more central cell with a big nucleus was a capsulogenic cell (Fig. 5L, cc). We could not observe any more cells (nuclei) besides the ones described, which means that 2 capsulogenic cells are missing in this stage. The next step was the elongation of the valvogenic cells that form the long processes present in the mature spore. Their nucleus becomes more slender and more difficult to observe in the more mature spores (Fig. 5P, Q). Meanwhile, in the capsulogenic cell, the development of the polar capsule seemed to begin with a vesicula or tube-like structure which, later on, and by swelling, assumed the form of a capsular primordium with an external tube (Fig. 5N, O). As the external tube disappeared (Fig. 5O), the polar filament started to become visible inside the polar capsule (Fig. 5P, Q). The nuclei of the capsulogenic cell, which was initially larger, started to reduce in size (Fig. 5P, Q), and became difficult to distinguish in the more mature spores (Fig. 5H, I). The sporoplasm, which became binucleated earlier (Fig. 5L, K), with 1 nucleus slightly smaller than the other, did not change until a later stage, when it grew in size, occupying the entire space of the body cavity bellow the polar capsule, and staining more intensely (Fig. 5Q), making the observation of its nucleus in the mature spores difficult (Fig. 5H, I).
Mature spores (Figs 6 and 7) were characterized by an elliptical spore body, 16·0±1·5 (14·3–18·8; n=10) μm long by 9·6±0·8 (8·8–11·5; n=10) μm wide, and exhibiting 3 long valve processes. One of these arose from one side of the spore body (the polar capsule side) and measured 110·1±7·5 (99·0–121·7; n=10) μm in length and 6·5±0·3 (5·9–7·1; n=10) μm wide, while the other 2 processes arose from the opposite side of the spore body (the sporoplasm side) and measured 117·3±8·1 (102·7–129·8; n=15) μm in length and 4·0±0·3 (3·6–4·7; n=10) μm wide. The total spore length, from one tip of the processes to the tip of the opposite process, was 241·3±13·7 (224·7–262·3; n=10) μm. The valve processes tapered quickly towards the tips and do not form nets with the processes of other nearby spores. The binucleate sporoplasm was 8·6±0·6 (7·8–9·5; n=10) μm long and 7·5±0·5 (6·5–8·3; n=10) μm wide. The spore had a single and large polar capsule, of oval and elongated shape, measuring 9·3±0·7 (8·1–10·3; n=20) μm in length and 4·1±0·3 (3·5–4·8; n=20) μm wide. Its length orientation was nearly perpendicular to the spore axis, with a slight slope (Fig. 1C) forming an angle of 82° (74–90°; n=14). The polar filament was folded in a longitudinal manner parallel to the capsule axis and, when extruded, it measured 24·3±1·1 (23·3–27·1; n=10) μm of total length. The polar filaments were extruded after a short period of time in a drop of salt water, or immediately when a drop of urea-saturated solution was added. After extrusion, the polar filament seemed to form a loop or a swelling at its extremity (Fig. 7C–E). This swelling in the polar filament extremity, in the air-dried smears from one host, measured 7·2±0·9 (5·4–8·7; n=10) μm in length (almost 30% of the polar filament length) and 4·0±0·6 (2·4–4·5; n=10) μm in width (Fig. 7D, E).
