Introduction
Staročeskéite, ideally Ag0.70Pb1.60(Bi1.35Sb1.35)Σ2.70 S6, has been found at the Staročeské pásmo Lode of the Kutná Hora base-metal ore district, Czech Republic, during an extensive search for bismuth sulfosalts in the ore district. It is named after the type locality. The history of the mineral goes back to 2005, when first samples of bismuth mineralization from Kutná Hora ore district were measured using an electron probe microanalyser (EPMA), including compositions corresponding to staročeskéite. Then, the compositions were considered as an intermediate member between gustavite and andorite-group minerals. The Bi–Sb intermediate member posed an interesting challenge, so a fragment of a homogenous grain ca. 40 µm by 30 µm in size of the holotype sample ST 37 was extracted for single-crystal X-ray diffraction (SC-XRD) analysis. The structure was solved and published in 2010 (Pažout and Dušek, Reference Pažout and Dušek2010) but the phase was still not recognized by the authors as a new mineral species. Later on, after the description of the new mineral terrywallaceite from Huancavelica, Peru (Yang et al., Reference Yang, Downs, Evans and Pinch2013), which is also a Bi–Sb member of the lillianite homologous series, it became apparent that the mineral found in Kutná Hora is a new mineral species. Therefore, in 2015–2016 another large set of polished sections with Bi-mineralization from Kutná Hora was measured using EPMA and more samples with staročeskéite compositions were discovered. Subsequently, the proposal for a new mineral was submitted and the new mineral and its name were approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA 2016-101; Pažout and Sejkora, Reference Pažout and Sejkora2017). The holotype sample has been deposited in the mineralogical collections of the National Museum in Prague, Department of Mineralogy and Petrology, Cirkusová 1740, Praha 9, Czech Republic under the catalogue number P1P 30/2016.
Staročeskéite is a member of the lillianite homologous series of sulfosalts with N = 4. The structural and chemical features of the lillianite homologues have been described in detail by Makovicky and Karup-Møller (Reference Makovicky and Karup-Møller1977a,Reference Makovicky and Karup-Møllerb), and Makovicky and Topa (Reference Makovicky and Topa2014). The pronounciation of the mineral name is best approximated as /´starotʃeski:ait/ using the International Phonetic Alphabet. The acute accent ´ symbol indicates that the main stress is on the /sta/ syllable.
Occurrence
Staročeskéite was found in twenty-one different samples (~50 analytical points) in the material collected from medieval mine dumps of the Staročeské pásmo Lode (Old Bohemian Lode) of the Kutná Hora Ag–Pb–Zn ore district in 1999–2015. Therefore no information is available on their in situ position within individual vein structures. The GPS coordinates of the type occurrence are 49°58.47882′ N, 15°16.15142′ E. The Kutná Hora Ag–Pb–Zn ore district (located 60 km east of Prague, Central Bohemia, Czech Republic) contains a hydrothermal vein type mineralization of Variscan age (Holub et al., Reference Holub, Hoffman, Mikuš and Trdlička1982, Žák et al., Reference Žák, Dobeš and Sztacho1996). It was one of the main European producers of silver in the 14th to 16th centuries, with hundreds of mines on twelve major lodes (Fig. 1). Each lode (also called pásmo in Czech or zug in German) represents a hydrothermally altered zone of several hundred metres to about three kilometres long and dozens of metres wide, with the depth range between several hundred metres to 1 km, each consisting of several, usually parallel veins (Holub et al., Reference Holub, Hoffman, Mikuš and Trdlička1982).
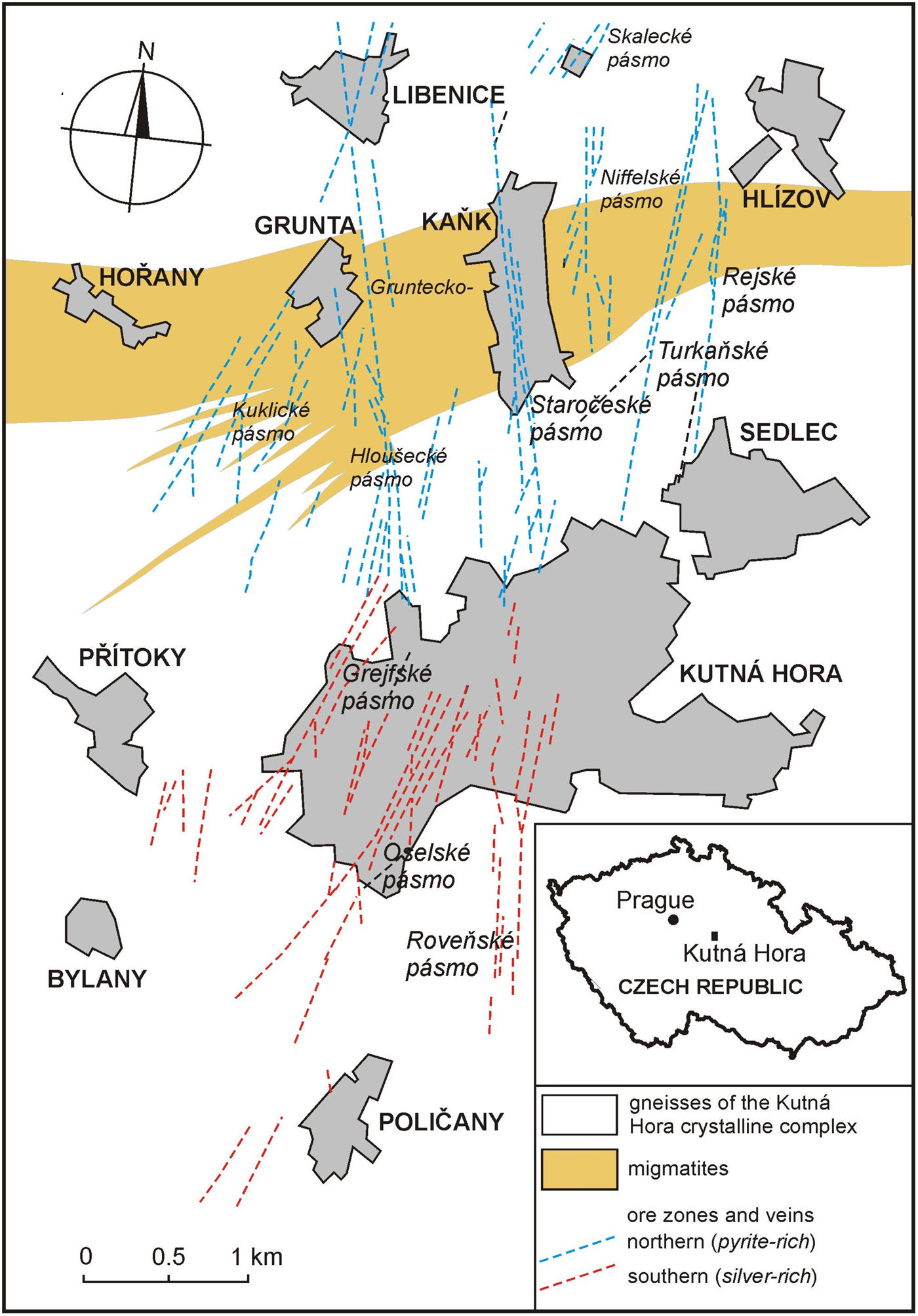
Fig. 1. Map of Kutná Hora ore district with major ore zones (lodes), after Malec and Pauliš (Reference Malec and Pauliš1997). Each lode (‘pásmo’ in Czech) consists of several veins.
