INTRODUCTION
Seed dispersal by vertebrates is a crucial process for plant regeneration, particularly in rain forests where as many as 90% of tree species disperse their seeds with the help of frugivorous animals (Jordano Reference JORDANO and Fenner2000). Among frugivores, the role played by primates as effective seed dispersers is well recognized worldwide (Kunz & Linsenmair Reference KUNZ and LINSENMAIR2008, Link & Di Fiore Reference LINK and DI FIORE2006, Wehncke & Domínguez Reference WEHNCKE and DOMÍNGUEZ2007). However, dispersal effectiveness (sensu Schupp Reference SCHUPP1993) varies greatly among primate species, and is affected by aspects such as social structure, diet, ranging behaviour and defecation pattern (Andresen Reference ANDRESEN1999, Reference ANDRESEN2002a; McConkey Reference McCONKEY2005a, Wehncke et al. Reference WEHNCKE, VALDEZ and DOMÍNGUEZ2004).
The dung that surrounds defecated seeds can have a significant effect on post-dispersal seed fate. In some cases the dung might protect seeds from the attack of seed-eating animals (Janzen Reference JANZEN1982, Ríos & Pacheco Reference RÍOS and PACHECO2006), while in other cases it might actually attract seed predators (Janzen Reference JANZEN, Estrada and Fleming1986, McConkey Reference McCONKEY2005b). In the particular case of seeds defecated by mammals, the faecal material also attracts dung beetles (Andresen Reference ANDRESEN2002b). Secondary seed dispersal by dung beetles, also known as coprochory (Engel Reference ENGEL2000), occurs when beetles move and/or bury some of the seeds, as ‘dung contaminants’, while processing the dung they will use for feeding or oviposition (Andresen & Feer Reference ANDRESEN2005).
Although the interaction between seeds, frugivorous mammals and dung beetles has been recognized in the literature as a diplochorous system (Vander Wall & Longland Reference VANDER WALL and LONGLAND2004), the number of studies focusing on it is still relatively low (Culot et al. Reference CULOT, HUYNEN, GÉRARD and HEYMANN2009, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006, Slade et al. Reference SLADE, MANN, VILLANUEVA and LEWIS2007), and many details of the interaction remain to be elucidated (Andresen Reference ANDRESEN2005, Andresen & Feer Reference ANDRESEN, FEER, Forget, Lambert, Hulme and Vander Wall2005, Nichols et al. Reference NICHOLS, SPECTOR, LOUZADA, LARSEN, AMEZQUITA and FAVILA2008). A few studies have been carried out in temperate biomes (D'Hondt et al. Reference D'HONDT, BOSSUYT, HOFFMANN and BONTE2007, Verdú et al. Reference VERDÚ, NUMA, LOBO, MARTÍNEZ-AZORÍN and GALANTE2009, Wicklow et al. Reference WICKLOW, KUMAR and LLOYD1984), but most have been conducted in tropical rain forests, where this interaction seems to be most conspicuous. On the one hand, in these forests, dung beetles are very abundant and many species (> 50) can co-exist (Hanski & Cambefort Reference HANSKI and CAMBEFORT1991). On the other hand, in these forests, most mammalian herbivore dung, which is the beetles’ preferred food source, contains seeds.
Secondary seed dispersal, in general, is a common process that mostly has a positive effect on seed fate (Forget et al. Reference FORGET, LAMBERT, HULME and VANDER-WALL2005). Secondary dispersal by dung beetles, in particular, decreases the probability of seed predation when seeds are buried by beetles (Andresen & Feer Reference ANDRESEN, FEER, Forget, Lambert, Hulme and Vander Wall2005). This in turn, can often translate into enhanced seedling establishment (Andresen Reference ANDRESEN2001, Andresen & Levey Reference ANDRESEN and LEVEY2004). However, whether a seed is dispersed by beetles depends on seed size and on the particular assemblage of beetles handling the seed-containing defecation (Andresen Reference ANDRESEN2002b, Larsen et al. Reference LARSEN, WILLIAMS and KREMEN2005, Slade et al. Reference SLADE, MANN, VILLANUEVA and LEWIS2007, Vulinec Reference VULINEC2002). In turn, the assemblage of beetles arriving at a defecation is influenced, among others, by factors related to the primary seed disperser, such as dung type, dung amount and defecation pattern (Andresen Reference ANDRESEN2002a, Culot et al. Reference CULOT, HUYNEN, GÉRARD and HEYMANN2009, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). However, very little comparative information exists to show to what extent the seed-dispersal effectiveness of different species of primary seed dispersers, is differentially affected by the activity of dung beetles.