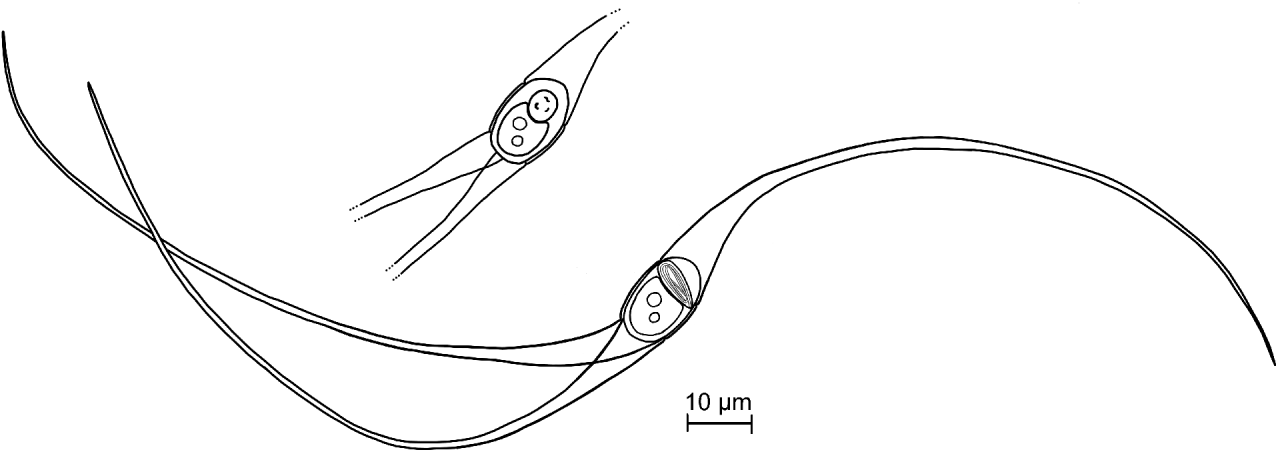
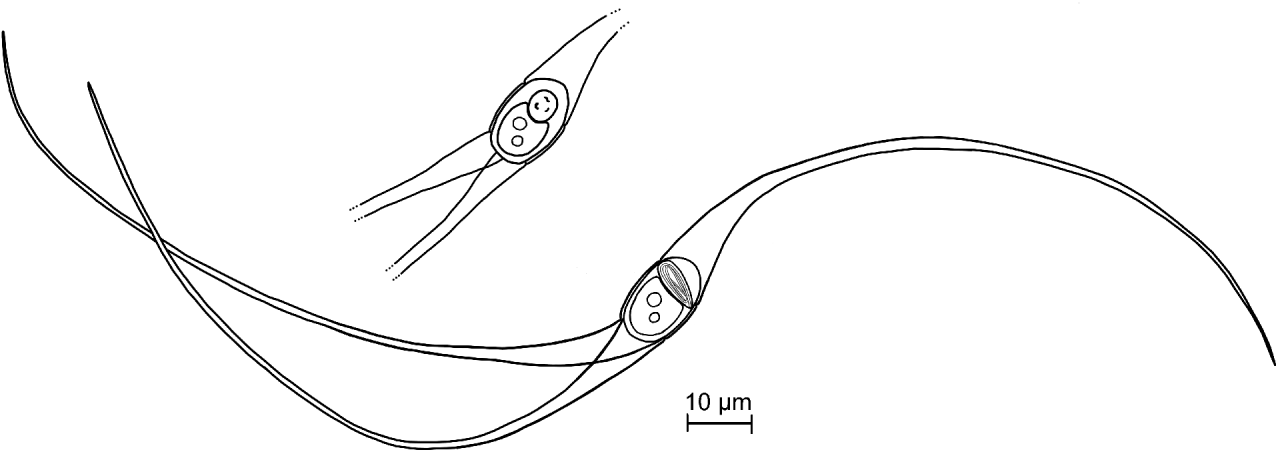
Fig. 6. Line drawings of the Unicapsulactinomyxon spores from the coelomic cavity of Diopatra neapolitana. A full spore showing the polar capsule in a lateral view and a spore body showing the polar capsule in an apical view.

Fig. 7. Unicapsulactinomyxon spores from the coelomic cavity of Diopatra neapolitana. (A) Actinospore in a smear of coelomic fluid. (B) Spore bodies of 3 fresh actinospores. (C) The extruded polar filaments of 2 fresh spores. Note the filament extremity with a loop or a swelling (arrows). (D–E) Extruded polar filaments in a smear stained with May-Grunwald and Giemsa.