Geologically and mineralogically, two mineral assemblages are present in this ore district, one ‘silver-rich’ in the southern part of the ore district and one ‘pyrite-rich’ in the northern part (Malec and Pauliš, Reference Malec and Pauliš1997). The Staročeské pásmo Lode belongs to the northern pyrite-rich lodes and it is the biggest lode of the Kutná Hora ore district, both in terms of the amount of extracted ore and the amount of extracted silver (~300–500 tons of Ag).
The silver-rich assemblage (southern part) consists mainly of miargyrite, pyrargyrite, freibergite, native silver, galena, pyrite, sphalerite, berthierite and Pb–Sb (–Ag) sulfosalts (boulangerite, jamesonite and owyheeite) in quartz–kutnohorite gangue. The pyrite-rich assemblage (northern part) comprises pyrite, arsenopyrite, sphalerite, Ag-bearing galena, pyrrhotite, marcasite, chalcopyrite, stannite, freibergite–tetrahedrite and Pb–Sb (–Ag) sulfosalts in quartz gangue without kutnohorite. Typical of the pyrite-rich assemblage is the presence of Bi and Sn (each from a different mineralization stage), completely absent in the former, Ag-rich assemblage. More than 150 mineral species are known from the Kutná Hora ore district (Malec and Pauliš, Reference Malec and Pauliš1997; Pauliš, Reference Pauliš1998; Pažout, Reference Pažout2017; Pažout et al., Reference Pažout, Sejkora and Šrein2017).
The new mineral was found in quartz gangue in the rich Ag–Pb–Bi–Sb sulfosalt association (gustavite, terrywallaceite, vikingite, treasurite, eskimoite, erzwiesite, Bi-rich fizelyite and Bi-rich ramdohrite) accompanied by Ag- and Bi-bearing galena and Pb–Bi–Sb sulfosalts (izoklakeite, cosalite and Bi-rich jamesonite) (Figs 2 and 3). The new mineral forms late in the Bi sulfosalt paragenesis, following the usual sequence of crystallization from early Bi-rich to later Sb-rich phases: native Bi → galenass → matildite → izoklakeite/cosalite → eskimoite/vikingite → gustavite → Sb-rich gustavite → terywallaceite/staročeskéite → Bi-rich andorite minerals → Bi-rich jamesonite (Pažout Reference Pažout2017; Pažout et al., Reference Pažout, Sejkora and Šrein2017). The origin of this Bi sulfosalt mineralization is related to the penetration of low-temperature fluids (~100–250°C) into tectonically opened fractures in older ore vein fillings (pyrite, arsenopyrite and stannite). Virtually no mobilization of elements from the earlier vein filling took place; hydrothermal fluids must have been relatively rich in Ag and Pb and simultaneously poor in Cu. A high Bi content was characteristic of the initial stage of the mineralization. Gradually, the Bi/Sb ratio decreased considerably with more Sb-rich minerals originating. At the final stage, Bi-rich Pb–Sb sulfosalts with lower Ag contents crystallized (Pažout et al., Reference Pažout, Sejkora and Šrein2017).
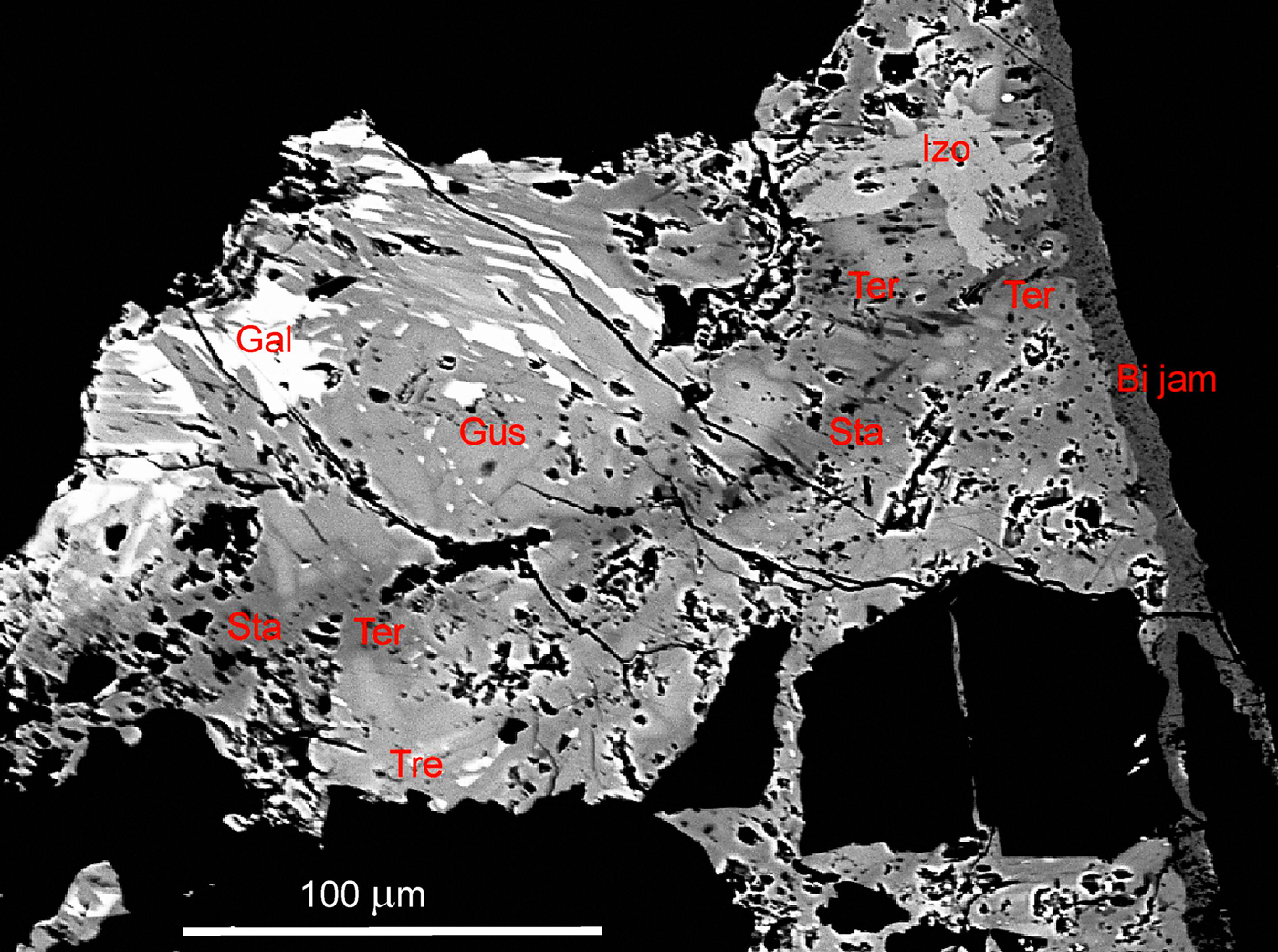
Fig. 2. The association of Sb-rich Bi-sulfosalts and Bi-rich Sb-sulfosalts from the Kutná Hora ore district. The earliest (oldest) Ag- and Bi-bearing galena (Gal) is replaced by izoklakeite (Izo) and treasurite (Tre), followed by Sb-rich gustavite (Gus), terrywallaceite (Ter) and staročeskéite (Sta). The right rim of the grain is formed by latest Bi-rich jamesonite (Bi jam). Back-scattered electron (BSE) image of sample ST 4 F.
