INTRODUCTION
Abalone (Haliotis species) are commercially important marine gastropod species worldwide. Whilst abalone fisheries declined from 19,720 to 7486 metric tonnes between 1970 and 2013, farming production exploded from 50 to 103,464 metric tonnes over the same period (Cook, Reference Cook2014). The decrease in abalone fisheries has been triggered by overexploitation, illegal catches and disease that lessened wild abalone stocks (Cook, Reference Cook2014). Haliotis tuberculata L. is the only species present in Europe (van Wormhoudt et al., Reference van Wormhoudt, Gaume, Le Bras, Roussel and Huchette2011). This species is relatively abundant on the Channel Islands and the Atlantic coast of France (Clavier & Richard, Reference Clavier and Richard1982; Gaty & Wilson, Reference Gaty and Wilson1986). In the wild, H. tuberculata is sedentary (Forster, Reference Forster1967) and found at the subtidal and low intertidal levels in shallow rocky habitats (Hayashi, Reference Hayashi1983). Their biology and ecology such as their reproductive cycle (Hayashi, Reference Hayashi1980a), population structure and growth (Hayashi, Reference Hayashi1980b; Clavier & Richard, Reference Clavier and Richard1986; Roussel et al., Reference Roussel, Huchette, Clavier and Chauvaud2011) have been well studied. Respiration rates of H. tuberculata from hatcheries (oxygen consumption rates in mg or ml O2 h−1) have previously been investigated with regard to factors such as body size (10–50 mm, Gaty & Wilson, Reference Gaty and Wilson1986; 10–90 g, Basuyaux et al., Reference Basuyaux, Mathieu and Richard2001) and simulated temperature (8, 16 and 24°C in Gaty & Wilson, Reference Gaty and Wilson1986, and 12, 15, 18, 21, 28°C in Basuyaux et al., Reference Basuyaux, Mathieu and Richard2001). Nonetheless, the natural variations (day–night and season cycles) in the metabolism of abalone from the natural habitat and the effect of body size upon respiratory rates in mature individuals (i.e. size over 50 mm, Hayashi, Reference Hayashi1980a), are still not well understood and represent important biological information for the recently developed industry in Europe (Roussel et al., Reference Roussel, Charreyron, Labarre, Van Wormhoudt and Huchette2013; Cook, Reference Cook2014; Lachambre et al., Reference Lachambre, Huchette, Day, Boudry, Rio-Cabello, Fustec and Roussel2017). Indeed, many abalone farms rely on coastal seawater and hence factors such as seawater temperature or dissolved oxygen, which vary according to season, day–night and tidal cycles, are likely to impact abalone physiology (Morash & Alter, Reference Morash and Alter2015). A better understanding of the natural temporal variations in physiological and metabolic rates which affect life-history traits such as growth and reproduction can thus contribute to improving fisheries management and aquaculture development (Young et al., Reference Young, Bornik, Marcotte, Charlie, Wagner, Hinch and Cooke2006; Cooke et al., Reference Cooke, Blumstein, Buchholz, Caro, Fernández-Juricic, Franklin, Metcalfe, O'Connor, Clair, Sutherland and Wikelski2014; Ragg & Watts, Reference Ragg and Watts2015; Gao et al., Reference Gao, Xian, Meijie, Changbin and Ying2016).
Several factors can modify abalone metabolism such as light quality (H. discus discus, Gao et al., Reference Gao, Xian, Meijie, Changbin and Ying2016), infection (H. rufescens and H. discus hannai, González et al., Reference González, Brokordt and Lohrmann2012; H. diversicolor, Lu et al., Reference Lu, Shi, Cai and Feng2017), farm stressors such as density, high temperature and ammonia concentration (see review from Morash & Alter, Reference Morash and Alter2015). To increase our understanding of the effects of environmental and farm stressors upon abalone physiology, it is first necessary to better comprehend the natural temporal variability in abalone metabolism and biology. In particular, seasons have a strong effect on chemical constituents in the muscle and viscera (H. diversicolor, Chiou et al., Reference Chiou, Lai and Shiau2001; H. laevigata and Haliotis rubra, Su et al., Reference Su, Antonas, Li and Nichols2006; farmed Jade Tiger hybrid abalone, Mateos et al., Reference Mateos, Lewandowski and Su2010), metabolic activity of digestive-gland cells (H. kamtschatkana, Carefoot et al., Reference Carefoot, Taylor and Donovan1998), textural proprieties (Haliotis discus, Hatae et al., Reference Hatae, Nakai, Shimada, Murakami, Takada, Shirojo and Watabe1995) and even immunity parameters of abalone (H. tuberculata, Travers et al., Reference Travers, Le Goic, Huchette, Koken and Paillard2008). These variations are partly related to reproductive cycle and energy transfer. However, to our knowledge, the season effect upon abalone (including H. tuberculata) metabolism has been poorly investigated to date.