To our knowledge, only two studies, one in Peru (Culot et al. Reference CULOT, HUYNEN, GÉRARD and HEYMANN2009) and one in Guatemala (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006), have made a quantitative comparison of secondary dispersal by dung beetles for seeds defecated by sympatric primates, and only the latter study focused on howler (Alouatta spp.) vs. spider (Ateles spp.) monkeys. These two primate genera, often sympatric, differ greatly in many ecological traits which, in turn, make their defecations differ in several aspects (Andresen Reference ANDRESEN1999, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). As a consequence of the latter, dung beetle assemblages attracted to their defecations may differ, and thus also the final fate of defecated seeds might differ (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). It was found in Guatemala, for example, that seeds surrounded by Alouatta dung were more likely to be buried by dung beetles, than seeds in Ateles dung (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006).
Spider and howler monkeys also differ in their defecation patterns. Spider monkeys produce a scattered pattern of dung and seed deposition, while howler monkeys produce a clumped pattern (Andresen Reference ANDRESEN1999, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). However, this generalization does not take into consideration that, even though in a smaller proportion, spider monkeys can also produce clumped patterns, and howler monkeys scattered patterns, depending on the particular behaviour/activity at the time of defecation (Andresen Reference ANDRESEN2002a, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006, Russo et al. Reference RUSSO, PORTNOY and AUGSPURGER2006). In particular, both primate genera often produce a clumped pattern when defecating in the morning at their sleeping site; in contrast, a scattered pattern is produced when monkeys defecate individually while travelling. Yet, so far, in previous studies, no significant effect of defecation pattern on seed dispersal by beetles has been detected (Andresen Reference ANDRESEN2002a, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006).
With the present study we aim to demonstrate how the differences in seed dispersal quality among sympatric primates can be shaped by the activity of dung beetles. With this study we also want to increase the scant existing information, to discover what general patterns might be emerging. To address these objectives we asked the following questions: (1) Do dung beetle assemblages attracted to howler monkey vs. spider monkey dung differ, and are these differences similar to the ones observed in an analogous study conducted in Guatemala? We hypothesized that differences such as the ones recorded in Guatemala would be observed in our Colombian site. (2) Is seed fate affected by dung type and defecation pattern, and are the effects similar to the ones observed in a previous Alouatta–Ateles comparison? We hypothesized that dung type would affect seed fate while defecation pattern would not.
METHODS
Study site
The study was conducted during July–December of 2006 in the Reserva Natural De Las Aves El Paujil (RNAP), a protected area located in the municipalities of Puerto Boyacá (Boyacá) and Cimitarra (Santander) in a region known as Serranía Las Quinchas (06°02′48″N, 74°16′00″W), in north-central Colombia (Balcázar et al. Reference BALCÁZAR, RANGEL and LINARES2000). The RNAP was created in 2004 and encompasses an area of 974 ha. Altitudes in the study site are around 180 m, and mean annual temperature is 26 °C. Mean annual precipitation at nearby Puerto Boyacá is 2072 mm. The rainfall has a bimodal distribution with a peak in April–May, and a second peak in September–November.
The main vegetation type in the region is tropical moist forest (Holdridge Reference HOLDRIDGE1967). The RNAP is a mosaic of forest patches (some isolated and others interconnected) within a matrix of secondary vegetation. Up until the creation of the protected area, the forest was subject to intensive selective logging. At the landscape level the RNAP is surrounded by cattle pastures and agricultural lands. This study was carried out in a 60-ha forest patch, within the RNAP. This patch is delimited by very narrow roads (which do not impede the movement of primates) and a river, beyond all of which the forest continues. This particular site was inhabited, at the time the study was conducted, by one troop of the brown spider monkey (Ateles hybridus I. Geoffroy-St. Hilaire, 1829) and one troop of the red howler monkey (Alouatta seniculus (Linnaeus, 1776)), with 16 and 7 individuals, respectively. Other mammals that were very frequently seen in this forest patch include tayra, Central American agouti, paca, two-toed sloth, giant armadillo, southern tamandua, white-fronted capuchin and lemurine night monkey.