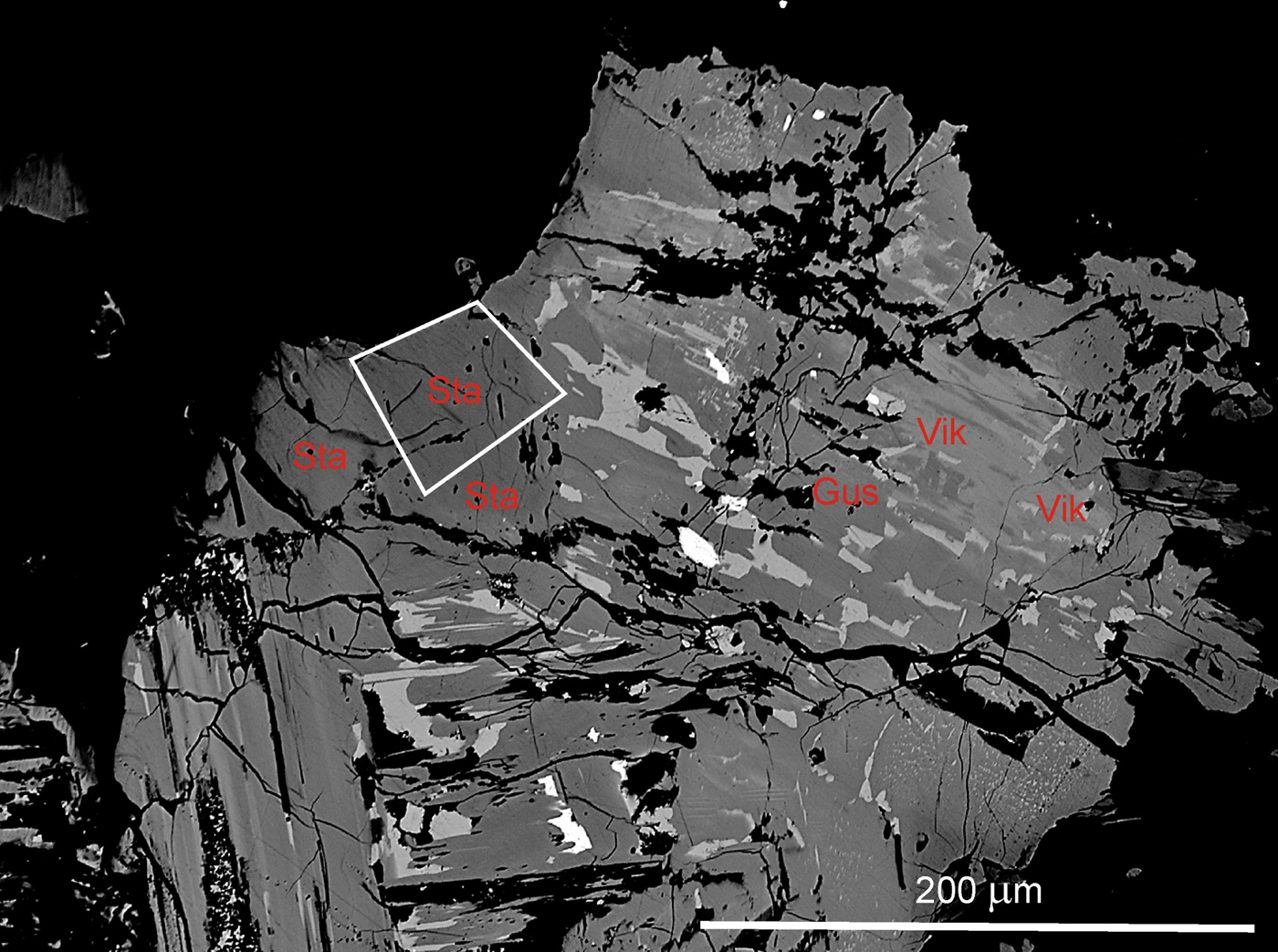
Fig. 3. Staročeskéite (Sta), gustavite (Gus) and vikingite (Vik), BSE image of holotype sample ST 37. Galena (white) is the earliest mineral. A fragment for SC-XRD analysis of staročeskéite was extracted from the area marked by white lines.
Physical and optical properties
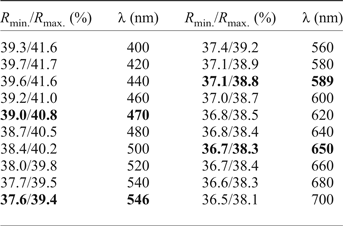
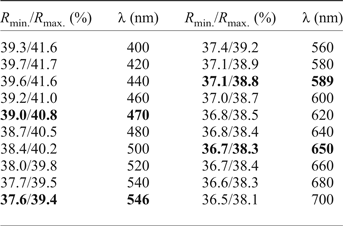
The mineral occurs as lath-shaped crystals or anhedral grains up to 80 µm × 70 µm, growing together in aggregates up to 200 µm × 150 µm across (Figs 2 and 3). Staročeskéite is steel-grey in colour and has a metallic lustre, the calculated density is 6.185 g/cm3. In reflected light staročeskéite is greyish white; bireflectance and pleochroism are weak with greyish tints. Anisotropy is weak to medium, with grey to bluish grey rotation tints. Internal reflections were not observed. Reflectance percentages for the four wavelengths required by the Commission on Ore Mineralogy (R min and R max) for staročeskéite from Kutná Hora are: 39.0/40.8 (470 nm), 37.6/39.4 (546 nm), 37.1/38.8 (589 nm) and 36.7/38.3 (650 nm). A full reflectance dataset is given in Table 1 and Fig. 4.