Molecular results
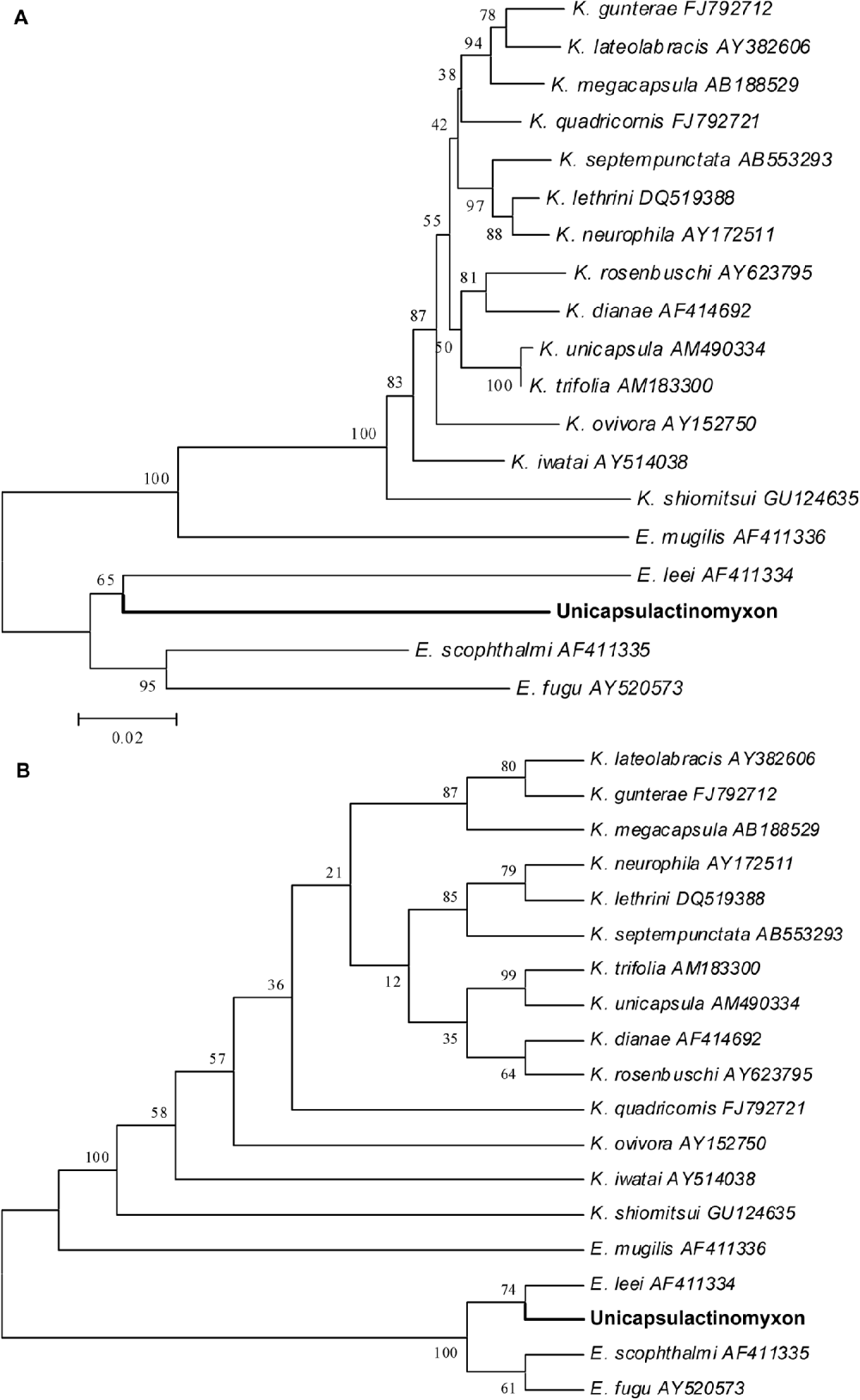
The amplification and sequencing of the 18S rDNA of the new actinospore type from the D. neapolitana sample was very difficult to achieve, and only 1009 bps were sequenced. The MYX1F and SPHr primers amplified 1650 bps, but only 1009 bps were sequenced by ACT1FR, ACT1F and ACT1R primers, and only at the 5′ end. Several other primers, designed for myxozoans (not reported), were also tested and failed at amplification and sequencing. The sequence of the new actinospore type was deposited in the GenBank under Accession number HQ692887. The actinospore sequence did not match any available myxospore sequences, and no close relatives could be found as shown in the phylogenetic tree (Fig. 8). The highest similarity was to Enteromyxum species; E. scophhtalmi, 84·1%; E. fugu, 83·6%; E. leei, 81·0%. The distant relatives are E. mugilis and various Kudoa species with 76–80% similarity.