Dung beetle sampling
We focused our study on dung beetles in the subfamily Scarabaeinae. To assess differences between the dung beetle assemblages attracted to both dung types, we used baited pitfall traps. Pitfall traps consisted of plastic containers, 11 cm high and 11 cm in diameter, which were filled to one third of their capacity with soapy water and buried level with the ground. A plastic plate was placed 20 cm above each trap to protect them from rain. A small mesh bag containing 30 g of fresh monkey dung was hung from the centre of each plate, approximately 5 cm above the top rim of the buried container. We chose to use 30 g because this is an average weight for the defecation produced by one howler monkey (Andresen Reference ANDRESEN2002a, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). Although spider monkey defecations tend to be smaller (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006) we used the same bait size for both dung types to avoid having dung amount as a confounding variable. We used a total of 20 pitfall traps: 10 baited with howler and 10 with spider monkey dung. Traps were placed along two transects, one for each dung type. Within each transect, the 10 traps were separated by 50 m to avoid trap interference (Larsen & Forsyth Reference LARSEN and FORSYTH2005). Distance between transects was 100 m; both transects were located >200 m from any linear habitat opening (small roads and river). Traps were opened in the late afternoon (16h00–18h00) and dung beetles were collected after 24 h. We repeated the sampling three times: in July, September and November, using the same trap locations each time. All individuals collected were identified by F. Z. Vaz-de-Mello at the Universidade Federal de Mato Grosso, Instituto de Biociências, Departamento de Biologia e Zoologia, Cuiaba, Brazil. Specimens were deposited both at the Universidade Federal de Mato Grosso and in the Entomology Laboratory at the Universidad Industrial de Santander, Colombia.
Secondary seed dispersal by dung beetles
To assess how seed fate is affected, through the activity of dung beetles, by dung type and/or defecation pattern, we carried out two complementary field experiments with the seeds of one tree species, Rollinia edulis Triana & Planch. (Annonaceae). We chose this plant species because it fruited abundantly during the study period and because its seeds are defecated intact by both primates. Also, seeds of this species are large enough (6.8 mm in diameter and 2.4 mm thick) to facilitate experimental manipulation, but small enough to have a high probability of being dispersed by dung beetles (Andresen Reference ANDRESEN2002b, Andresen & Levey Reference ANDRESEN and LEVEY2004).
Experiment 1: Effects of dung type and defecation pattern on seed fate. Seeds of R. edulis were collected from fruits found underneath fruiting trees. Seeds were washed and allowed to dry in the shade. Once dry, seeds were marked individually by attaching a 60-cm-long nylon thread with epoxy cement. We carried out a factorial experiment using two factors with two levels each: (1) dung type (howler vs. spider monkey) and (2) defecation pattern (clumped in sleeping sites vs. scattered in random sites). Previous studies assessing the effect of defecation pattern on secondary seed dispersal by dung beetles have not placed experimental seeds in real defecation sites (Andresen Reference ANDRESEN2002a, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). In this study we aimed to increase the degree of realism of our experimental set-up by using actual defecation sites under sleeping trees for the clumped defecation pattern. For this, we followed monkey troops in the late afternoon to determine the location of their sleeping sites. The following morning we went back to those sites and waited for the monkeys to defecate upon waking. We then collected 100 g of fresh dung and divided it into ten 10-g portions. The amount of 10 g was used because when howler and spider monkeys defecate the dung breaks up into smaller pieces while falling through the vegetation, and individual dung piles of this size are common for both primate species (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). Each of the marked seeds, surrounded by 10 g of dung, was placed on the forest floor under the sleeping site, in an area of approximately 1 m2, maintaining a distance of approximately 40 cm among seeds. The exact position of each seed-containing dung pile was marked with a small wooden stick. For scattered defecation pattern we also used 10 seed-containing dung piles, but these were placed individually, one every 10 m, along transects located randomly in the forest. We had a total of six replicates for the howler-clumped and howler-scattered treatment combinations, and five replicates for the spider-clumped and spider-scattered treatment combinations, for a total of 220 seeds in all the experiment.
The replicates of this experiment were set out between August and September. Seed fate was recorded daily during the first 3 days and once a week thereafter, until completing 16 wk. We recorded whether a seed had been buried and/or moved horizontally. For seeds that had not been buried we recorded whether it had been killed (by vertebrates, insects or pathogens), removed (when the seed could not be found), or was still intact. When seeds had been moved horizontally we measured the distance of movement. We did not manipulate any of the seeds during the period of fate assessment. After the 16-wk period we unearthed all buried seeds and determined whether they were dead or still intact. We also measured the depth at which the seed had been buried. Horizontal and vertical distances were measured to the nearest 1 mm.
Experiment 2: Effects of dung type on horizontal distance and burial depth. Much variability exists both in the depth that seeds are buried and the horizontal distance they are moved by dung beetles (Andresen Reference ANDRESEN2002b). Thus, to obtain an independent estimate for the mean values of these variables, based on a larger sample size, we conducted a separate experiment with R. edulis seeds, having dung type as the only factor. To avoid seed predation or removal by rodents we sun-dried the seeds and then painted them with white vinyl paint (Andresen Reference ANDRESEN2002b) before attaching the nylon thread. These seeds were surrounded by 10 g of fresh monkey dung and placed on the forest floor individually, every 10 m, along transects. After 48 h both horizontal distances and burial depths were measured to the nearest 1 mm. Seeds were re-used until completing eight replicates for each dung type, with 10 seeds per replicate. This experiment was carried out during October and November.