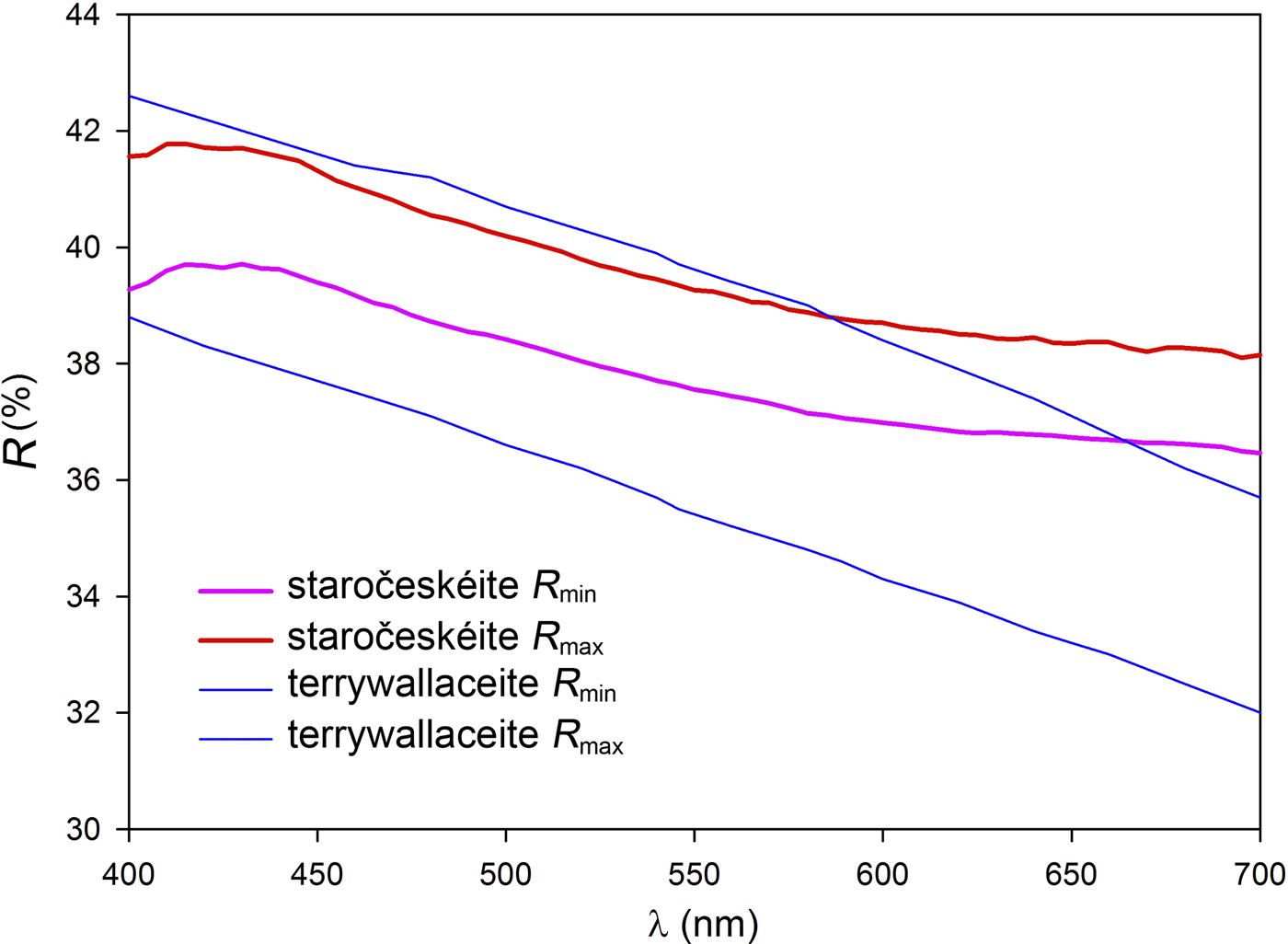
Fig. 4. Reflectivity curves for staročeskéite (this paper) and terrywallaceite (Yang et al., Reference Yang, Downs, Evans and Pinch2013).
Chemical composition
Chemical analyses of the holotype sample were performed using a JEOL JXA-8600 electron probe microanalyser in wavelength-dispersive spectroscopy (WDS) mode (25 kV and 35 nA) and a beam diameter of 5 µm. The following standards and X-ray lines were used: CuFeS2 (CuKα and FeKα), Ag (AgLα), PbS (PbLα), Bi2S3 (BiLα and SKα), Sb2S3 (SbLα), CdTe (CdLβ and TeLα), Bi2Te2S (BiLα and TeLα) and Bi2Se3 (SeKα). Raw data were corrected with an online ZAF-4 procedure.
A second set of polished sections with staročeskéite was measured on a CAMECA SX 100 electron probe microanalyser at the National Museum, Prague in 2015. The analytical conditions were as follows: WDS mode, accelerating voltage of 25 kV, beam current of 20 nA, electron-beam diameter of 2 µm and standards: chalcopyrite (SKα), Bi2Se3 (BiMβ), PbS (PbMα), Ag (AgLα), halite (ClKα), Sb2S3 (SbLα), CdTe (CdLα), HgTe (HgMα), pyrite (FeKα), Cu (CuKα), ZnS (ZnKα), NiAs (AsLα) and PbSe (SeLβ). Measured data were corrected using PAP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985).
Analytical data for the holotype sample are given in Table 2. Its empirical formula (calculated on the basis of 11 atoms per formula unit) is:
Table 2. Chemical data (wt.%) for staročeskéite (n = 5). Holotype sample ST 37.

S.D. – standard deviation.
(Ag0.68Cu0.01)Σ0.69(Pb1.56Fe0.01Cd0.01)Σ1.58(Bi1.32Sb1.37)Σ2.69(S6.04Se0.01)Σ6.05, corresponding to N chem = 3.94, Bi/(Bi + Sb) = 0.49 and L% = 70.5. An ideal formula, corresponding to: (1) 70% of the Ag+ + (Bi3+, Sb3+) ↔ 2 Pb2+ substitution relative to the Ag-free ideal composition of lillianite Pb3Bi2S6 and (2) the Bi:Sb ratio 1:1 (i.e. Bi/(Bi + Sb) = 0.50), can be written as Ag0.70Pb1.60(Bi1.35Sb1.35)Σ2.70S6, which requires Ag 7.22, Pb 31.70, Bi 26.97, Sb 15.72 and S 18.39 wt.%, total 100.00 wt.%.
