Introduction
Contagious ecthyma (CE) is a global viral disease with elevated prevalence in regions that have dense sheep and goat populations (Kumar et al., Reference Kumar, Narayan, Bhatt, Haq, Khurana, Tiwari, Karthik, Malik, Dhama and Chandra2015). The disease was reported in sheep and goats in many parts of the world: since 1920 in South West Africa, then in Greece in 1922, in France in 1923, in the USA (Texas) in 1932, and in Malaysia in 1935 (Asiah, Reference Asiah1990). The global outbreaks of CE cause great economic threats and losses due to indistinct origin and development of the disease (Cheng et al., Reference Cheng, Li, Li, Fan, Tang, Liu and Chen2018; Zamri-Saad et al., Reference Zamri-Saad, Kamal and Aziz1989). CE is considered a neglected tropical disease due to its perceived limited impact and the fact that it is self-limiting (Bala et al., Reference Bala, Balakrishnan, Abdullah, Mohamed, Haron, Jesse, Noordin and Mohd-Azmi2018a; Jesse et al., Reference Jesse, Latif, Abba, Hambali, Bitrus, Peter, Haron, Bala, Balakrishnan, Abdullah and Mohd Lila2018a). Sheep and goat rearing is regarded as an important livestock production venture with numerous economic benefits toward the livelihood of producers. It serves as a vital community economic activity for low income people, to sustain their living expenses via exchange of products and animals (Abdullah et al., Reference Abdullah, Ismail, Balakrishnan, Bala, Hani, Abba, Awang, Jesse, Arshad, Nazariah, Abdullah, Noordin and Mohd-Lila2015). Livestock production provides a crucial means for many people in the rural communities to exit from poverty. Unfortunately, animal rearing is always facing threats from various endemic animal diseases. CE is synonymous to the orf disease, contagious pustular dermatitis, sore mouth, infectious labial dermatitis, and scabby mouth (Winter and Charmley, Reference Winter and Charmley1999; Kerry et al., Reference Kerry, Anna and Glenda2012). The disease has been considered economically important due to its endemicity in sheep and goat herds globally, with greater severity in the latter species (Nfi, Reference Nfi1991; De La Concha-Bermejillo et al., Reference De La Concha-Bermejillo, Guo, Zhang and Waldron2003; Guo et al., Reference Guo, Zhang, Edwards, Ermel, Taylor and De La Concha Bermejillo2003; Jesse et al., Reference Jesse, Latif, Abba, Hambali, Bitrus, Peter, Haron, Bala, Balakrishnan, Abdullah and Mohd Lila2018a). CE has been reported in other wild ruminant species, camel and camelids, and members of the cervidae (Gitao, Reference Gitao1994; Khalafalla et al., Reference Khalafalla, El-Sabagh, Al-Busada, Al-Mubarak and Ali2015). This zoonotic viral infection was first reported in humans in 1934 by Newsome and Cross (Reference Newsome and Cross1934).
The Orf virus is transmissible to humans, causing skin lesions following an occupational exposure with infected animals or feeding troughs (Rebecca, Reference Rebecca2012; Maor et al., Reference Maor, Yu and Brand2017). This fact is synonymous with other infectious diseases such as Leptospirosis (Bashiru and Bahaman Reference Bashiru and Bahaman2018; Garba et al., Reference Garba, Bahaman, Khairani-Bejo, Zakaria and Mutalib2017; Reference Garba, Bahaman, Zakaria, Bejo, Mutalib, Bande and Suleiman2018), Pnuemonic Manheimiasis (Zamri-Saad et al., Reference Zamri-Saad, Effendy, Israf and Azmi1999). People, particularly small ruminant herders, veterinarians, members of the family who care for the young animals through bottle feeding as well as butchers and meat sellers can be infected by CE virus via direct contact with clinically infected animals and their carcasses during processing or via contaminated feeding materials (Nourani and Maleki, Reference Nourani and Maleki2006; Nandi et al., Reference Nandi, De and Chowdhury2011; Shapiro, Reference Shapiro, Cohen, Powderly and Opal2017). Lesions develop mostly on fingers, hands, forearms, face, and sometimes on genitalia (Hagis and Ginn, Reference Hagis, Ginn, McGravin, Carlton and Zachary2001). CE among butchers reportedly had a prevalence of 24.4% before the feast of the sacrifice and 40.5% among slaughterers after the feast of the sacrifice. Personnel involved in slaughtering animals were extremely exposed to the disease. The preponderance before the feast was among males and preponderance among females was after the feast of the sacrifice, at 30.4 and 32.7%, respectively (Saçar et al., Reference Saçar, Uyar, Saçar and Duran2015). There were cases of CE lesion development associated with erythema multiform and bullous pemphigoid-like eruption and large vesiculo-pustular structures (Alian et al., Reference Alian, Ahangarkani and Arabsheybani2015). The human form of CE is more persistent in livestock-rearing communities and meat processors (Buchan, Reference Buchan1996; Jesse et al., Reference Jesse, Hambali, Abba, Lin, Chung, Bitrus, Abdullah, Balakrishnan, Bala and Lila2018b; Bala et al., Reference Bala, Balakrishnan, Jesse, Abdullah, bin Noorzahari, Ghazali, Mohamed, Haron, Noordin and Mohd-Azmi2020). The inter-human transmission of CE is rare. The disease has a vivid self-limited course and it resolved in 4–8 weeks after advancing through several stages (Maor et al., Reference Maor, Yu and Brand2017).
In infected animals, the stress due to the disease and skin lesions will cause lower milk production, lower weight gain, lower quality of animal skin for leather, and lower value of animal wool (Nandi et al., Reference Nandi, De and Chowdhury2011; Yu et al., Reference Yu, Tan, Zhao, Zhang, Ma, Wu, Zhu and Cui2017). Although CE is endemic, the actual prevalence of infections within the livestock herds is greatly underrated (Hosamani et al., Reference Hosamani, Scagliarini, Bhanuprakash, McInnes and Singh2009). This review is aimed to provide useful information about the virus infection, viral disease, epidemic, and zoonotic conditions of CE in various parts of the world.