Data analyses
Dung beetle assemblages. To analyse the total frequency of individuals and species captured with each type of dung, we conducted goodness-of-fit Chi-squared tests. To assess differences at the trap-level, among dung types, in the mean number of individuals and the mean number of species, we carried out repeated-measures ANOVAs on square-root-transformed data. The analyses were done using the program Systat (Systat 11 for Windows, SPSS Inc., Chicago, USA), with type of dung as the between-subjects factor, and month of capture (July, September, November) as the within-subjects factor.
To assess differences in community-level species richness (i.e. number of species for a given number of captured individuals; Gotelli & Colwell Reference GOTELLI and COLWELL2001) we constructed rarefaction curves (sample-based rarefaction), re-scaling the y-axis based on the accumulated number of individuals captured, using the program EstimateS 7.5 (free software: http://viceroy.eeb.uconn.edu/index.html).
To determine dung preference of individual dung beetle species we conducted goodness-of-fit Chi-squared tests on each species for which at least 10 individuals had been captured. Finally, we analysed, with G-tests of independence, the frequency of large (≥10 mm) dung beetle individuals and species attracted to the dung of both primate species. It has been shown that secondary seed dispersal by dung beetles is positively related to beetle size, and that for large seeds (≥5 mm in length) beetle species with lengths ≥10 mm are the most important seed dispersers (Andresen Reference ANDRESEN2002b, Vulinec Reference VULINEC2002).
Secondary seed dispersal. To analyse the effects of dung type, defecation pattern and of their interaction on seed fate, we conducted 2-way ANOVAs, using the program Systat 11 for each of the following response variables: proportion of seeds buried, proportion of seeds moved horizontally, proportion of seeds killed (including removed seeds), depth of burial for buried seeds, and horizontal distance for moved seeds. For analyses, all proportion data were transformed using the angular transformation, and horizontal distances were log-transformed. It is important to mention that the first two variables are not mutually exclusive, as a seed could both have been buried and moved horizontally. Also, to assess the existence of a significant relationship between seed death and seed burial, a G test of independence was carried out.
For the additional experiment conducted to more accurately assess horizontal distances and burial depths, we performed one-way ANOVAs for the same response variables analysed in the factorial experiment, with the exception of seed death. For analyses, all proportion data were transformed using the angular transformation and burial depths were log-transformed.
RESULTS
Dung beetle assemblages attracted to howler and spider monkey dung
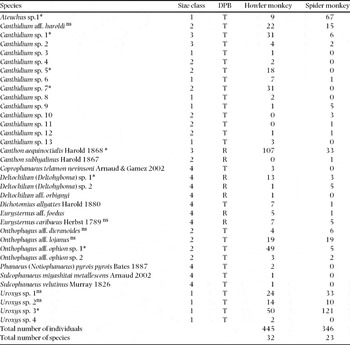
In total, we captured 791 dung beetles belonging to 35 species of Scarabaeinae. Most of the species (80%) and individuals (77%) were tunnellers, and the rest were rollers (Table 1). With howler-monkey dung we captured more individuals than with spider-monkey dung (445 vs. 346, χ 2 = 12.4, df = 1, P = 0.0005), but there was no significant difference in the number of species captured with howler monkey (32) and spider monkey (23) dung (χ 2 = 1.16, df = 1, P = 0.28).
Table 1. Dung beetle species and number of individuals captured with howler monkey (Alouatta seniculus) and spider monkey (Ateles hybridus) dung in a total of 10 independent pitfall traps opened for a 24-h period, three times (July, September and November). Body length size classes: 1: ≤ 5 mm, 2: 6–9 mm, 3: 10–14 mm, 4: ≥ 15 mm. Dung processing behaviour (DPB): R = roller, T = tunneller (dung beetles in the genus Eurysternus, although classified as rollers, have a unique behaviour in which dung balls are only lightly covered by soil). Species with ≥10 individuals captured were tested for dung preference; *indicates preference (P ≤ 0.05), while ns indicates no significant preference (P > 0.05).