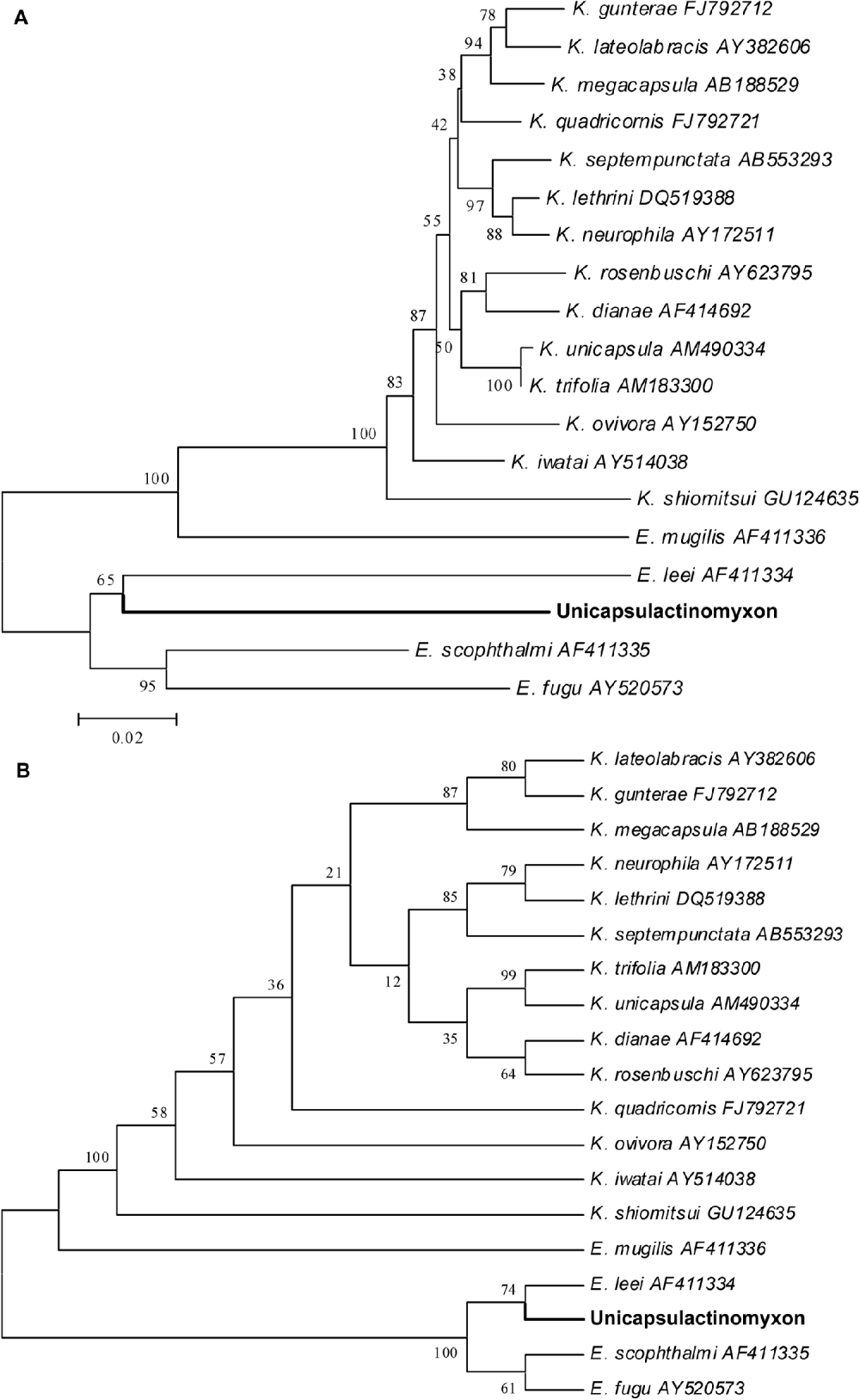
Fig. 8. Phylogenetic tree generated by minimum evolution (A) and maximum parsimony (B) analysis of the 18S rDNA sequences of the new Unicapsulactinomyxon type presented in this paper and by some other marine myxozoan relatives. Bootstrap values are presented in the order of NJ, MP, ML, BI. Sequences downloaded from GenBank are presented with their Accession numbers.
Taxonomic summary
Unicapsulactinomyxon, novel actinospore collective group
Spores with an elliptical spore body, holding a binucleated sporoplasm, 1 large polar capsule and 3 long valve processes (Fig. 6). Spore development in groups of 8 inside pansporocysts formed by 2 enveloping cells. Free spores do not form nets.
Type-host: Diopatra neapolitana (Delle Chiaje, 1841)
Type-locality: Aveiro estuary, Portugal.
Prevalence of infection: 1·0% in 2007 and 0·3% in 2009, in a total of 517 and 942 worms analysed, respectively.
Pathology: A major reduction in the amount of gametes in the infected hosts (all males).
Site of infection: Coelomic cavity.
Myxosporean form: Unknown.
Etymology: The prefix Unicapsula reflects the presence of a single polar capsule – unique in the actinosporean group – that constitutes the main morphological characteristic of this new spore type.