Etiology and disease
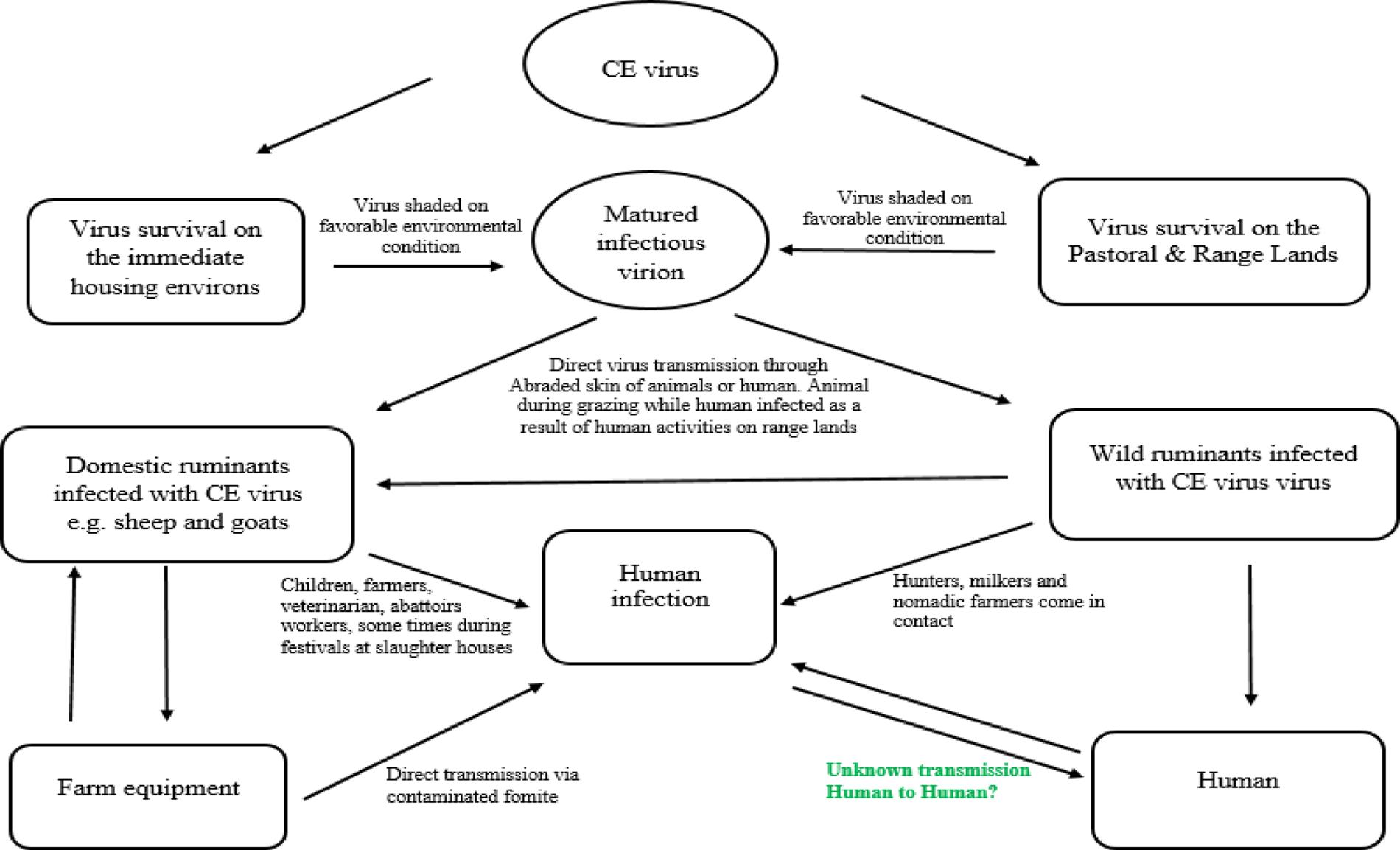
CE is caused by a parapoxvirus of the Poxviridae family. Other members within the Parapoxvirus genus with CE virus include Bovine papular stomatitis virus (BPSV), Pseudocowpox virus (PCPV), Red deerpox virus or parapoxvirus of red deer in New Zealand (PVNZ), Chamois CE virus, seal pox virus, and Auzdik disease virus (Buchen, Reference Buchen2003). The genome of the virus is a linear double-stranded DNA molecule with a G+C content up to 64% (Wang et al., Reference Wang, Xiao, Zhang, Chen, Li, Li, Hao and Luo2016) and genomic length of 138 kb (Chi et al., Reference Chi, Zeng, Li, Hao, Li, Huang, Huang, Rock, Luo and Wang2015). Usually, B2L and VIR are considered as conserved genes among different isolates of CE virus (Friederichs et al., Reference Friederichs, Krebs, Blum, Wolf, Lang, von Buttlar and Buttner2014; Yogisharadhya et al., Reference Yogisharadhya, Kumar, Ramappa, Venkatesan, Bhanuprakash and Shivachandra2017). Therefore, B2L and VIR genes of CE virus were used frequently as markers for virus detection and diagnosis. Similar to other parapoxviruses, its virion nature is structurally ovoid with a criss-cross pattern (Bala et al., Reference Bala, Balakrishnan, Jesse, Abdullah, bin Noorzahari, Ghazali, Mohamed, Haron, Noordin and Mohd-Azmi2020). The structural morphology for Orf virus viroid from infected goat is depicted in Figure 1.
Fig. 1. Transmission Electron microscopy of negatively stained CE virus particles from infected female goat. CE virus-like particles with ovoid shaped structure (white arrow) appeared with a criss-cross pattern (green arrow) typical for Orf virus. Bar represents 100 nm.
Animals infected with CE virus will normally develop acute cutaneous pustular lesions especially in less hairy areas (Maor et al., Reference Maor, Yu and Brand2017). Interestingly, the lesions are established and evolve through several stages, from macule to papule, vesicle, pustule, and scab formation (Fleming et al., Reference Fleming, Wise and Mercer2015). During disease manifestation, blisters usually occur first and later progress from wet to dry scabs. The lesions produced by the disease persist for 3 weeks. However, when animal care is inadequate, the lesion site is normally vulnerable to secondary bacterial infection. Painful scabs on mouth and lips usually prevent infected animals from proper feeding. An infected udder can result in inflammation of the mammary gland or mastitis in sheep and goats (ICTV, 2006).