Abalone metabolism might also vary according to diurnal rhythm. Indeed, abalone are nocturnal gastropods. They are active, move and feed mainly overnight in laboratory conditions, and in their natural habitat (H. discus hannai, Momma & Sato, Reference Momma and Sato1970; H. laevigata, H. roei, H. ruber, H. cyclobates, H. scalaris, Shepherd, Reference Shepherd1973). One can thus expect that abalone (and thus H. tuberculata) metabolism is greater at night-time in order to fulfil the metabolic needs related to individuals’ nocturnal behaviour.
Finally, others stressors related to commercial practices such as high water ammonia concentration, high density and air exposure (see review from Morash & Alter, Reference Morash and Alter2015) are likely to impact the physiological rates of abalone. Nitrogenous waste can be toxic and limit production in aquaculture (Morash & Alter, Reference Morash and Alter2016) and thus likely impact the physiology of abalone. Both fished abalone (H. iris, Wells & Baldwin, Reference Wells and Baldwin1995; Ragg & Watts, Reference Ragg and Watts2015, H. australis, Wells & Baldwin, Reference Wells and Baldwin1995) and farmed abalone (H. tuberculata, Lachambre et al., Reference Lachambre, Huchette, Day, Boudry, Rio-Cabello, Fustec and Roussel2017) can be exposed to air during handling procedures. Abalone have been demonstrated to remain metabolically active during air exposure at a much lower rate than during immersion (H. iris, Baldwin et al., Reference Baldwin, Wells, Low and Ryder1992). Haliotis tuberculata can be found at low shore levels on rocky shores during extreme spring tides or even at higher levels in large rockpools (Crofts, Reference Crofts1929). It is thus expected that H. tuberculata is physiologically adapted to aerial exposure. Nevertheless, periods of exposure to air longer than those experienced in natural conditions, and at different temperature, can occur especially during live shipments. A greater understanding of abalone (and hence H. tuberculata) metabolic capacities under aerial conditions is therefore important to improve the transport and handling of live animals.
This study proposed to study the natural temporal variations in physiological parameters of H. tuberculata. We quantified the seasonal, diurnal variations as well as the effect of individual size on four physiological parameters i.e. carbon aerial and aquatic respiration, calcification and excretion rates which are key parameters to understand abalone physiology (Morash & Alter, Reference Morash and Alter2015). In addition, aerial respiration was studied during a 6 h exposure and in different temperature conditions to study the effect of temperature on the aerial metabolic rate.
MATERIALS AND METHODS
Sampling
Abalone were collected by divers equipped with diving tanks in the Bay of Brest, in Brittany, NW of France. The sampling site was situated on the east part of the ‘Ile des Morts’ in the Bay of Roscanvel located on the SW part of the Bay of Brest (48°18,225′N 4°32,134′W, France). Abalone were found under rocks at a depth between 3 and 10 m depending on the tide. They were carefully removed by hand, placed into a net until the end of the diving session and subsequently placed within a closed transportable tank containing seawater.
Laboratory conditions
In the laboratory, abalone were placed into a flow-through system and kept unhandled for at least 24 h before starting any measurements to minimize handling stress. Filtered seawater was provided from the Bay of Brest by the nearby pumping station of the Institut français pour l'exploitation de la mer (Ifremer) at Sainte-Anne du Portzic. Seawater temperature was thus at the same temperature as in situ. A mix of algae from their natural habitat was added for food.
Methodology
SEASON COMPARISON IN ALL PHYSIOLOGICAL PARAMETERS
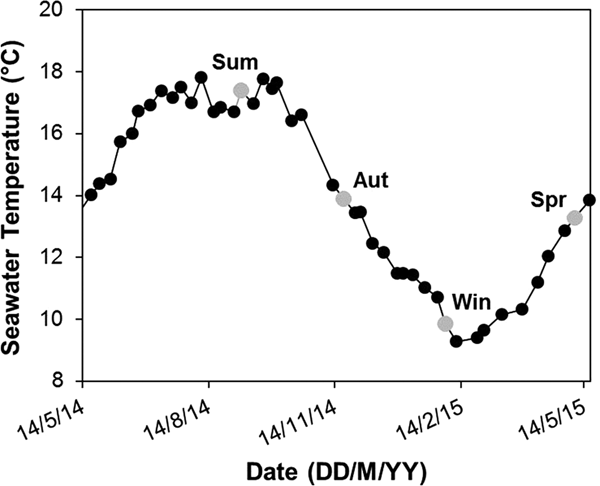
Aquatic and aerial respiration rate, calcification rate and ammonium excretion rate were calculated in adult abalone collected in the four seasons (Figure 1, N = 16 in summer, N = 15 in autumn, N = 17 in winter and in spring). These sampling periods were especially chosen to test the effect of seawater temperature upon the measured parameters. For instance, seawater temperature recorded in 2014–2015 was on average 17.1 ± 0.4°C (![]() $\bar x \pm {\rm SD}$, mean ± standard deviation) in summer (18.3°C in September experimental day), 15.1 ± 2.2°C in autumn (14.6°C in November experimental day), 10.5 ± 0.9°C in winter (11°C in January experimental day) and 13.2 ± 1.8°C in spring (14.1°C in May experimental day) (SOMLIT: Service d'Observation en Milieu Littoral, INSU-CNRS, St Anne du Portzic; Figure 1).