Deposited material: H&E and Giemsa-stained slides of histological sections of infected D. neapolitana and air-dried spores at the Natural History Museum, London, Catalogue numbers: NHMUK 2011.1 and NHMUK 2011.2, respectively.
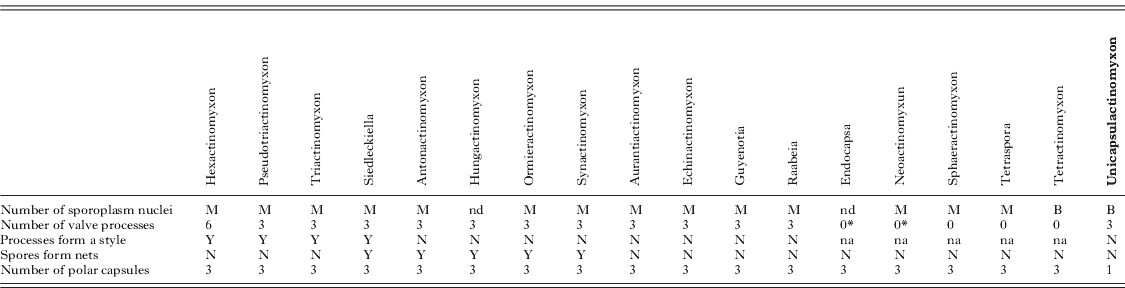
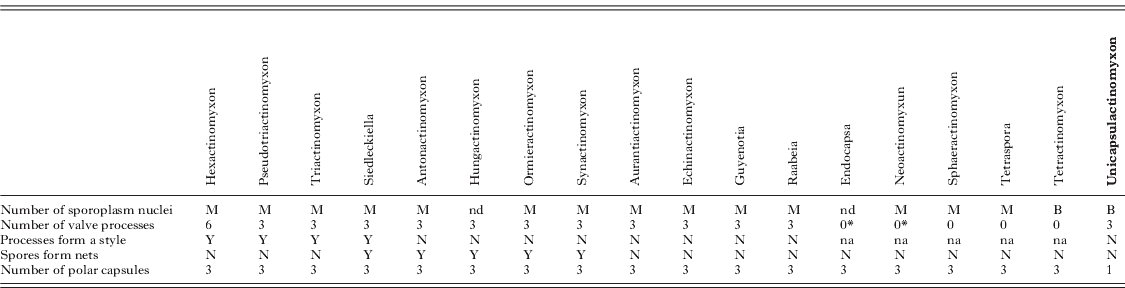
Remarks: The main features of the 18 actinosporean collective groups can be seen in Table 3. The most important feature from this newly described spore type, Unicapsulactinomyxon, is that it differs from all other spore types previously reported in the literature, in that it has a single and large polar capsule.
Table 3. Summary of the main features of 18 actinosporean collective groups according to Janiszewska (Reference Janiszewska1955, Reference Janiszewska1957) and Lom and Dyková (Reference Lom and Dyková2006) compared with the Unicapsulactinomyxon type
DISCUSSION
With the demise of the Actinosporea class, the different types of actinospores are ascribed to actinospore collective groups (Lom et al. Reference Lom, McGeorge, Feist, Morris and Adams1997; Kent and Lom, Reference Kent and Lom1999; Kent et al. Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Longshow, Palenzeula, Siddall and Xiao2001; Lom and Dykova, Reference Lom and Dyková2006). The distinction between different spore types is based on their morphology and nuclei number of the sporoplasm (Janiszewska, Reference Janiszewska1955, Reference Janiszewska1957; Lom and Dyková, Reference Lom and Dyková2006) and also on 18S rDNA data (see Bartholomew et al. Reference Bartholomew, Whipple, Stevens and Fryer1997; Hallett et al. Reference Hallett, Erséus and Lester1999, Reference Hallett, Atkinson, Schöl and El-Matbouli2003; Negredo et al. Reference Negredo, Dillane and Mulcahy2003; Rácz et al. Reference Rácz, Eszterbauer and Molnár2005; Lom and Dyková, Reference Lom and Dyková2006). The novel type of spore, Unicapsulactinomyxon, reported here, shares most characteristics with the tetractinomyxon type i. e. it is a marine parasite with a binucleated sporoplasm which uses the body cavity of polychaetes as its habitat. However, it contrasts with the tetractinomyxon type definition (Ikeda, Reference Ikeda1912) by having 3 long valve processes. The lack of style, the binucleated sporoplasm and 3 valve processes, which do not form nets, distinguish this spore from all other spore types. In conclusion, not only does this spore have a combination of morphological characteristics that distinguishes it from all other types of actinospores described to date, it also has one exclusive characteristic: a single polar capsule. This feature justifies the establishment of a new actinospore collective group, Unicapsulactinomyxon.