However, at the trap level the results of the statistical analyses were opposite to the results for total frequencies. An average of 15 and 12 individuals were captured per trap baited with howler- and spider-monkey dung, respectively, with no significant differences between dung types (F 1,18 = 1.36, P = 0.26). On the other hand, the mean number of species captured per trap was significantly higher for howler monkey dung than for spider monkey dung (6 species vs. 4 species; F 1,18 = 5.86, P = 0.026). For both variables, the effect of time (month of capture) was significant: higher mean numbers of individuals and species were captured, per trap, in July (individuals/species = 20/7) than in September (11/4) or November (9/3; individuals: F 2,36 = 17.5, P < 0.001; species: F 2,36 = 18.2, P < 0.001). For none of the variables was the interaction between dung type and time significant (individuals: F 2,36 = 2.43, P = 0.10; species: F 2,36 = 0.98, P = 0.385). Despite the differences in species density (sensu Gotelli & Colwell Reference GOTELLI and COLWELL2001) at the trap-level, rarefaction curves showed that the overall species richness at the community level (for an equal capture intensity of 346 individuals) were the same for howler monkey and spider monkey dung (Figure 1).

Figure 1. Rarefaction curves (sample-based) showing the expected number of dung beetle species as a function of the accumulated number of individuals captured with howler monkey (Alouatta seniculus) dung (filled circles) and with spider monkey (Ateles hybridus) dung (open circles). Error bars represent 95% confidence intervals.
Regarding community assemblage patterns we observed that nine species represented 81% of all captures. In the beetle assemblage associated with howler monkey dung Canthon aequinoctialis was the dominant species (24%), while in the assemblage associated with spider monkey dung Uroxys sp. 3 was the dominant species (35%). Of the 35 species found, only 14 were represented by ≥10 individuals and were tested for dung preference (Table 1). Six species showed no significant preference. Two species, both of them ≤ 5 mm in body length, showed preference for spider monkey dung (Ateuchus sp. 1, χ 2 = 42.8, df = 1, P < 0.0001; Uroxys sp.3, χ 2 = 28.7, df = 1, P < 0.0001). Finally, six species (all > 5 mm in body length) preferred howler monkey dung (Canthon aequinoctialis, χ 2 = 38.1, df = 1, P < 0.0001); Onthophagus aff. ophion sp. 1, χ 2 = 34.2, df = 1, P < 0.0001); Canthidium sp. 1 (χ 2 = 15.6, df = 1, P < 0.0001); Canthidium sp. 5 (χ 2 = 16.1, df = 1, P < 0.0001; Canthidium sp. 7, χ 2 = 28.0, df = 1, P < 0.0001; Deltochilum (Deltohyboma) sp. 1, χ 2 = 5.06, df = 1, P = 0.0245).
Of the 35 species, 13 were large-bodied (length ≥10 mm; Table 1). All of these were captured with howler monkey dung, but only eight were captured with spider monkey dung. In terms of species, the dung beetle assemblage associated with howler monkey dung had a similar percentage of large dung beetle species to the assemblage associated with spider monkey dung (41% vs. 35%; G = 0.194, df = 1, P = 0.659). However, in terms of individuals, the assemblage attracted to howler monkey dung had a higher percentage of large individuals than that attracted to spider monkey dung (41% vs. 16%; G = 60.1, df = 1, P < 0.0001).
Secondary dispersal of Rollinia edulis seeds by dung beetles
Experiment 1: Effects of dung type and defecation pattern on seed fate. In general, 61% of all seeds (220) used in the factorial experiment were buried by dung beetles. More seeds were buried by beetles when they were surrounded by howler monkey dung (68.3%) than when surrounded by spider monkey dung (53.0%; F 1,18 = 8.09, P = 0.011). Also, the mean percentage of seeds buried was higher when seeds were placed in a clumped pattern (68.0%) than when placed in a scattered pattern (53.3%; F 1,18 = 7.40, P = 0.014). Finally, the interaction between both factors was also significant (F 1,18 = 4.42, P = 0.05), as the difference in the mean percentage of seeds buried in scattered vs. clumped pattern was only large in the case of spider monkeys (Figure 2).
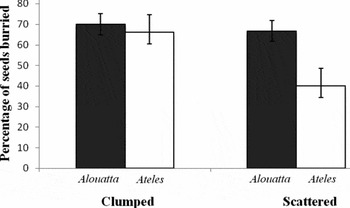
Figure 2. Results of a factorial experiment showing the percentage of Rollinia edulis seeds buried by dung beetles. Seeds were imbedded in howler monkey (Alouatta seniculus) dung or spider monkey (Ateles hybridus) dung, and placed on the forest floor in a scattered defecation pattern or a clumped defecation pattern. Sample sizes were N = 6 in the case of both howler monkey treatment combinations, and N = 5 in the case of both spider monkey treatment combinations; 10 seeds were used in each replicate, for a total of 220 seeds in the whole experiment. Error bars represent 1 SE.