$\bar x \pm {\rm SD}$, mean ± standard deviation) in summer (18.3°C in September experimental day), 15.1 ± 2.2°C in autumn (14.6°C in November experimental day), 10.5 ± 0.9°C in winter (11°C in January experimental day) and 13.2 ± 1.8°C in spring (14.1°C in May experimental day) (SOMLIT: Service d'Observation en Milieu Littoral, INSU-CNRS, St Anne du Portzic; Figure 1).

Fig. 1. Seawater temperature variations recorded at the SOMLIT station (St Anne du Portzic site) over 12 months in 2014–2015. Grey circles indicate the field seawater temperature at each sampling period (Sum: summer; Aut: autumn; Win: winter; Spr: spring).
Abalone ranging from 70 to 80 mm in length were sexually mature i.e. size over 50 mm (Hayashi, Reference Hayashi1980a). This size range, corresponding to adult individuals, is the most abundant in the sampling area and was selected to facilitate sampling. Aquatic incubations were performed around midday in each season. Aerial respiration rates were measured upon emersion as soon as individuals were removed from their aquatic incubation bottle. As animals were free to feed prior to experiments, measured metabolic rate may include postprandial metabolic demand.
DIURNAL COMPARISON IN AQUATIC PHYSIOLOGICAL PARAMETERS
In order to compare metabolic rates which can vary through time at a diurnal scale (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015), aquatic incubations were repeated overnight in autumn (November 2014) on the same individuals to compare aquatic respiration, calcification and excretion rates during day and night periods.
INDIVIDUAL SIZE EFFECT UPON AQUATIC PHYSIOLOGICAL PARAMETERS
Calcification, aquatic respiration and ammonium excretion rates were calculated in abalone (N = 45) from a wide range of size (length: 35–106 mm) in September 2014. Smaller individuals (<50 mm, N = 15 abalone for a total of 107 abalone tested) were provided by the France Haliotis abalone farm (48°36′46N 4°33′30W, Plouguerneau, France). These abalone were the third generation bred in the farm and resulted from systematic mating between wild and farmed broodstock (either males or females were wild broodstock) for each generation.
AERIAL RESPIRATION DURING 6 H AIR EXPOSURE AND TEMPERATURE EFFECT
Abalone (N = 8) were gently detached from the laboratory tank and emersed for 6 h. Individual aerial respiration rates were measured each hour in spring (May 2015) at 14°C. Incubations were also conducted at 18 and 10°C on successive days. These air temperatures were chosen as they are representative of the temperature that can be experienced by abalone during either ambient temperature or cooled temperature transport. The spring season was chosen because it corresponds to the usual fishing period for H. tuberculata (fishing is forbidden in summer in Europe and practically difficult for the fishermen in winter due to poor weather conditions).
Physiological measurements
Aquatic incubations of 1.5–2 h (dark conditions) were conducted to calculate abalone aquatic respiration (dissolved inorganic carbon, DIC), calcification/decalcification (CaCO3) and ammonium excretion (NH4+ fluxes). Individuals were gently detached and placed into 2 l watertight plastic bottles filled with natural filtered seawater (1 individual/bottle) immersed into a 580 l tank filled with running seawater to maintain a constant temperature during incubations. Bottles were gently rotated once in the middle of incubations to ensure homogenization.
AQUATIC RESPIRATION RATE
Aquatic respiration within each bottle (R, μmol DIC h−1) was calculated as the variation in the dissolved inorganic carbon (DIC) concentration between the start and the end of the incubation using the following equation: R = (ΔDIC × ν)/(Δt × 103) − G, where ΔDIC is the variation in DIC during incubation (μmol DIC l−1), Δt is the incubation time (h), ν is the bottle volume (l) and G is the calcification rate (μmol CaCO3 h−1). To determine the dissolved inorganic carbon (DIC) concentration (μmol l−1), seawater samples were taken from incubation bottles at the start and at the end of each incubation in order to measure total alkalinity (TA), pH and seawater temperature.