The skin lesions caused by CE virus infection usually begin from oral commissures and later spread to the muzzle and other parts of the oral cavity (Mahmud et al., Reference Mahmud, Rahman, Dey, Islam and Talukder2014). In severe cases, infected younger animals usually cannot properly feed due to intense pain induced by the lesions in their mouth cavity and this results in severe body weight loss (Nfi, Reference Nfi1991; Zamri-saad et al., Reference Zamri-Saad, Al-Ajeeli and Ibrahim1992; Bala et al., Reference Bala, Balakrishnan, Abdullah, Mohamed, Haron, Jesse, Noordin and Mohd-Azmi2018a). The lesions can be multifocal and appear extensively on the muzzle, tongue, ears, nose, and sometimes on the eyelids. In some severe cases, genitalia, udders, and feet were also affected (Maganga et al., Reference Maganga, Relmy, Bakkali-Kassimi, Ngoubangoye, Tsoumbou, Bouchier, N'Dilimabaka, Leroy, Zientara and Berthet2016). When viral infection is co-habited with a secondary bacterial infection, such as staphylococci or streptococci, usually the death rate can approach up to 90%, particularly in lambs and kids (Gelaye et al., Reference Gelaye, Achenbach, Jenberie, Ayelet, Belay, Yami, Loitsch, Grabherr, Diallo and Lamien2016; Maganga et al., Reference Maganga, Relmy, Bakkali-Kassimi, Ngoubangoye, Tsoumbou, Bouchier, N'Dilimabaka, Leroy, Zientara and Berthet2016; Fleming et al., Reference Fleming, McCaughan, Lateef, Dunn, Wise, Real and Mercer2017). The CE incubation period usually varies from 3 to 7 days, depending on predisposing conditions. In human beings, CE usually occurs as single skin sore or a few lesions visible on a finger and/or hand of the infected person. It is associated with pain, pruritis, and fever, and 95% of the lesions occurred in the skin of hand. If the lesion condition is painful, a secondary bacterial infection may have accompanied by the virus infection (Maor et al., Reference Maor, Yu and Brand2017). Systemic symptoms associated with redness, inflammation, lymphadenopathy, and erysipelas-like lesions or fungus like lesions frequently occur in human cases of CE (Georgiades et al., Reference Georgiades, Katsarou and Dimitroglou2005). The fungus-like lesions sometimes can be mistaken as CE in burn patients and also in those with atopic dermatitis or are undergoing immunosuppressive therapy due to scleroderma issues (Hsu et al., Reference Hsu, Rokni, Aghazadeh, Brinster, Li, Muehlenbachs, Goldsmith, Zhao, Petersen, McCollum and Reynolds2016). Some clinical signs of CE observed among two important goats breed in Nigeria (Sokoto cross-breed red and Kano brown goats) were presented in Figure 2 showing a scabby lesions on the nose and oral labial commissure typical of contagious ecthyma disease.

Fig. 2. Clinical signs of CE observed among some goat breeds in Nigeria. (a) Scabs on the mouth and nose. (b) Showing scabs on oral commissure of the cross-breed red Sokoto goat. (c) CE lesions and scabs on the mouth of Kano brown goats. (d) CE lesions on the muzzle, eye, and lips of red Sokoto goat.
Host range and virus transmission
CE occurs mostly in both domestic and wild ruminants such as sheep, goats, deer, big horn sheep, antelope, deer, musk oxen, and domestic Shetland sheep. Apart from cattle, other permissive species include alpacas, camels and other camelids, seal squirrels, dogs, and occasionally humans (Azwai et al., Reference Azwai, Carter and Woldehiwet1995; Robinson and Mercer, Reference Robinson and Mercer1995; Hagis and Ginn, Reference Hagis, Ginn, McGravin, Carlton and Zachary2001; Kerry et al., Reference Kerry, Anna and Glenda2012; Khalafalla et al., Reference Khalafalla, El-Sabagh, Al-Busada, Al-Mubarak and Ali2015; Adedeji et al., Reference Adedeji, Adole, Chima, Maguda, Dyek, Jambo, Anefu, Shallmizhili and Luka2018b). Usually camel CE is transmissible to sheep and vice versa (Abu Elzein et al., Reference Abu Elzein, Housawi, Al-Afaleq, Ramadan, Gameel and Al-Gundi2004). Some animals may or may not have visible lesions (sores) but the animals may still be able to spread the virus. Figure 3 shows a diagram indicating the main possible routes and mode of transmissions of CE viruses.

Fig. 3. Transmission cycle of Orf virus that causes CE. The CE virus is presence at the dormant stage on the environs such as the range/pastoral lands as well as the immediate housing areas that are engaged with sheep and goat rearing. Direct transmission of infectious virions occurs in the domestic and wild ruminants via abraded skin. Animal to animal infection occurs via direct contact (e.g. during suckling of milk) while human infection also occur by direct contact with diseased animals.
CE virus usually remains stable on the body surfaces for several months after affected animals have recovered from the disease. Animals can be infected by CE virus through broken skin following a cut or abrasion and physical contact with clinically infected animals, subclinical carriers and fomites such as contaminated farm materials. Some animals may or may not have visible lesions (sores), but these animals may still be able to spread the virus (Zhang et al., Reference Zhang, Xiao, Yu, Liu, Wang, Tao, Liu and Ning2016).
Experimentally, the virus is readily transmissible by scarifying the lips or buccal mucosa of a healthy animal with an emulsion of scabs from an infected case. Naturally, interspecies transmission of CE virus between sheep and goats may occur, but experimental transmission from one species to another may not yield a typical disease outcome (De la Concha-Bermejillo et al., Reference De La Concha-Bermejillo, Guo, Zhang and Waldron2003). There is a need to revisit the probable differences in factors that influences the pathogenicity (Suppiah et al., Reference Suppiah, Sakinah, Chan, Wong, Bala, Lawal, Benelli, Subbiah and Chee2018; Aliyu et al., Reference Aliyu, Kumurya, Bala and John2017; Balakrishnan et al., Reference Balakrishnan, Abdullah, Bala, Abba, Sarah, Jesse, Nazariah, Noordin and Mohd-Azmi2017), immune responses and immunity against CE virus, similarly to other viruses causing diseases (Camalxaman et al., Reference Camalxaman, Zeenathul, Quah, Loh, Zuridah, Hani, Sheikh-Omar and Mohd-Azmi2013; Azmi & Field, Reference Azmi and Field1993; Loh et al., Reference Loh, Mohd-Azmi, Lai, Sheikh-Omar and Zamri-Saad2003; Bala et al., Reference Bala, Kawo, Mukhtar, Sarki, Magaji, IA and Sani2012).
Laboratory diagnosis
Cell culture is a gold standard which is suitable for isolation and propagation of pox viruses (Hautaniemi, Reference Hautaniemi2012; Abdullah et al., Reference Abdullah, Ismail, Balakrishnan, Bala, Hani, Abba, Awang, Jesse, Arshad, Nazariah, Abdullah, Noordin and Mohd-Lila2015). Usually, to estimate the extent of virus proliferation in cell culture, examining both infected and control host cell monolayers is involved. Morphologic changes (cytopathic effect, CPE) in the cell monolayer, such as shrinking, granulation, rounding, ballooning, and degeneration of the cells, show the presence of viruses (Hematian et al., Reference Hematian, Sadeghifard, Mohebi, Taherikalani, Nasrolahi, Amraei and Ghafourian2016). An example of typical CPE of the virus on Vero cells is shown in Figure 4.