In terms of depth, 60% of buried seeds were found between 0.5 and 2.5 cm. Mean depth was 2.6 cm and the maximum depth recorded was 9.5 cm. Dung type had no effect on this variable (F 1,18 = 1.70, P = 0.208), and neither was the interaction between dung type and defecation pattern significant (F 1,18 = 1.15, P = 0.297). However, defecation pattern had a significant effect on burial depth, as seeds deposited in a clumped pattern were buried more deeply than seeds in a scattered pattern (3.1 vs. 2.1 cm; F 1,18 = 9.40, P = 0.007).
Eighty per cent of the seeds were moved horizontally by dung beetles, regardless of them having been buried or not. The mean distance of horizontal movement was 11 cm (median = 8 cm), with a maximum recorded distance of 49 cm. Given the small horizontal distances observed, as well as the structure of the dung beetle assemblages (Table 1), it is very likely that large (≥10 mm) tunneller species were responsible for most of the secondary dispersal (both vertical and horizontal) of seeds in this study. Neither type of dung (F 1,18 = 2.26, P = 0.150), nor defecation pattern (F 1,18 = 0.121, P = 0.732), or the interaction of these factors (F 1,18 = 0.023, P = 0.150), had a significant effect on the mean percentage of seeds dispersed horizontally by dung beetles. Similarly, none of these factors had a significant effect on horizontal distances (dung type: F 1,18 = 0.18, P = 0.677; defecation pattern: F 1,18 = 1.70, P = 0.209; interaction: F 1,18 = 0.026, P = 0.867).
Of all seeds, 35% were killed by seed predators (28% by vertebrates and 5% by insects) or pathogens (2%). Similar to the previous variable, the mean proportion of seeds killed was not affected by dung type (F 1,18 = 2.34, P = 0.143), defecation pattern (F 1,18 = 0.674, P = 0.422) or their interaction (F 1,18 = 0.006, P = 0.941). Yet, it is worth noting that in the case of the factor dung type, an interesting trend of higher proportion of killed seeds was observed for spider monkey dung (42%) vs. howler monkey dung (28%).
A G test of independence showed a significant negative relationship between seed burial and seed death (G = 12.2, df = 1, P < 0.001). Of seeds that had not been buried by dung beetles, 73% suffered predation or pathogen attack, while only 53% of seeds buried by dung beetles suffered this fate.
Experiment 2: Effects of dung type on horizontal distance and burial depth. In this additional experiment, carried out with the main purpose of providing an independent assessment of burial depths and horizontal distances, the percentage of seeds buried by beetles was notably higher than in the factorial experiment. Of seeds in howler monkey and spider monkey dung, 74% and 73% of seeds, respectively, were buried by beetles. Unlike in the previous experiment, this time we found no effect of dung type on the number of seeds buried by beetles (F 1,14 = 0.233, P = 0.637). Mean burial depth was 2 cm, equal to the value obtained in the factorial experiment for the scattered defecation pattern. Again, great variability was observed (0.5–15 cm depth), with most of the buried seeds (75%) being found between 0.5 and 2.5 cm. Dung type had no effect on burial depth (F 1,14 = 0.805, P = 0.385).
In this experiment 70% of the seeds were moved horizontally by dung beetles, a somewhat lower percentage than in the factorial experiment (80%), but again, dung type had no effect on this variable (F 1,14 = 0.391, P = 0.542). Mean horizontal distances were also smaller in this experiment (7 cm vs. 11 cm in the factorial experiment), with a maximum of 36 cm, but similarly, no effect of dung type was detected (F 1,14 = 1.02, P = 0.329).
DISCUSSION
In terms of total frequencies of dung beetles captured with howler monkey vs. spider monkey dung, number of species was similar between monkeys while number of individuals was higher for howler monkeys. The rarefaction analysis also showed similar species richness at the community level for the dung beetle assemblage attracted to both dung types. These are the same patterns obtained for total frequencies in another howler vs. spider monkey comparison (same genera but different species) carried out in Guatemala (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). On the other hand, in a comparison between howler monkey and coati (an omnivorous procyonid) dung in Mexico (Estrada et al. Reference ESTRADA, HALFFTER, COATES-ESTRADA and MERITT1993), while the total number of species captured with the two dung types was again similar, in this case the total frequency of individuals captured was lower for howler monkey dung when compared to coati dung.
Even though the results discussed above are useful for a community-level comparison, when one wants to compare particular assemblages of beetles attracted to single defecations, it is necessary to consider the results obtained at the trap level. Comparison at this level becomes particularly relevant when the ultimate purpose is to relate dung beetle assemblages with secondary seed dispersal, as this interaction occurs at the level of single defecations. At this level, interestingly, the analyses led to different conclusions: the mean number of individuals attracted to a trap was similar for both bait types, but the mean number of species was higher for traps baited with howler monkey dung. Although the difference in the number of species attracted per trap may seem small (6 vs. 4), the effect on seed dispersal might be biologically important if species attracted to a defecation belong to different functional groups (e.g. rollers and tunnellers, nocturnal and diurnal). It has been shown that an increase in functional group richness causes an increase in the quantity of dung and seeds removed by beetles (Slade et al. Reference SLADE, MANN, VILLANUEVA and LEWIS2007).