This novel type spore does not correspond to any of the myxospores already sequenced, and the closest relatives found were, according to the SSU rDNA investigations, the Enteromyxum species, but with very low similarity (81·0–84·0%) in their 18S rDNA sequences. This explains the difficulty encountered with the attempt to amplify and sequence the 18 rDNA of this novel type spore. Nevertheless, 1009 bps are sufficient to ensure that this spore is not the alternative life stage of any sequenced myxospore.
The development of this type of spore, in D. neapolitana, as seen in light microscopy, is similar to the development pattern described for the majority of the actinospore types (see Ikeda, Reference Ikeda1912; Mackinnon and Adam, Reference Mackinnon and Adam1924; Janiszewska, Reference Janiszewska1955, Reference Janiszewska1957; Ormières and Frézil, Reference Ormières and Frézil1969; El-Matbouli and Hoffmann, Reference El-Matbouli and Hoffmann1998; Hallett et al. Reference Hallett, O'Donoghue and Lester1998; Lom and Dyková, Reference Lom and Dyková2006; Meaders and Hendrickson, Reference Meaders and Hendrickson2009; Rangel et al. Reference Rangel, Santos, Cech and Székely2009; Morris, Reference Morris2010), with the exception that this spore only develops one single functional polar capsule. The earliest stages of infection observed were binucleated cells. These cells should result from a schizogony multinucleated cell stage (El-Matbouli and Hofmann, Reference El-Matbouli and Hoffmann1998; Meaders and Hendrickson, Reference Meaders and Hendrickson2009) which, however, was not observed in this work. The binucleated cells then form a tetranucleate cell that undergoes plasmotomy and forms 4 cells. Two of these will form the pansporocyst surrounding the other two internal cells, which afterwards will form the gametes. This sequence is in agreement with the work of Janiszewska (Reference Janiszewska1957) on the development of Raabeia gorlicensis, and with the description made by El-Matbouli and Hoffmann (Reference El-Matbouli and Hoffmann1998) explaining the origin of the early pansporocysts in the development of the triactinomyxon stage of Myxobolus cerebralis. The two internal cells then divide 3 times and undergo meiosis, originating 16 gametes and 16 polar bodies. The polar bodies started to appear from the pansporocyst stage with 12 internal cells, which is consistent with the actinosporean development described by other previously conducted studies (see Ikeda, Reference Ikeda1912; Mackinnon and Adam, Reference Mackinnon and Adam1924; Janiszewska, Reference Janiszewska1955, Reference Janiszewska1957; Ormières and Frézil, Reference Ormières and Frézil1969). During gametogenesis, the starting of the α cell meiosis process is independent of the start of the second mitotic division of the β cells. Therefore, meiosis of the α cells can be initiated either before, or after, the two β cells start their second mitotic division or even after the β cells have concluded their second mitotic division. Janiszewska (Reference Janiszewska1957) described a second meiotic division occurring in the developing spore, before the sporoplasm mother cell starts to divide mitotically. We cannot assert the occurrence of this second meiotic division in the species studied here, but the illustration in Fig. 5K is very similar to the drawing in Fig. 19 of Janiszewska's work (Reference Janiszewska1957), representing the expulsion of the second polar body. However, our illustration could also represent the division of the sporoplasm mother cell, which becomes binucleated after this stage.