Staročeskéite was found in 21 samples (~50 analytical points), representative analyses are given in Table 3. It can be differentiated chemically from similar members of the lillianite homologous series on the basis of the Ag content L% and the Bi/(Bi + Sb) ratio (Figs 5 and 6).
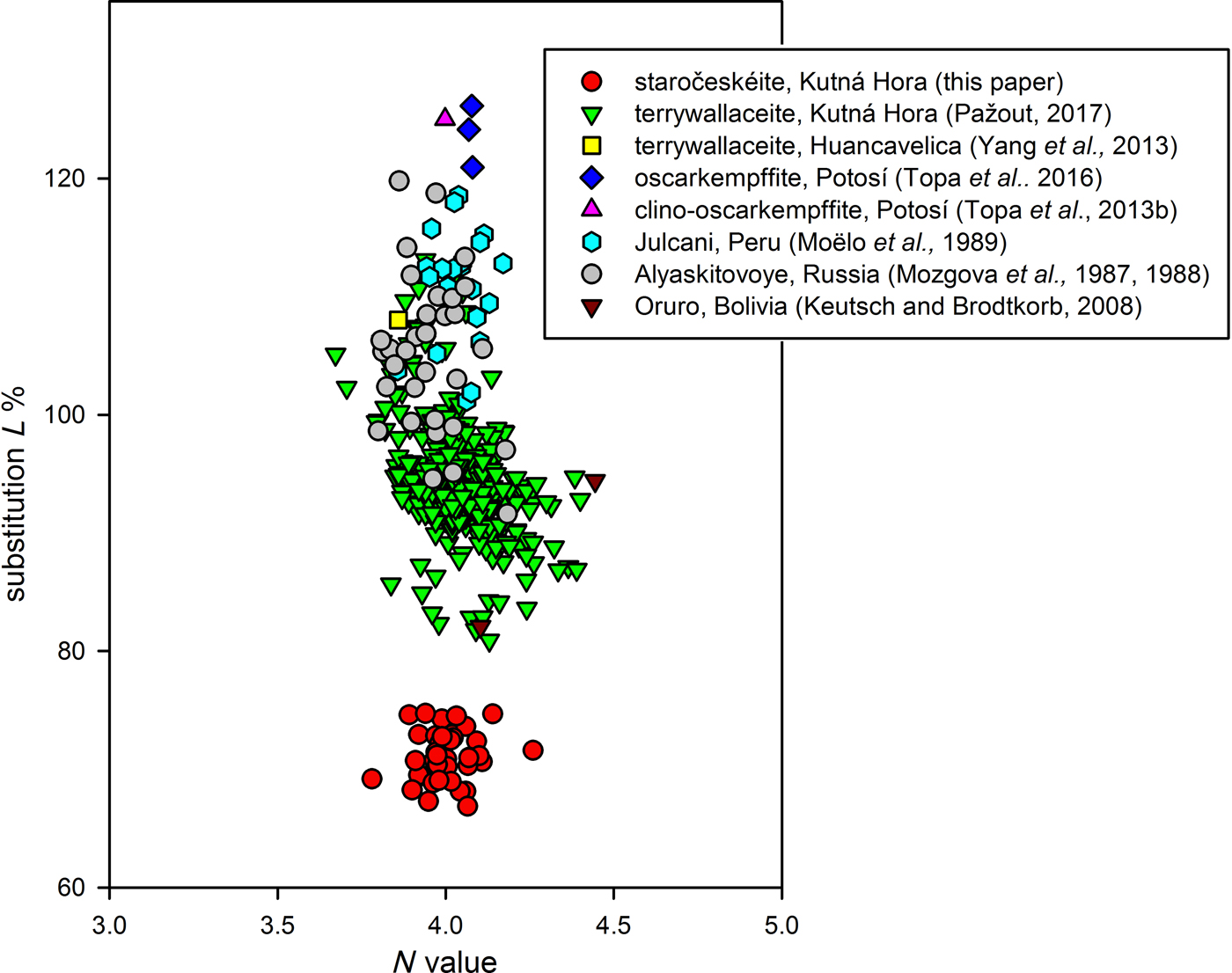
Fig. 5. The N chem value vs. Ag++(Bi,Sb)3+↔ 2 Pb2+ substitution (L%) plot for lillianite homologues with N = 4 and distinct Bi/Sb substitution.
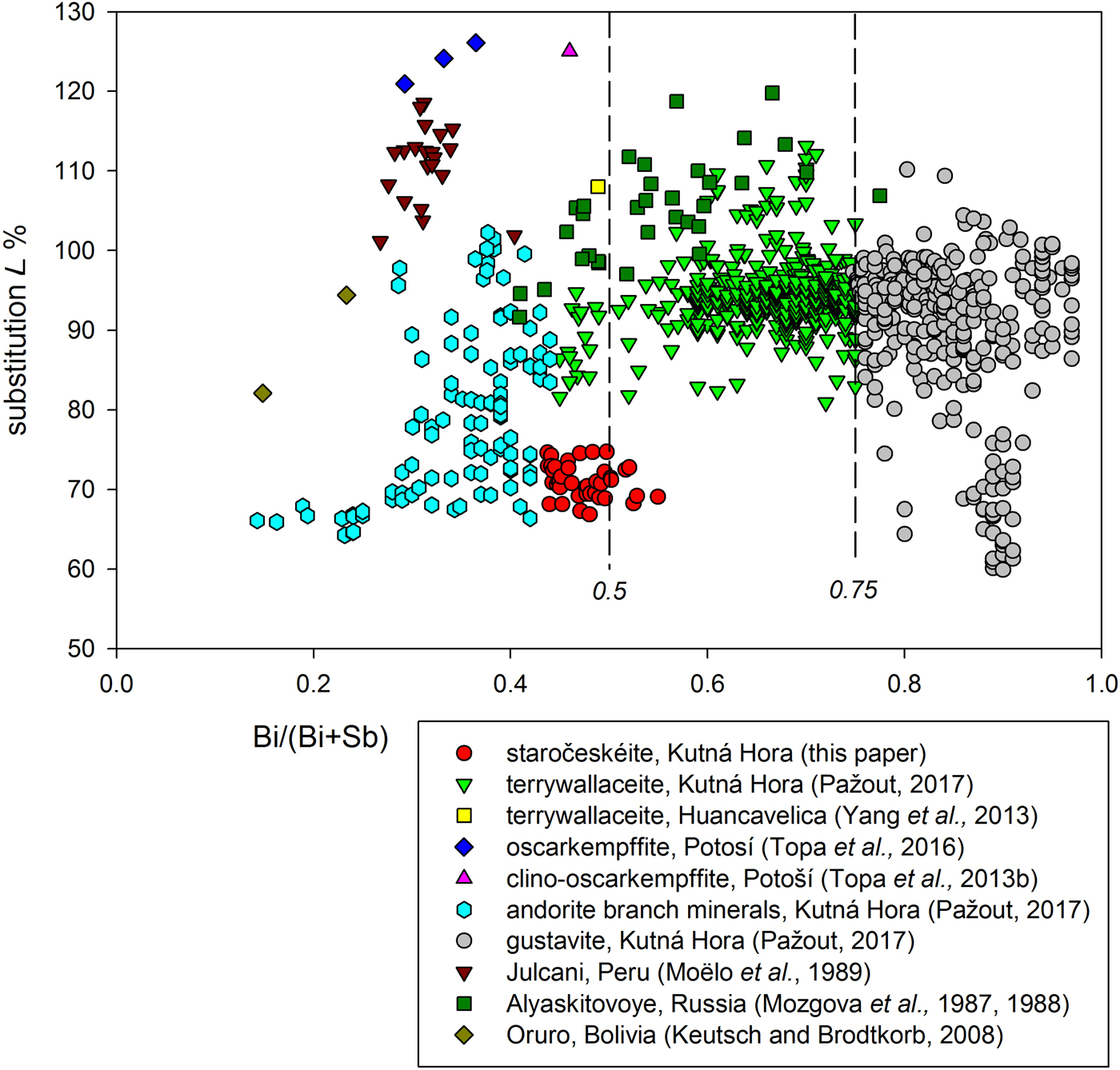
Fig. 6. The Bi/(Bi + Sb) (at.%) vs. Ag++(Bi, Sb)3+↔ 2 Pb2+ substitution (L%) plot for lillianite homologues with N = 4 and distinct Bi/Sb substitution.