Clearly a trap-level comparison of dung beetle assemblages is appropriate when assessing differences in secondary dispersal of seeds defecated by different primary dispersers. Yet, the methodology used here, which consists of killing beetles as they fall in the pitfall traps, and although it is the methodology commonly used, very likely overestimates the number of individuals and species that are in reality responsible for processing a given faecal clump. In this sense, the methodology used by Culot Reference CULOT2005 (see also Andresen Reference ANDRESEN1999) which involves live-trapping, might yield more realistic results. In this case traps contain soil, instead of liquid, and the dung bait is placed inside the trap on top of the soil, instead of hung above the trap. This allows for captured beetles to process the dung within the trap. It might be advisable to adopt this trapping methodology in future studies focusing on seed dispersal by dung beetles.
In terms of species composition of the dung beetle assemblages attracted to both dung types, 12 species were exclusively captured with howler monkey dung, while only three species were captured exclusively with spider monkey dung. However, abundances of these unique species were low (≤ 3 individuals in most cases; Table 1). Similarly, while only two beetle species (out of 14 tested) showed preference for spider monkey dung, six preferred howler monkey dung. Both for the dominant species, as well as those species that showed dung preference, dung beetle species were in general larger in the case of howler monkey dung. Thus, not surprisingly, the overall abundance of large individuals (≥10 mm) was significantly higher for howler monkey dung than for spider monkey dung. In Guatemala, although a similar number of dung beetle species showed preference for howler and spider monkey dung, larger beetles (≥ 15 mm) were also more frequent in howler monkey dung (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006).
These differences in the structure of the dung beetle assemblages attracted to different dung types can cause differences in dung burial rates and also in seed removal. In particular, dung beetle size has been previously shown to have a direct effect on the amount of ecological function performed (Larsen et al. Reference LARSEN, WILLIAMS and KREMEN2005), including secondary seed dispersal (Andresen Reference ANDRESEN2002b, Feer Reference FEER1999, Vulinec Reference VULINEC2002).
Indeed, differences in seed dispersal by dung beetles were observed in this study between the two dung types. As in the other study comparing howler and spider monkey dung (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006), the proportion of buried seeds was also higher for seeds surrounded by howler monkey dung. This might be due to a combination of the higher mean number of species attracted per trap and the higher proportion of large beetles, as discussed above. In contrast with the study in Guatemala, the proportions of seeds buried, for the dung of both primate species, were much lower in that study (2–23%), than in the present one (53–68%). This difference among study sites is likely to be a consequence of the differences in the local dung beetle communities, as well as the difference in experimental characteristics. Regarding the latter, in the previous study, size of experimental seeds was larger (10 mm vs. 7 mm in this study) and the amount of dung surrounding seeds was lower (5 g vs. 10 g in this study). These two factors have been shown to influence seed burial by dung beetles. In particular, seed burial by beetles shows a negative relationship with seed size and a positive relationship with the amount of dung surrounding a seed (Andresen Reference ANDRESEN2001, Reference ANDRESEN2002a, Reference ANDRESEN2002b; Andresen & Levey Reference ANDRESEN and LEVEY2004, Culot et al. Reference CULOT, HUYNEN, GÉRARD and HEYMANN2009, Feer Reference FEER1999).
Seed burial by dung beetles was not only affected by dung type, but we also found a significant effect of defecation pattern. A higher proportion of seeds was buried when deposited in a clumped pattern vs. a scattered pattern, but it is important to note that this was the case only for spider monkey dung (significant interaction term; Figure 2). In previous studies using similar experimental set-ups, either only with howler monkey dung (Andresen Reference ANDRESEN2002a), or with both howler and spider monkey dung (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006), no effect of defecation pattern on seed burial by beetles was detected. One important methodological difference in this study, with respect to the others, was that the seeds for the clumped pattern were set out under sleeping trees, shortly after the monkeys defecated in the morning. Thus, the total concentration of dung in the clumped-defecation sites was higher than just the 10 experimental piles of 10 g of dung each. Also, large quantities or urine are present in sleeping sites, which can further increase the olfactory attraction for dung beetles. Finally, some dung beetle species are known to ‘monitor’ the activity of certain mammal species, thus arriving quickly on the defecations (Hanski & Cambefort Reference HANSKI and CAMBEFORT1991). All these factors, related to placing clumped-pattern seeds in real defecation sites under sleeping trees, could be responsible for detecting, in this study, a difference between the clumped and the scattered defecation pattern. However, one would expect to detect the effect for both howler, and spider monkey dung. Yet, as mentioned above, this effect was only conspicuous for spider monkey dung. This suggests that the difference in the intensity of odour cues, at sleeping sites vs. isolated dung piles was much more pronounced in the case of spider monkeys. What the ultimate cause for this might be remains unknown, and the effect of defecation pattern on seed burial by dung beetles needs to be investigated further.