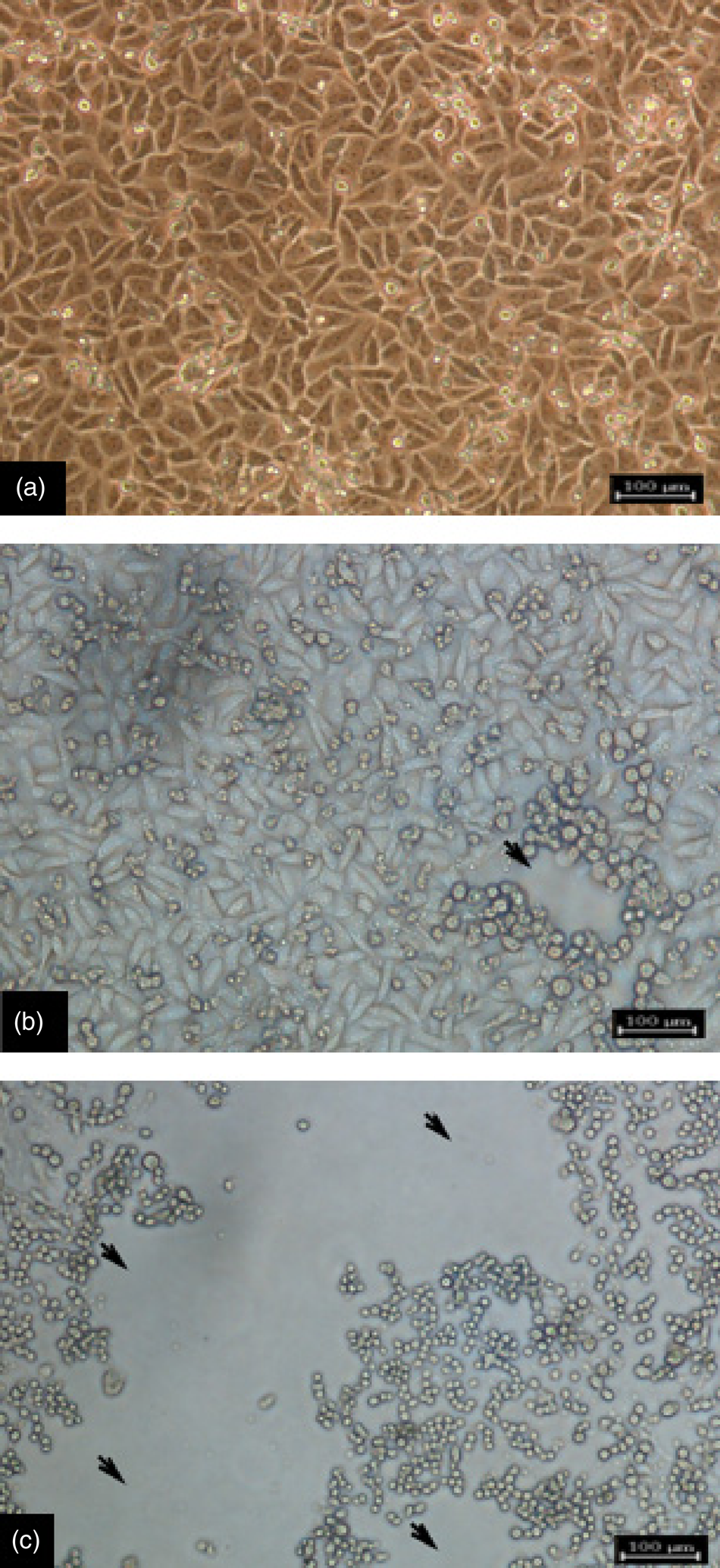
Fig. 4. Cytopathic effect (CPE) of CE virus on Vero cells. (a) Negative control non-inoculated Vero cells. (b) Infected vero cells showing the progression of CPE at 72 hours post-infection: features are rounding and aggregation of cells. (c) Infected Vero cells at 192 hours post-infection showing rounded cells, extensive formation of CPE, and detachment of the monolayer (magnification × 40). Bar = 100 µm.
Different primary cells such as lamb testis and lamb kidney cells were initially used for Orf virus isolation in tissue culture. Interestingly, ovine, bovine, and caprine primary cells were preferred by many researchers due to their optimum ability to produce large quantity of cells and support of poxviruses replication (Babiuk et al., Reference Babiuk, Parkyn, Copps, Larence, Sabara, Bowden, Boyle and Kitching2007). Often, this situation were synonymous with an approach to establish cultures that improved biological products such as insulin for diabetic remedy employing the goats' islets for xenotransplantation (Hani et al., Reference Hani, Ibrahim, Othman, Lila and bt Allaudin2010; Vakhshiteh et al., Reference Vakhshiteh, Allaudin, Mohd Lila and Hani2013; Hani et al., Reference Hani, Allaudin, Mohd-Lila, Ibrahim and Othman2014; Abdull Razis et al., Reference Abdull Razis, Ismail, Hambali, Abdullah, Ali and Mohd Lila2009). Similar scenario stands in the selection of suitable cells for study of apoptosis (Abdullah et al., Reference Abdullah, Ahmad, Ku Ahmad, Ghazali, Jaafar, Ideris, Ali, Omar, Yusoff, Lila and Othman2007; Shen Ni et al., Reference Shen Ni, Allaudin, Mohd Lila, Othman and Othman2013; Razis et al., Reference Razis, Ismail, Hambali, Abdullah, Ali and Lila2006). Despite all these potentialities, still there are some detriments with regard to the use of primary or secondary cell cultures due to inherent nature of primary cells such as cell heterogeneity, endogenous viruses, and limited virus susceptibility to only a few passages. Thus, variabilities may distort the virus ability to replicate at each passage (Nashirudddullah et al., Reference Nashirudddullah, Pathak, Barman, Ahmed, Rajbongshi and Kumar2016). Therefore, continuous cell lines have now become the alternative use for Orf virus isolation and propagation (Babiuk et al., Reference Babiuk, Parkyn, Copps, Larence, Sabara, Bowden, Boyle and Kitching2007).
Notably, the CE virus usually only grows well in primary cell cultures unless the particular virus isolate has been adapted to grown on a particular cell line. In practice, this can be very difficult to achieved (Kumar et al., Reference Kumar, Narayan, Bhatt, Haq, Khurana, Tiwari, Karthik, Malik, Dhama and Chandra2015; McInnes et al., Reference McInnes, Wood, Nettleton and Gilray2001; Mazur and Machado, Reference Mazur and Machado1990). Even adapting CE virus to grow in primary cultures is not always straight forward and may take several blind passages of the cells before the obvious CPEs start to appear due to replication of the virus (Greig, Reference Greig1956; Fleming and Mercer, Reference Fleming and Mercer2007; McInnes et al., Reference McInnes, Wood, Nettleton and Gilray2001). The use primary cultures of fetal lamb muscle, skin or testes cells were found adequate for isolating CE virus particularly from infected scabs, as these types of cultures tends to be fibroblastic in nature; however, these primary cell lines do support the growth of virus once it has been adapted to growth in continuous cell lines. Once these primary cultures have been passaged more than 20 times, they tend to become refractory to infection (Bala et al., Reference Bala, Balakrishnan, Abdullah, Mohamed, Haron, Jesse, Noordin and Mohd-Azmi2018a; McInnes et al., Reference McInnes, Wood, Nettleton and Gilray2001). Alternatively, embryonated chicken eggs can be used to grow CE viruses. Eggs inoculated with CE virus showed reproducible pathological changes ranging from hemorrhages, odema, small grayish white foci, thicknening and development of pock lesions in the corrio-allantoic membrane (Ali et al., Reference Ali, Ahmed, Tamam, Arafa, Saad, Ali and Madbouly2013).