Table 3. Representative compositions of staročeskéite from Kutná Hora* ordered according to increasing Bi/(Bi + Sb).

* Samples: 1 – ST 37, 2 – ST 37, 3 – ST 55 gr1, 4 – ST 4 F, 5 – ST 90 A, 6 – ST 4 F, 7 – ST 4 F, 8 – ST 90 A, 9 – ST 42 C gr1, 10 – ST 70C.
N, L% and x values were calculated according to Makovicky and Karup-Møller (1977a) – see text.
Crystallography and crystal structure
X-ray diffraction data were collected at ambient temperature on the single-crystal diffractometer Gemini (Oxford Diffraction) with CCD area detector Atlas using monochromatic MoKα radiation (λ = 0.71073 Å). A small fragment of the sample, 0.0442 mm × 0.0270 mm × 0.0214 mm in size, was used for collection of X-ray data at ambient temperature. An orthorhombic cell was found with a = 4.2539(8), b = 13.3094(8) and c = 19.6253(12) Å. Unlike a typical natural gustavite, images from data collection clearly showed a C-centred unit cell with no reflections breaking the cell centring. Also, no reflections were observed that would double the size of the short a parameter, as occurs in the unit cell of fizélyite and ramdohrite. Atomic coordinates, displacement parameters, selected interatomic distances, details of data collection and refinement and the structure description are given in Pažout and Dušek (Reference Pažout and Dušek2010). There are only three distinct cation sites, giving the stoichiometry M3(M2)2(M1)2S6. Staročeskéite has M3 completely occupied by Pb, M2 dominated by trivalent cations with Sb >> Bi, and M1 occupied mainly by trivalent cations with Bi >> Sb, but also with substantial Ag+ for charge balance.
The structure of staročeskéite (Fig. 7), a natural Sb–Bi orthorhombic 4,4L homologue of the lillianite homologous series, consists of one Pb site (M3) in trigonal prismatic coordination and two octahedral mixed sites M1 and M2 each consisting of three elements (Pažout and Dušek, Reference Pažout and Dušek2010). The octahedral site M1 positioned at the margin of the diagonal chain of four octahedra is formed by a mixed site with the occupancy 52% Bi, 35.6% Ag and 12.4% Sb, the central octahedral site M2 is formed by one mixed site with the occupancy 60.1% Sb, 25.9% Pb and 14% of Bi. The final empirical formula is Ag0.71Pb1.52Bi1.32Sb1.45S6, which is in a good agreement with the EPMA-established composition Ag0.69Pb1.56(Bi1.32Sb1.37)Σ2.69(S6.04 Se0.01)Σ6.05. Cation charge of the final formula is 12.059 against anion charge 12.0. The final Rw was 5.08% for observed reflections. On the basis of site occupancies, the formula of staročeskéite is M3PbM2(Sb0.60Pb0.26Bi0.14)Σ2 M1(Bi0.52Ag0.356Sb0.124)Σ2S6, which can be simplified to give M3PbM2Sb2M2(AgBi)Σ2S6,which has two different-valence cations in only one site, and hence is an end-member in the sense of Hawthorne (Reference Hawthorne2002). The structures of gustavite (Pažout and Dušek, Reference Pažout and Dušek2009) and staročeskéite were compared by Pažout and Dušek (Reference Pažout and Dušek2010).