The depth of seed burial is a very important variable in the seed–beetle interaction. On the one hand, increasing depth diminishes seed predation, but on the other hand, it also diminishes seedling emergence (Andresen Reference ANDRESEN2001, Andresen & Levey Reference ANDRESEN and LEVEY2004, Estrada & Coates-Estrada Reference ESTRADA and COATES-ESTRADA1991, Feer Reference FEER1999, Shepherd & Chapman Reference SHEPHERD and CHAPMAN1998). In previous studies, no effect of dung type or defecation pattern was observed on burial depth (Andresen Reference ANDRESEN2002a, Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006). In the present study, however, seeds were buried more deeply when deposited in the clumped pattern. However, the difference in depth between treatments was not large (1 cm), and it remains to be evaluated whether such a difference is of biological significance.
Defecated seeds are also moved horizontally during dung beetle activity, regardless of seed burial. As has been reported in previous studies (Andresen & Feer Reference ANDRESEN, FEER, Forget, Lambert, Hulme and Vander Wall2005), we also found that, while the percentage of seeds moved horizontally was high, horizontal distances were small (< 50 cm), and that neither variable was affected by dung type or defecation pattern. Finally, while experimental factors had also no effect on seed survival, it was found, as previously (Andresen & Feer Reference ANDRESEN, FEER, Forget, Lambert, Hulme and Vander Wall2005), that seeds buried by dung beetles had higher chances of surviving than seeds remaining on the soil surface after being defecated.
The results obtained in the second experiment yielded an unexpected outcome. While the values for depths and distances were comparable to those obtained in the first (factorial) experiment, the overall percentage of seeds buried by dung beetles was considerably higher in the second experiment, and, furthermore, no difference between dung types was detected this time. This is an important finding, and calls attention to the large number of factors, known and unknown, that can affect the outcome of this plant–animal interaction (Andresen Reference ANDRESEN2005, Andresen & Feer Reference ANDRESEN, FEER, Forget, Lambert, Hulme and Vander Wall2005). The first experiment was carried out in August–September and the second experiment in October–November of the same year. Higher numbers of beetles (both species and individuals) were captured in July, when compared to the September and November captures. As we did not sample dung beetles in August or October, we have not enough information to assess the role that seasonality, or even short-term temporal variability (Andresen Reference ANDRESEN2008), might be playing in causing the contrasting results of both experiments. However, what we did observe was a change in the texture of spider monkey dung. During the first experiment, spider monkey dung was more watery and had a less consistent texture than during the second experiment. In fact, during the second experiment, the texture of the two dung types was equally firm. Apparently, when dung is more watery, it might be easier for dung beetles to separate seeds from the fecal material. This difference in texture was also reported in Guatemala (Ponce-Santizo et al. Reference PONCE-SANTIZO, ANDRESEN, CANO and CUARÓN2006) and Peru (T. Larsen, unpubl. data), and was proposed as a causal factor for the lower proportion of seeds buried by dung beetles when placed in more watery spider monkey dung (Guatemala) and human dung (Peru) compared with less watery howler monkey dung.
In conclusion, this study corroborates the fact that the interaction between seeds, primates and dung beetles is a very complex one, where specific outcomes can change idiosyncratically, depending on many factors. However, among all the variability, certain general patterns continue to emerge, such as the fact that, for a seed deposited in mammal dung, survival probability is greatly increased when it is buried by dung beetles. Also, that different mammal species, even those that might appear to be very similar (Culot Reference CULOT2005, Culot et al. Reference CULOT, HUYNEN, GÉRARD and HEYMANN2009), produce seed depositions, through defecation, that vary in certain characteristics that will ultimately affect seed fate differently. As post-dispersal fate greatly determines the effectiveness of a primary seed disperser, ecologists studying seed dispersal through endozoochory by mammals ought to consider assessing the effects of dung beetles in their study systems.
ACKNOWLEDGEMENTS
This study was made possible through the financial assistance of Fundación ProAves in Colombia and the National Autonomous University of Mexico (UNAM). We are thankful for the logistical support provided by Reserva Natural de las Aves El Paujil (RNAP). We are particularly grateful to Fernando Vaz de Mello for the identification of dung beetle species.