CE can be diagnosed and confirmed by various techniques among which electron microscopy is to observe the typical parapoxvirus virions in skin biopsies or fluid from the skin lesions, even though this technique has some limitations in distinguishing the Orf virus from other parapoxviruses. Molecular techniques, especially polymerase chain reaction (PCR) assays, are required for definitive diagnose (Inoshima et al., Reference Inoshima, Morooka and Sentsui2000; Abdullah et al., Reference Abdullah, Ismail, Balakrishnan, Bala, Hani, Abba, Awang, Jesse, Arshad, Nazariah, Abdullah, Noordin and Mohd-Lila2015; Dalal et al., Reference Dalal, Kumar, Chaudhary, Bansal, Kumar, Kakker and Maan2017). Histopathology and serologic techniques for the detection of viral antigens by using enzyme-linked immunosorbent assay (ELISA), agar gel precipitation test (AGPT), virus neutralization test (VNT), and viral isolation in cell culture are also helpful (Vikoren et al., Reference Vikoren, Lillehaug, Åkerstedt, Bretten, Haugum and Tryland2008; Zeedan et al., Reference Zeedan, Abdalhamed, Ghoneim and Ghazy2015; Bora et al., Reference Bora, Bora, Barman, Borah and Das2016) (Table 1).
Table 1. Diagnostic methods for CE

Treatment of contagious ecthyma
Currently, there is no specific antimicrobial therapy for CE virus, as it produces an array of defences such as virulence factors that counteract the host immune response; for several decades, research has been geared to search for effective treatment and control of CE (Bala et al., Reference Bala, Balakrishnan, Abdullah, Mohamed, Haron, Jesse, Noordin and Mohd-Azmi2018a; Kumar et al., Reference Kumar, Narayan, Bhatt, Haq, Khurana, Tiwari, Karthik, Malik, Dhama and Chandra2015). Lesions on animals can be treated with 3% iodine solution in a single application with long-acting oxytetracycline, which can be administered at 20 mg kg−1, alongside topical application of gentian violet on the skin lesions to improve healing (Adedeji et al., Reference Adedeji, Adole, Chima, Maguda, Dyek, Jambo, Anefu, Shallmizhili and Luka2018b). However, in other cases of CE, animals were treated with amoxicillin and ivermectin followed by fly repellent spraying in the animal pen so as to prevent further spread of the infection by insects (Abbas and Mughal, Reference Abbas and Mughal2014).
Human CE cases are usually treated by using antiseptics alongside topical mupirocin and moist dressings. Supportive care alongside antibiotics was important in preventing secondary bacterial infections as well as facilitating wound healing (Peng et al., Reference Peng, Chen, Zheng, Li, Du and Zhang2016). Many approaches such as curettage, imiquimod, cryotherapy, cidofovir, shave excision, and electrocautery were all used and revealed good result in the treatment of human CE.
Vaccination and vaccine designs
Vaccination against CE usually reduces the number of animals with lesions and facilitates quick recovery of the re-infected animals. Even though there are major challenges about the vaccine efficacy and function due to the Orf virus strain variability and evasive abilities from the host immune response (Zhang et al., Reference Zhang, Liu, Shang, Liu and Cai2014). In order to maintain efficient livestock production among private and government-owned farms, animals should be vaccinated against CE for long-lasting and desirable protection. Annual vaccines should be developed based on strain variability so as to curtail the shedding of the viral particles in the environment (Bala et al., Reference Bala, Balakrishnan, Abdullah, Adamu, bin Noorzahari, May, Mangga, Ghazali, Mohamed, Haron, Noordin and Lila2019). Vaccine designs for CE involves approaches such as live tissue attenuation (Musser et al., Reference Musser, Taylor, Guo, Tizard and Walker2008), recombinant DNA technology (Zhao et al., Reference Zhao, He, Gao, Lu, Han, Li and Gao2011). Often a similar situation stands for the production of plant based vaccine that involve the technologies of integrating of the preferred genes encoding specific antigen and its protein manipulated in the genome of plant tissues (Laere et al., Reference Laere, Ling, Wong, Koh, Mohd Lila and Hussein2019), this approached may signifies application of the molecular techniques for complicated scientific operations such as DNA fingerprinting design (Mohd-Azmi et al., Reference Mohd-Azmi, Ali and Kheng2000) and scaling production of Plasmids and its expression (Ramanan et al., Reference Ramanan, Tik, Memari, Azaman, Ling, Tey, Lila, Abdullah, Rahim and Ariff2010; Ismail et al., Reference Ismail, Allaudin and Lila2012).
The earliest strategy employed for Orf virus control was the use of an autogenous vaccine to immunize animals against the disease, using the infected scab material from diseased animals (Zamri-Saad et al., Reference Zamri-Saad, Roshidah, Al-Ajeeli, Ismail and Kamarzaman1993). Although such vaccines were able to reduce mortality due to Orf virus, the difficulties associated with their lack of standardization, coupled with emergence and re-emerging of naturally evolving strains of CE viruses circulating in the field, made the use of those vaccines less popular in disease control (Mahmud et al., Reference Mahmud, Rahman, Dey, Islam and Talukder2014). Undoubtedly, those autogenous vaccines have contributed so much in the control of CE. Among the most popular vaccines from earlier efforts are the Scabivax and Ecthymavax which were live vaccines used to curtail the menace of Orf virus in sheep and goats in some countries (Musser et al., Reference Musser, Taylor, Guo, Tizard and Walker2008; Tan et al., Reference Tan, Ueda, Heath, Mercer and Fleming2012; Musser et al., Reference Musser, Waldron and Taylor2012; Zhao et al., Reference Zhao, He, Gao, Lu, Han, Li and Gao2011). Moreover, the genetic characteristics and diversity of several isolates of Orf virus necessitates the need to improve the current vaccine in order to optimally protect against the prevailing strain in circulation (Bhanuprakash et al., Reference Bhanuprakash, Hosamani, Venkatesan, Balamurugan, Yogisharadhya and Singh2012; Chi et al., Reference Chi, Zeng, Hao, Li, Li, Huang and Luo2013). In this regard, potential use of protein segments either in their naked form (Zhao et al., Reference Zhao, He, Gao, Lu, Han, Li and Gao2011) or loaded on carrier vehicles such as nano particles may perhaps improve the long-term immune response for CE control. A similar situation exists for the production of a plant-based vaccine that involve the technologies of integrating of the preferred genes that encodes specific antigen and its protein manipulated in the genome of plant tissues (Laere et al., Reference Laere, Ling, Wong, Koh, Mohd Lila and Hussein2019), this approach involves application of molecular techniques for complicated operations such as DNA construct design (Mohd-Azmi et al., Reference Mohd-Azmi, Ali and Kheng2000) and scaling production of plasmids and their expression (Ramanan et al., Reference Ramanan, Tik, Memari, Azaman, Ling, Tey, Lila, Abdullah, Rahim and Ariff2010; Ismail et al., Reference Ismail, Allaudin and Lila2012).