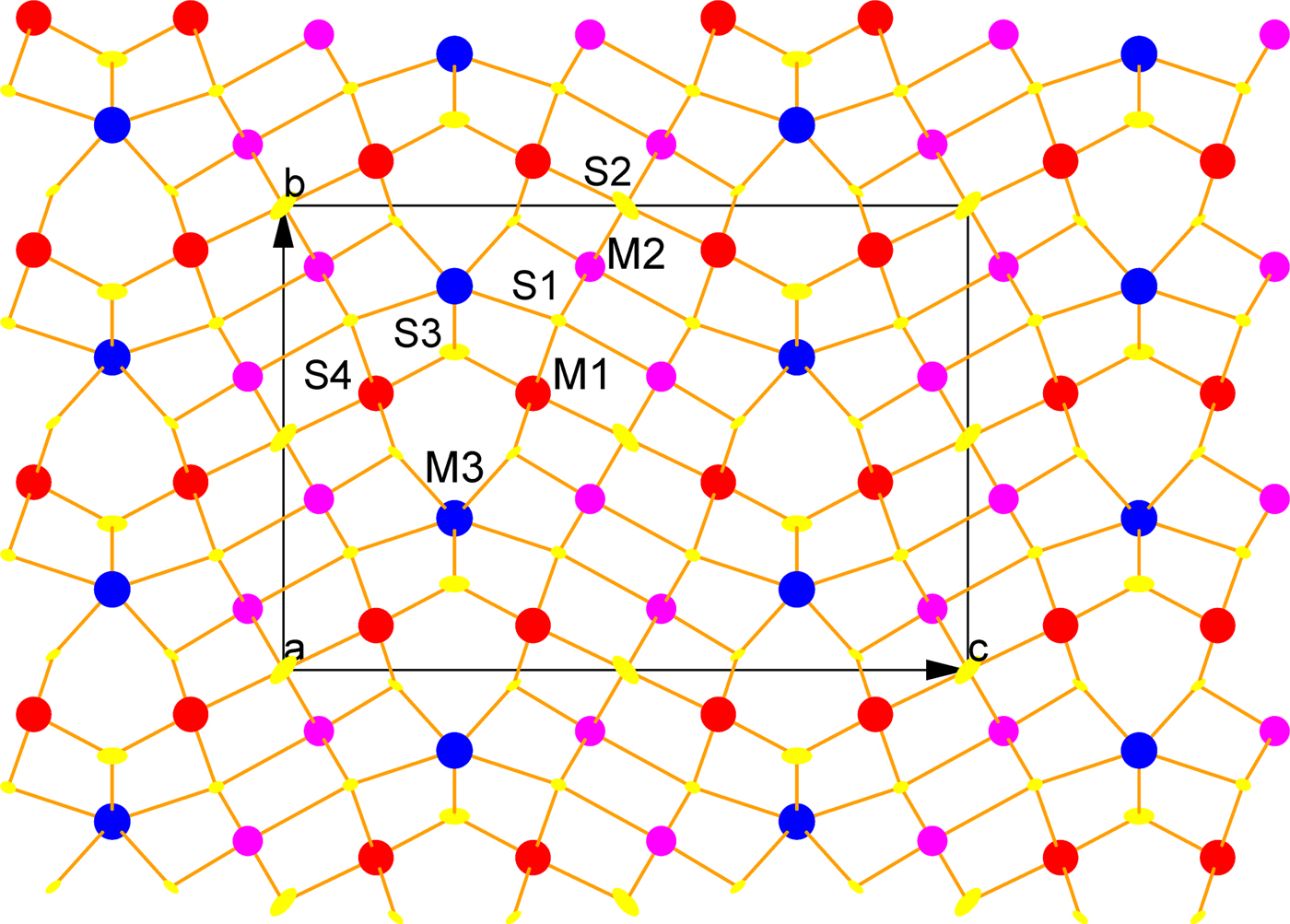
Fig. 7. The crystal structure of staročeskéite. M3–Pb in bicapped trigonal prismatic coordination (CN 8), M1–Bi/Ag/Sb mixed site (Bi is major component), M2–Pb/Sb/Bi mixed site (Sb is a major component), both in octahedral coordination, S – sulfur atoms. Viewed along the a axis.
To assess the composition boundaries within which the name staročeskéite applies we consider the following: for staročeskéite, M3 = Pb; M2 is mainly trivalent, Pb < (Sb + Bi) with Sb >> Bi; M1 is dominated by (Bi + Sb) + Ag with Bi > Sb. If we assume exclusively Pb in M3, allow up to 50% Pb in M2, and, given the absence of Pb in M1, set Ag ≥ 0.5 and Bi ≥ Sb conditions there, then limiting compositions for the name to be applicable are given in Table 4.
Table 4. Limiting compositions for the mineral staročeskéite.

Thus the name staročeskéite would be valid for a lillianite structure with composition AgxPb3–2xBiySb2+x–yS6 in a two-dimensional composition space with the boundaries ½ ≤ x ≤ 0.8 and 1–½x ≤ y ≤ 2, where the parameter x = Ag content = L% expressed as a fraction and y = total Bi content. The top range of the x parameter is assumed to be not more than 0.8 because for x = 1 the phase would chemically correspond to Bi-rich andorite-VI (with L% = 100 and Bi/(Bi + Sb) = 0.19) or terrywallaceite (with L% = 100 and Bi/(Bi + Sb) = 0.66), depending on the Sb content, and for x = 0.9 the phase would chemically correspond to Bi-rich andorite-IV (with L% = 90 and Bi/(Bi + Sb) = 0.19) or terrywallaceite (with L% = 90 and Bi/(Bi + Sb) = 0.69). Thus it is evident that for staročeskéite to exist, there must be some Pb in M2 (because Pb just in M3 results in other clearly defined minerals). Therefore, from these empirical assumptions and tests of gustavite and terrywallaceite grains with various L% by single-crystal diffraction (Pažout Reference Pažout2017), it can be concluded that staročeskéite compositional limits regarding the Ag + (Bi,Sb) = 2 Pb substitution are from L% = 50 to ~L% =80. Nevertheless, the ultimate answer to which phase is dealt with is given by SC-XRD.
Although it is possible to define an ideal end-member composition for staročeskéite as noted above, the solid-solution ranges of complex sulfosalts do not necessarily include such members, and several structurally related species with different observed solid-solution ranges may share the same end-member(s). Therefore, we prefer to define the composition range corresponding to the name ‘staročeskéite’ in terms of the parameter L% (Ag content) and Bi/(Bi + Sb) ratio. In addition, staročeskéite possesses a simple lillianite structure with no doubling of a repeat or other superstructure, and no distortion away from Cmcm symmetry, in contrast to related species such as terrywallaceite and the andorite-group minerals.
For the purpose of this article only points with L% between 65 and 75 and the Bi/(Bi + Sb) = 0.44–0.55 were considered to represent staročeskéite. To check the validity of the ‘maximum Sb’ situation, grains with compositions corresponding to L ≈ 70% and Bi/(Bi + Sb) between 0.35 and 0.43 should be extracted for single-crystal analysis and characterized structurally. Grains with these compositions were found in the Kutná Hora ore district in the Bi-sulfosalt mineralization and were interpreted as Bi-rich fizélyite if L% was between 64 and 70, and Bi-rich ramdohrite if L% was between 70 and 75 (Pažout, Reference Pažout2017). Even if the single-crystal data of the extracted fragments were not good enough to solve the structure, the data collection images should clearly show a C-centred cell with the ‘short 4 Å’ cell parameter of staročeskéite or a primitive P cell with the ‘double 8 Å’ cell parameter of fizélyite or ramdohrite. This direction will be exploited further in the future.
As already mentioned, the cell parameters given were refined from single-crystal data. The amount of material was not sufficient for experimentally obtained powder X-ray data. A theoretical pattern (Table 5) was calculated with Jana2006 software (Petříček et al., Reference Petříček, Dušek and Palatinus2006) employing a wavelength corresponding to that of CuKα1 radiation (λ = 1.540598 Å). Cell parameters, space group, atom positions, site-occupancy factors and isotropic displacement factors from the crystal-structure determination were used.
Table 5. Calculated powder XRD data for staročeskéite.

The strongest lines are given in bold.