The 16 gametes form 8 zygotes. Each zygote should divide several times giving rise to an early spore with at least 7 cells (or nuclei), representing 3 capsulogenic and 3 valvogenic cells and the sporoplasm mother cell (Ikeda, Reference Ikeda1912; Mackinnon and Adam, Reference Mackinnon and Adam1924; Janiszewska, Reference Janiszewska1955, Reference Janiszewska1957; Ormières and Frézil, Reference Ormières and Frézil1969; El-Matbouli and Hoffmann, Reference El-Matbouli and Hoffmann1998). But, in our species, the zygote's divisions resulted in an early spore with only 5 cells, which is in contrast to what was described by other authors. As discussed above, Fig. 5K could either represent a hypothetical second meiosis, or the division of the sporoplasm mother cell, which, after this stage becomes binucleated and remains as such until the end of the sporogony. The 5 cells in this early spore then start to position themselves in their final locations – with 3 cells in the ‘corners’ of the spore, representing the 3 valvogenic cells; 1 binucleated cell, representing the sporoplasm; and 1 cell with a large nucleus, representing 1 capsulogenic cell. There was no evidence of the other 2 capsulogenic cells, which could be an indication that these 2 cells are not formed. After this stage, the development of this spore is once again similar to what is described by other authors, with the exception that it only develops 1 polar capsule. The polar bodies resulting from the gametogony meiosis are still visible within the pansporocysts during the sporogony phase, and start to disappear when the spores initiate the development of their processes. This is in agreement with the studies of Ikeda (Reference Ikeda1912) on the development of Tetractinomyxon intermedium and the studies of Ormières and Frézil (Reference Ormières and Frézil1969) on the development of Aurantiactinomyxon eiseniellae. The development of the polar capsule in this spore initiates with a small vesicule or tube-like form inside the capsulogenic cell that, later on, grows into a swelling, forming a large vesicular structure prolonged by a tube. This vesicular structure is the capsular primordium and its tubular prolongation is the external tube. Then, the external tube disappears, and a polar filament starts to appear inside the polar capsule.
The apparent absence of 2 capsulogenic cells diverges from what is known in the actinosporeans. Nevertheless, we must consider the hypothesis that these 2 capsulogenic cells are indeed formed, and that their polar capsules remain only as capsular rudiments, as happens with the myxospores of the Unicapsula genera, which have 1 functional polar capsule and 2 capsular rudiments only discernible by electron microscopy (Lom and Dyková, Reference Lom and Dyková2006).
Unicapsulactinomyxon has a polar filament coiled in a longitudinal manner that is an uncommon feature in the Myxozoa spores. In the majority of the myxospores and actinospores, the polar filament is positioned inside the polar capsule with a more or less helicoidal arrangement. There are a few species with polar filaments arranged differently. Among these are members of the families Auerbachiidae and Sphaeromyxidae (Lom and Dyková, Reference Lom and Dyková2006), and Hennegoides longitudinalis (Myxobolidae) (Lom et al. Reference Lom, Tonguthai and Dyková1991) whose polar filaments are arranged longitudinally inside the polar capsules of their myxospores. As the life cycles of these species are still unknown, it will be interesting to determine whether this feature is common to both spore stages or not.
Prevalence of infection of actinosporeans in the oligochaete hosts obtained in several previous studies (Yokoyama et al. Reference Yokoyama, Ogawa and Wakabayashi1993; El-Mansy et al. Reference El-Mansy, Székely and Molnár1998a, Reference El-Mansy, Székely and Molnárb; Xiao and Desser, Reference Xiao and Desser1998; Özer and Wootten, Reference Özer and Wootten2000; Oumouna et al. Reference Oumouna, Hallett, Hoffmann and El-Matbouli2003) cannot be compared directly with our results because we calculated the prevalence from the observed presence of the infection in the host, whereas the prevalence in the referred works is underestimated by the spore release (missing immature infections) and their consequent presence in the water column. In fact, El-Mansy et al. (1998a,b) found that the infection in the oligochaete host persists throughout the year, but the spore release was reported only in the warmer months (spring and summer). Similarly, Xiao and Desser (Reference Xiao and Desser1998) also reported that the abundance of waterborne spores peaked from late June to August when water temperatures ranged from 18 to 24°C and coincided with the feeding and growing season of larval fish.
Among the species in which the reported infection was detected in the host tissues, Hallett et al. (Reference Hallett, O'Donoghue and Lester1998) did not find any seasonal variation of S. ersei in the oligochate D. diverticulatus. In the polychaete Spirorbis spirorbis, Køie (Reference Køie2002) found actinosporean infections during the summer months, and in Hydroides norvegica, in September and January, even taking into account that the sampling was not made throughout the year. E. mugilis was found in N. diversicolor only during the winter and spring (Rangel et al. Reference Rangel, Santos, Cech and Székely2009). In the current work, actinosporean infection was detected during all seasons, except winter. In the 2 first studies, the actinospores are released from the host either via the intestine (D. diverticulatus) or through gonopores (S. spirorbis and H. norvegica), while in the case of N. diversicolor, the actinospores may have a different release mechanism. In this latter polychaete, which is a monotelic species, and dies immediately after spawning (Andries, Reference Andries2001), the infected males release their gametes and the actinospores at the same time by the rupture of its body wall (Rangel et al. Reference Rangel, Santos, Cech and Székely2009). Therefore, in some cases, the actinosporean seasonal prevalence seems to be coordinated with the vertebrate host life cycle (e.g. Xiao and Desser, Reference Xiao and Desser1998) or the invertebrate host life cycle (Rangel et al. Reference Rangel, Santos, Cech and Székely2009). This correspondence among gamete and spore release is difficult to establish for D. neapolitana, since its reproduction is still unknown. Another problem is that the low prevalence (0·3 to 1·0%) made it difficult to detect any seasonal patterns of prevalence of the actinosporeans in this species, thus a higher sampling size would be required to extract a possible seasonal pattern.