Zhao et al. (Reference Zhao, He, Gao, Lu, Han, Li and Gao2011) stated that subunit vaccines plays a crucial role and may be considered as a potentially effective approach in vaccine development. An application of reverse genetics was applied to produce vaccine candidate strains against several virus agents, such as highly pathogenic avian influenza viruses and Newcastle disease virus (Soda et al., Reference Soda, Sakoda, Isoda, Kajihara, Haraguchi, Shibuya, Yoshida, Sasaki, Sakamoto, Saijo and Hagiwara2008; Uchida et al., Reference Uchida, Takemae and Saito2014). Due to the variable characteristics of CE vaccine viruses and their refractory nature to culture, along with reported vaccines failures, adoption of a reverse-genetic vaccine approach could be useful to alleviate the global CE burden. This vaccine strategy provides good quantities of vaccine antigen as well as proper matching between a vaccine antigen and a circulating strain.
Contagious ecthyma in Europe, South America, North America, and Australia
Table 2 summarizes the major cases reported for CE in europe, South America, North America, and Australia. CE was first reported in sheep by Steeb in 1787 and similarly in goats by Danish in 1879 (Kerry et al., Reference Kerry, Anna and Glenda2012; Glover, Reference Glover1928). The term contagious pustular dermatitis is use to describe the disease.
Table 2. CE cases in Europe, South America, North America, and Australia

Transmission of CE to humans has been described as the most persistent occupational zoonosis in the UK (Buchan, Reference Buchan1996) and Australia (Crumbie, Reference Crumbie1998). It affected most of the animal health workers, veterinarians, and workers from dairy industries due to their high degree of interactions with animals. High frequency of the disease was observed during religious sacrifices (Uzel et al., Reference Uzel, Sasmaz, Bakaris, Cetinus, Bilgic and Karaoguz2005). More human cases of CE were recorded in the Western USA by which approximately 40% of sheep producers experienced CE cases in their various operation(s) (NAHMS, 2001). From 2001–2012, CE virus transmission from Alaskan wildlife including mountain goats, Dall's sheep, muskox, sitka black-tailed deer, and caribou was recorded. The gross lesions of typical CE developed in younger Dall's sheep (lambs) were similar to those of mountain goats, but not as severe compared to the former cases (Tryland et al., Reference Tryland, Beckmen, Burek-Huntington, Breines and Klein2018). The outbreak was also observed in 2003 among 6 month-old boer goats, whereby the skin lesions diagnosed and confirmed the presence of Orf virus (Guo et al., Reference Guo, Zhang, Edwards, Ermel, Taylor and De La Concha Bermejillo2003). Typically, degenerated keratinocytes composed of many typical 200–300 nm Parapoxvirus virions were detected in the haired skin at the lip (Kinley et al., Reference Kinley, Schmitt and Stephens-Devalle2013).
In 2004, a CE outbreak was recorded in Dovre, Norway involving free-ranging musk oxen calves and yearlings. Moreover, neutralizing antibodies against Parapoxvirus were detected in 8.6% of musk oxen sampled between 2004 and 2006 (Vikoren et al., Reference Vikoren, Lillehaug, Åkerstedt, Bretten, Haugum and Tryland2008). Again, another case of CE was diagnosed in the early part of the summer of 2007 in an organic dairy farm. In 2009, a suspected outbreak of CE disease in Brazil was reported. Based on phylogenetic analysis, there was a high level of similarity of those CE viruses with CE virus-India 82/04 isolate (Abrahão et al., Reference Abrahão, Campos, Trindade, Guedes, Lobato, Mazur and Kroon2009). In July of 2013, an outbreak of CE occurred in flocks of sheep from Pilcaniyeu Town, Río Negro Province of Argentina (Peralta et al., Reference Peralta, Robles, Martinez, Alvarez, Valera, Calamante and Konig2015). In England, based on 3000 farms surveyed between 2011 and 2012, the prevalence rates recorded were 1.88 and 19.53% for ewes and lambs, respectively (Onyango et al., Reference Onyango, Mata, McCormick and Chapman2014).
There were numbers of CE cases among humans, particularly among workers in animal-related food industry or in children who usually have contact with animals either at home, at companion animal zoos, or at livestock shows. There were several cases of CE among women and men, with clinical symptoms after cutting their hands with bones from the slaughtered goat, injury during meat processing or being bitten by an infected animal (Rebecca, Reference Rebecca2012). The scab lesions developed in humans showed similar characteristic to lesions on udders and lips of CE-infected animals. Histopathology of the lesions showed epidermal necrosis and dermal infiltration of neutrophils, eosinophils, and lymphocytes. Multiple typical Orf viral particles were shown by transmission electron microscopy (Koufakis et al., Reference Koufakis, Katsaitis and Gabranis2014).
Contagious ecthyma in Africa
Cases of CE were reported in numerous countries from Africa. In November 1976 and February–April 1977, two suspected outbreaks of CE were reported in the Research Farm of the University of Ibadan, Nigeria. The first suspected outbreak involved a herd of lambs while the second outbreak involved goats (Obi and Gibbs, Reference Obi and Gibbs1978). In Cameroon, between 1980 and 1989, CE cases were more obvious in goats compared to sheep, with the incidence rate of 88.5 and 51%, respectively, while the kids showed a higher susceptibility rate than adults. Kids of less than 2 to 3-months-old suffered high mortality (60%). CE cases occurred at high morbidity of about 80–90% with higher incidence (75%) during the dry season (Nfi, Reference Nfi1991). Ali et al. (Reference Ali, Kheir, Damir and Barri1991) reported CE cases among camels in Western Sudan with the younger camels seriously affected by the disease.
In 1994, in Turkana district of Kenya, the prevalence rate of CE in camels was 11.2% with a morbidity rate of 100% in all affected herds. Eventually, CE infection was also observed among the kids in one herd of goats close to the area (Gitao, Reference Gitao1994). Later, in South Africa, there were 54 CE outbreaks recorded within the 7-months of investigation among sheep and goats, with 44 outbreaks in rural areas in private farms. The observation was concluded based on scab samples subjected to PCR for the amplification of a specific 594 base pair fragment of the B2L gene (Scagliarini et al., Reference Scagliarini, Piovesana, Turrini, Savini, Sithole and McCrindle2012).