Relation to other species
Staročeskéite is a new member of the lillianite homologous series, class 3.1.1. of the Sulfosalt systematics (Moelo et al., Reference Moëlo, Makovicky, Mozgova, Jambor, Cook, Pring, Paar, Nickel, Graeser, Karup-Møller, Balić-Žunić, Mumme, Vurro, Topa, Bindi, Bente and Shimizu2008). A comparison of selected data for lillianite Pb3Bi2S6, terywallaceite AgPb(Sb,Bi)(Bi,Sb)2S6 and gustavite AgPbBi3S6 is given in Table 6. Structurally, staročeskéite has a unit cell and symmetry similar to those of lillianite. However, chemically, staročeskéite differs from lillianite by the Bi/(Bi + Sb) ratio which is always close to 1 for lillianite and by the Ag content L% which is usually close to zero but can be between 0 and 50% for lillianite and 50 to 80% for staročeskéite. In fact, in the light of the research presented in this paper, ‘lillianite’ with L% > 50 should be called staročeskéite if the most straightforward definitions of compositional boundaries are defined (see above). While ideal gustavite is PbBi2(AgBi)S6, gustavite in Nature is chemically similar to lillianite with regard to L% as it can have a varying degree of Ag+ + (Bi3+, Sb3+) ↔ 2 Pb2+ substitution. Verified gustavite is known only for L% = 85% and above (D. Topa, pers. comm.) However, the first author of this paper detected by single-crystal diffraction the gustavite unit cell in a crystal fragment with L% = 70 (Pažout, Reference Pažout2017). The Bi/(Bi + Sb) ratio for gustavite is always above 0.75. In addition, structurally gustavite has the ordered monoclinic superstructure, as does terrywallaceite. Terrywallaceite can have the same amount of Sb and Bi as in staročeskéite, but the Ag+ + Bi3+ ↔ 2 Pb2+ substitution is between 85 and 110% and it has a monoclinic cell and symmetry similar to those of gustavite. There are uncertainties for the case of Bi-rich ramdohrite in the Kutná Hora ore district because of a very close substitution percentage and occasionally a very high Bi content, reaching up to Bi/(Bi + Sb) = 0.42. Bi-rich fizélyite from Kutná Hora shows a narrower limit of Bi for Sb substitution with Bi/(Bi + Sb) between 0.16 and 0.38 (Pažout, Reference Pažout2017). However, both fizélyite and ramdohrite display P21/n symmetry with a cell volume double that of staročeskéite.
Table 6. Comparative data for staročeskéite and related minerals†.

* – data calculated from the structure; ** – measured powder XRD data.
† The space group and cell parameters of lillianite, gustavite and terrywallaceite are transformed into axial settings corresponding to staročeskéite.
Thus staročeskéite stands as a unique mineral both structurally and chemically, distinctly different from lillianite, gustavite, terrywallaceite and other known members of the lillianite homologous series. It is defined as a lillianite homologue with the following requirements: N = 4, L (Ag+ + Bi3+,Sb3+ ↔ 2 Pb2+ substitution) ≈ 70% (with structurally derived limits of L% between 50 and 80), and ~50 at.% of bismuth is replaced by antimony (Bi/(Bi + Sb) ≈ 0.5), with structurally derived limits from Bi/(Bi + Sb) ≈ 0.3 to 0.8.
More Bi–Sb and Sb–As mixed members are known in the oversubstituted end of the series (L% > 100): arsenquatrandorite Pb12.8Ag17.6Sb38.08As11.52S96 (And110 – Topa et al., Reference Topa, Makovicky, Putz, Zagler and Tajjedin2013c) and jasrouxite Pb4Ag16Sb24As16S72 (And136.5 – Topa et al. Reference Topa, Makovicky, Favreau, Bourgoin, Boulliard, Zagler and Putz2013a); and two Sb–Bi members oscarkempffite Pb4Ag10Sb17Bi9S48 (And124) and clino-oscarkempffite Pb6Ag15Sb21Bi18S72 (And125). Oscarkempffite is characterized by the Bi/(Bi + Sb) range between 0.29 and 0.37 (Topa et al., Reference Topa, Makovicky, Stanley and Robetzs2016), clino-oscarkempffite has the Bi/(Bi + Sb) ratio derived from the formula equal to 0.46 (Topa et al., Reference Topa, Makovicky and Paar2013b).
Notable occurrences of mixed Bi–Sb members with N = 4 (Sb-rich gustavite and Bi-rich andorite) were in the past described on the basis of the EPMA data from Alyaskitovoye deposit, Yakutia, Russia (Mozgova et al., Reference Mozgova, Nenasheva, Borodaev, Sivcov, Ryabeva and Gamayanin1987, Reference Mozgova, Nenasheva, Jefimov, Borodaev, Cepin and Sivcov1988), from Julcani, Peru (Moëlo et al., Reference Moëlo, Makovicky and Karup-Møller1989) and Oruro, Bolivia (Keutsch and Brodtkorb, Reference Keutsch and Brodtkorb2008). The extraordinary extent of the Bi–Sb substitution in minerals of the lillianite homologous series was described recently from the Kutná Hora ore dictrict, Czech Republic (Pažout, Reference Pažout2017). Comparing the data from Kutná Hora with published data from the past (Figs 5 and 6) it is apparent that a mineral phase corresponding to staročeskéite has not been described before. Mineral phases from Alyaskitovoye deposit (Mozgova et al., Reference Mozgova, Nenasheva, Borodaev, Sivcov, Ryabeva and Gamayanin1987, Reference Mozgova, Nenasheva, Jefimov, Borodaev, Cepin and Sivcov1988) show L% between 94 and 114 and Bi/(Bi + Sb) = 0.41–0.70, and thus correspond mainly to terrywallaceite which is also supported by their diffraction data. Samples from Julcani (Moëlo et al., Reference Moëlo, Makovicky and Karup-Møller1989) with L% = 101–118 and Bi/(Bi + Sb) = 0.31–0.40 correspond to Bi-rich andorite-VI (analyses with L% ≈ 101) or oscakempffite (analyses with L% ≈ 118). The majority of analyses show L% between 105 and 115 and precise identification of these minerals would be possible on the basis of structural characterization by single-crystal diffraction. Samples from Oruro (Keutsch and Brodtkorb, Reference Keutsch and Brodtkorb2008) with L% = 94.4 and Bi/(Bi + Sb) = 0.23 correspond to Bi-rich andorite-IV.
Acknowledgements
The authors thank Dan Topa (University of Salzburg) for the WDS measurements. This work was financed by the Czech Science Foundation (GAČR project 15-18917S) to RP and by the Ministry of Culture of the Czech Republic (DKRVO 2017/01; National Museum 00023272) to JS. Thanks go to reviewer Andrew Christy for valuable hints on compositional limits and a dominant cation approach. This paper benefited from the comments of two unknown reviewers as well as from the editorial care of Peter Williams and Stuart Mills.