In order to assure the infection in the fish host population, one way to overcome such low prevalence value in the polychaete population is to ensure a high potential of actinosporean production. This relates directly with the number of spores per segment, the size of the worm (number of segments), and for this particular species, with the number of segments bearing male gonads, once the occurrence of the spores is associated with them. The median of D. neapolitana body segments is 269 (Rodrigues et al. Reference Rodrigues, Pires, Mendo and Quintino2009). The male gonads start to appear from segment 51 (Dağli et al. Reference Dağli, Ergen and Çinar2005). This gives a median number of 218 segments with gametes and, in the case of infection, actinosporeans. If we consider a potential production of 147 266 spores per segment, then 1 individual host has the potential to produce around 32 104 000 spores during the final stages of sporogenesis. Such a huge number of spores is not a surprise if we realize that this polychaete species can reach a median length of 364 mm. Meaders and Hendrickson (Reference Meaders and Hendrickson2009) estimated a median spore production per host of 4759 spores of Ceratomyxa shasta in the polychaete Manayunkia speciosa within the final stages of sporogenesis, which they estimated to have a duration of 14 days. So, there is a huge difference in the potential for spore production between these two polychaetes species. Of course, this is mainly due to the significant difference in their body sizes, but also in the location of the developmental stages of these parasites. The coelomic cavity of D. neapolitana offers much more space for spore development than the epidermal layer of M. speciosa, within which C. shasta spores develop (Bartholomew et al. Reference Bartholomew, Whipple, Stevens and Fryer1997). This means that only 1 segment in D. neapolitana harbours 31 times more spores then 1 entire individual of M. speciosa. In fact, Parvicapsula minibicornis – another parasite infecting M. speciosa – develops inside the coelomic cavity and Bartholomew et al. (Reference Bartholomew, Atkinson and Hallett2006) counted a maximum of 7000 spores in an individual host in a given moment. As Bartholomew et al. (Reference Bartholomew, Atkinson and Hallett2006) stated, the location at which spores develop affects the host spore production potential.
The actinosporean infection seems to have a negative effect on the sexual development of the invertebrate host, especially in the male host. Køie (Reference Køie2002) found infections only in the coelomic fluid of segments with the male gonads in the hermaphroditic polychaetes S. spirorbis and H. norvegica. She noticed a reduction in the male gametes accompanied by the elongation and swelling of the infected segments, while the female gonads seemed unaffected. Bartholomew et al. (Reference Bartholomew, Atkinson and Hallett2006) realized that the sex of the dioecious polychaete M. speciosa infected by P. minibicornis was very hard to determine, due to the absence of gonads and gametes in the majority of the infected individuals and they suggested that the infection may result in destruction or absorption of the gonads. Meaders and Hendrickson (Reference Meaders and Hendrickson2009) had the same problem with specimens of M. speciosa infected by C. shasta. The male gametes were highly deteriorated, and only adult females could be easily sexed. It should be noted though that the two latter referenced parasites infect specimens of M. speciosa of both sexes (Bartholomew et al. Reference Bartholomew, Atkinson and Hallett2006; Meaders and Hendrickson, Reference Meaders and Hendrickson2009). In N. diversicolor, which is also a dioecious species, Rangel et al. (Reference Rangel, Santos, Cech and Székely2009) found that E. mugilis only infect male individuals, and noticed a reduction in the amount of male gametes. D. neapolitana is a dioecious species, and only the male individuals were infected. It is worth noting that the infection was confined to the segments with gonads, similar to the 2 spionid species (Køie, Reference Køie2002).
In conclusion, the study of the marine actinosporeans is still uncommon and studies like ours will probably continue to expose new and interesting forms of spores.
FINANCIAL SUPPORT
The authors would like to thank CIIMAR – CIMAR Associated Laboratory and its Pluriannual Program for founding the work and for the partial funding for the Hungarian Research Fund (Project no. OTKA K 71837).