In Egypt, 2010, about 5% of sheep were seropositive to CE virus as detected by AGPT (Said et al., Reference Said, Mohamed, Elhamid, Hosny and Baheeg2013). Some camels were affected by Parapoxvirus in certain parts of Sudan between 1993 and 2013. In 2013, a CE outbreak in camels caused morbidity and mortality rates of 20 and 1.5%, respectively (El-Tholoth et al., Reference El-Tholoth, Elnaker and Shiha2015; Khalafalla et al., Reference Khalafalla, El-Sabagh, Al-Busada, Al-Mubarak and Ali2015). Meanwhile, phylogenetic analysis on VIR and B2L genes of CE viruses obtained from Gabon showed they are closely related to Asian CE viruses (Maganga et al., Reference Maganga, Relmy, Bakkali-Kassimi, Ngoubangoye, Tsoumbou, Bouchier, N'Dilimabaka, Leroy, Zientara and Berthet2016) (Table 3).
Table 3. Examples of CE morbidity and mortality from different countries of the world

A CE outbreak was also reported in Zambia (Simulundu et al., Reference Simulundu, Mtine, Kapalamula, Kajihara, Qiu, Ngoma and Mweene2017). In AkwaIbom State, Nigeria, CE cases were reported with 100% morbidity and 3.3% mortality as confirmed by using PCR (Adedeji et al., Reference Adedeji, Maurice, Wungak, Adole, Chima, Woma, Chukwuedo and Shamaki2017). Similarly, outbreaks of CE were reported between 2014 and 2016 in West African dwarf goats and Kano brown goats in the Plateau and among camels in Jos, Plateau state and Bauchi state of Nigeria, as confirmed by PCR (Adedeji et al., Reference Adedeji, Adole, Chima, Maguda, Dyek, Jambo, Anefu, Shallmizhili and Luka2018b). Recently, the status of CE in three districts in Ethiopia was determined. Approximately 12% of sheep and goats examined by PCR were positive for CE virus (Tedla et al., Reference Tedla, Berhan, Molla, Temesgen and Alemu2018) (Table 4).
Table 4. CE cases in Africa

Contagious ecthyma in Asia
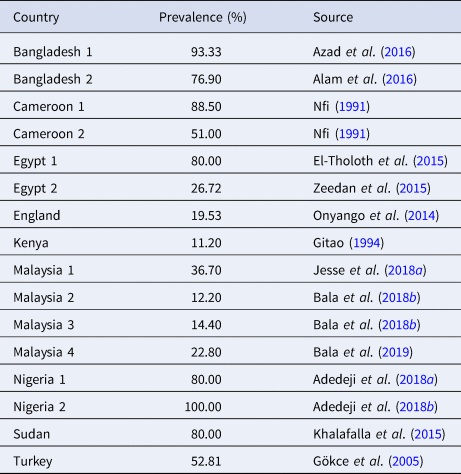
In 1998, in the eastern region of Saudi Arabia, several outbreaks of CE were reported in two local breeds of sheep. The younger animals were seriously affected by the disease. Interestingly, it was a different form of CE which presented with myiasis (Housawi and Abu Elzein, Reference Housawi and Abu Elzein2000). In the Kars region of Turkey, the seroprevalence of CE was determined to be 52.81 and 5% in lambs and humans, respectively. The mortality rate of 2.81% was observed in lambs (Gökce et al., Reference Gökce, Genç and Gökçe2005). Numerous outbreaks of CE were recorded in Central Taiwan (Chan et al., Reference Chan, Lin, Lee, Liao, Tsai, Hsu, Wong and Shih2007). The morbidity of these CE cases was between 2 and 6% with mild lesions and mortality of less than 0.8% in lambs. Many findings indicated that CE is endemic in some parts of Taiwan (Chan et al., Reference Chan, Hsu, Wang, Yang, Lin, Chulakasian and Wong2009). About a decade ago, an investigation was carried out on an acute sudden appearance of CE in Han sheep in the Jilin Province of China (Zhao et al., Reference Zhao, Song, He, Lu, Zhang, Li, Chen and Gao2010). An outbreak of CE was also reported in the Hubei Province of China in 2009, after transporting about 655 goats in herds from a long distance, with 60% morbidity and 24.7% mortality rates (Zhang et al., Reference Zhang, Lu, Shang, Zheng, Jin, He and Liu2010). Several cases of CE in Korean black goats between 2010 and 2011 were diagnosed. Meanwhile, in Mongolia, CE outbreaks were recorded with morbidity ranging from 10 to 80 and 50 to 70% in adults and suckling camels, respectively (Dashtseren et al., Reference Dashtseren, Solovyev, Varejka and Khokhoo1984). In Bangladesh, based on PCR detection in a limited study, overall morbidity and mortality rates of 23.89 and 1.02%, respectively, were recorded (Azad et al., Reference Azad, Saha, Alam, Monoura, Giasuddin, Islam and Alam2016) (Table 5).
Table 5. The high prevalence of CE in some countries
In Fujian Province in the southern part of China, between 2011 and 2012, 16% out of 106 goat sera of healthy herds and 83% out of 182 goat sera from recovering herds were seropositive for CE (Chi et al., Reference Chi, Zeng, Hao, Li, Li, Huang and Luo2013). Later, in Shaanxi, Shandong, and Yunnan Provinces of China, 34.89% of goats were positive for CE virus as confirmed by PCR of the B2L gene. Meanwhile, 7.32% of 874 buccal swab samples were positive for the Orf virus (Gao et al., Reference Gao, Zhao, Liu, Zhou, Liu, Liu, Yang and Chen2016) (Table 6).
Table 6. Prevalence (%) of CE multiple cases from China, Nigeria, and Egypt

In Uttar Pradesh, India, at least 6% sheep of different ages and sex were affected by CE (Kumar et al., Reference Kumar, Wadhwa, Chaubey, Singh, Gupta, Sharma and Mishra2014). However, in Iran, the digestive form of CE was recorded in 2013 in a 3-week old male yeanling, followed by an outbreak in the entire herd (Mashayekhi et al., Reference Mashayekhi, Mohajeri and Akbarzadeh2013). The outbreak was confirmed based on PCR targeting ORF011 (Oryan et al., Reference Oryan, Mosadeghhesari, Zibaee and Mohammadi2017).
CE was first reported in Malaysia in 1935, 1939, and 1949, followed by documented cases of the disease between the period of 1959 and 1960 in various parts of Malaysia (Asiah, Reference Asiah1990). Outbreaks of CE with a mortality rate of as high as 30% and as low as 1% could be due to different strains of Orf viruses in the particular area (Robinson and Balassu, Reference Robinson and Balassu1981). Murty and Singh (Reference Murty and Singh1971) revealed that CE lesions were seen in four of 587 Bikanery and Mandya sheep after keeping the animals in the same pen with Rajasthan sheep on arrival. Later, more CE cases were diagnosed in farms (Sepre and Kalia, Reference Sepre and Kalia1980). Zamri-saad et al. (Reference Zamri-Saad, Kamal and Aziz1989) revealed that 54% of 260 goats examined due to CE were kids of less than 3 months old and the youngest kid with lesions was 20-days old. Again, in 1992, CE cases were reported from the herd of 186 goats, with 47 younger having a with persistent history of CE in the herd (Zamri-saad et al., Reference Zamri-Saad, Al-Ajeeli and Ibrahim1992). Recently, in 2017, a study on seroprevalence of CE has been conducted in Selangor, Malaysia. Interestingly, IgG levels to CE virus were analyzed by ELISA and revealed prevalence rates of 12.2 and 14.4% in sheep and goat herds, respectively (Bala et al., Reference Bala, Krishnan, Abdullah, Yi, Bitrus, Abba, Aliyu, Peter, Hambali, Mohamed, Jesse, Haron, Noordin and Mohd-Lila2018b). All these reports indicated that CE is endemic among sheep, goats, and other farm and wild animals, which can lead to set backs in the economy of farmers worldwide due to the high mortality rate, particularly among kids and lambs (Table 7).
Table 7. CE cases in Asia

Strategies to reduce global cases of contagious ecthyma
To prevent CE from spreading to uninfected herds and to minimize re-infection, the following integrated approach should be put in place. A good management system plays a vital role in prevention and control measures. Animals should be given more nutritious diets based on the physiologic condition of each animal. Water should always be available in order to enhance their metabolic activities. Well-constructed and ventilated housing reduces the risk associated with overcrowding and activities of microorganisms at different levels (Nandi et al., Reference Nandi, De and Chowdhury2011; Bora et al., 2016; Bala et al., Reference Bala, Balakrishnan, Abdullah, Adamu, bin Noorzahari, May, Mangga, Ghazali, Mohamed, Haron, Noordin and Lila2019). Routine clearance of dirt and debris in the animals' compartments is a key to successful animal health management. Bedding materials should not contain any rough and abrasive materials that can injure the animals and should be changed regularly. Rough and harsh materials, when seen in the pasture or pens, should be immediately removed in order to reduce the risk of injury to the animals. The viruses causing CE are difficult to eradicate after entering a herd, because some animals may be carriers with no clinical signs. Therefore, keeping proper records will enable quick identification of re-infections and modes of virus transmission. Figure 5 highlighted on the integrated approach for the preventive measures at different point in the transmission cycle for the control of CE disease.

Fig. 5. Integrated approach for CE prevention and control. These scenarios indicate good management practice within the farm incorporated with collective efforts by governments, NGOs, farmer cooperative societies and individuals, in order to achieve effective and efficient prevention and control measures of CE.
During outbreaks, all the infected animals should be isolated to minimize further infection to healthy animals (Abdullah et al., Reference Abdullah, Ismail, Balakrishnan, Bala, Hani, Abba, Awang, Jesse, Arshad, Nazariah, Abdullah, Noordin and Mohd-Lila2015). Unnecessary movement of humans, vehicles, and fomites in and out of farms should be limited at that time. Isolated animals should be treated accordingly in order to facilitate quick healing. CE medication in some cases consists of moist dressings and local antiseptics. For treatment of secondary bacterial infections, iodine tincture and antibiotics have been used in most incidences (Bora et al., Reference Bora, Bora, Barman, Borah and Das2016; Bala et al., Reference Bala, Balakrishnan, Abdullah, Adamu, bin Noorzahari, May, Mangga, Ghazali, Mohamed, Haron, Noordin and Lila2018c). Supportive care includes tube feeding for younger animals. Insect repellents can be used to control flies from further spreading of the virus. Close observation of animal health status should be taken into consideration, which will enable early detection of any abnormalities in the animals (Sadiq et al., Reference Sadiq, Abba, Jesse, Chung, Bitrus, Abdullah, Balakrishnan, Bala and Mohd Lila2017; Kumar et al., Reference Kumar, Narayan, Bhatt, Haq, Khurana, Tiwari, Karthik, Malik, Dhama and Chandra2015). Precautions should be taken when moving equipment and other fomites from one farm to another, so as to prevent virus introduction. Borders should be strictly controlled in order to monitor the movement of animals and animal products from one country to another. New arrivals (animals) should be quarantined and any confirmed cases treated accordingly (Nandi et al., Reference Nandi, De and Chowdhury2011; Jesse et al., Reference Jesse, Hambali, Abba, Lin, Chung, Bitrus, Abdullah, Balakrishnan, Bala and Lila2018b; Bala et al., Reference Bala, Balakrishnan, Abdullah, Adamu, bin Noorzahari, May, Mangga, Ghazali, Mohamed, Haron, Noordin and Lila2019).
Public awareness should be organized by the concerned authority in order to guide farmers and enable them to seek first-hand information about animal disease control measures from veterinarians and other animal health experts. Farmers or animal attendants should avoid contact with suspected diseased animals. Personal protective equipment such as rubber or latex hand gloves, face masks, boots, and aprons should be used during treatment to avoid transmission between animals and humans. Well-constructed animal health facilities should be provided at various levels so as to enhance quick responses to the disease at any moment (Nandi et al., Reference Nandi, De and Chowdhury2011; Kumar et al., Reference Kumar, Narayan, Bhatt, Haq, Khurana, Tiwari, Karthik, Malik, Dhama and Chandra2015).
Vaccination strategy against contagious ecthyma
A great extent of biosecurity must be achieved for the prevention and control of CE. Vaccination against the infection is paramount. There are various vaccines ranging from inactive to whole live virus that can be used for the control of parapoxvirus viruses. Even though Orf virus strain variability can threaten Orf vaccine efficacy (Musser et al., Reference Musser, Taylor, Guo, Tizard and Walker2008; Zhang et al., Reference Zhang, Liu, Shang, Liu and Cai2014), vaccination against CE should be used as a tool for enhancing herd immunity against the disease. Animals vaccinated against CE should be isolated from unvaccinated animals, and vaccines should be used cautiously to avoid contaminating uninfected premises.
Conclusion
CE is a serious health issue for even-toed ungulates and an extreme threat to the small ruminants industry (sheep and goats), with an economic impact on the human population. The lesions caused by this disease jeopardize productivity and reduce the market value of meat, leather, and wool, and obstruct the national and international trade of animals and animal products. CE can even cause the condemnation of products due to severe lesions on the animal body. Though it is a self-limiting disease, and neglected by countries, CE has recently increased due to the re-emergence of Orf viruses in various locations globally. Frequent re-infection of previously infected animals and intra- and inter-species transmission need to be given more attention for proper prevention and control measures and to reduce the economic losses due to damages imposed by the disease.
Acknowledgements
The authors wish to acknowledge the local farmers in Kazaure LGA, Jigawa, Nigeria, for their assistance and consent for taking photos of the several affected animals with contagious ecthyma in their farms.
Financial support
The work was supported by the grant Ministry of Energy, Science, Technology, Environment & Climate Change (MESTECC), Biotechnology Cluster 02-01-04-SF2459 (Grant no. 5450820).
Conflict of interest
The authors have no conflict of interest to declare.