TA samples were obtained by filtering water through 0.7 µm Whatman GF/F filters. Samples were then stored with 250 ml plastic bottles in the dark. Laboratory analysis consisted in estimating TA (μmol l−1) within 20 ml subsamples (average of 6 subsamples per bottle) by automatic potentiometric titration (Radiometer, Titrilab TIM 865). Subsamples were titrated by adding small increments of 0.01 mol l−1 HCL with 0.7 mol kg−1 NaCl to approximate the ionic strength of seawater (Dickson & Goyet, Reference Dickson and Goyet1994) until about pH 3. TA was determined using the modified Gran method by determining the second endpoint of the titration curve.
The pH (total scale) was measured using a pH probe (Radiometer pHC2401) which was standardized with buffer solutions in synthetic seawater of 35‰ (Tris-HCL: 2-amino-2 hydroxymethyl-1,3-propanediol hydrochloride; 2-aminopyridine/HCL). pH values were measured immediately after opening bottles in order to prevent effects of CO2 exchange with the air.
DIC concentration was calculated from pH, TA, temperature, salinity, phosphate and silicate concentrations using the CO2SYS program (Pierrot et al., Reference Pierrot, Lewis and Wallace2006). The natural salinity, phosphate and silicate concentrations were obtained using data collected in the Bay of Brest by the French coastal observation service (SOMLIT: Service d'Observation en Milieu Littoral, INSU-CNRS, St Anne du Portzic). Dissociation constants for carbonic acid K1 and K2 were taken from Roy et al. (Reference Roy, Roy, Vogel, Porter-Moore, Pearson, Good, Millero and Campbell1993).
CALCIFICATION RATE
Calcification was determined using the alkalinity anomaly technique (Smith & Key, Reference Smith and Key1975) in each incubated bottle. Calcification rate (G in μmol CaCO3 h−1) was calculated as follows:
ΔTA, Δt and ν corresponds to the variation in TA during incubation (μmol l−1), incubation time (h) and bottle volume (L), respectively. This equation is based on the evidence that the precipitation of one mole of CaCO3 implies the consumption of 2 moles of ![]() ${\rm HCO}_3^ - $ which decreases TA by 2 equivalents (Frankignoulle et al., Reference Frankignoulle, Canon and Gattuso1994). G > 0 is indicative of calcification, whilst G < 0 indicates carbonate dissolution.
${\rm HCO}_3^ - $ which decreases TA by 2 equivalents (Frankignoulle et al., Reference Frankignoulle, Canon and Gattuso1994). G > 0 is indicative of calcification, whilst G < 0 indicates carbonate dissolution.
AMMONIUM EXCRETION RATE
Ammonia is assumed to be the primary end product of catabolism of amino acids in molluscs (Bayne & Newell, Reference Bayne and Newell1983). Besides providing us with information regarding H. tuberculata ammonium excretion, NH4+ concentrations were measured to correct TA since ammonium excretion is one of the processes that may affect TA in our experiments (Gazeau et al., Reference Gazeau, Alliouane, Bock, Bramanti, López Correa, Gentile, Hirse, Pörtner and Ziveri2014, Reference Gazeau, Urbini, Cox, Alliouane and Gattuso2015). Ammonium excretion was obtained by collecting seawater samples at the beginning and end of each incubation in 10 ml vials and kept at −20°C until analysis. The phenol-hypochlorite method (Koroleff, Reference Koroleff1969; Sororzano, Reference Sororzano1969) was used to determine NH4+ concentration (μmol l−1). After adding reagents, the colouration of each sample was measured at 630 nm using a spectrophotometer. Nitrification process was not assessed here since it can be considered to be negligible under short incubations (Tagliarolo et al., Reference Tagliarolo, Grall, Chauvaud and Clavier2013b; Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015).
AERIAL RESPIRATION MEASUREMENTS
Each abalone was detached and placed into a 0.1 l airtight dark chamber connected to a closed circulation system with an integrated infrared CO2 analyser (Li-Cor, Li-820) and a desiccation column filled with anhydrous calcium sulphate (Drierite, Xenia, USA) just after emersion. An adjustable pump maintained air flow at 0.8–0.9 l min−1. Aerial respiration within each chamber was estimated by the linear slope of CO2 concentration increase measured every 5 s over 3 min using the Li-820 software (Clavier et al., Reference Clavier, Castets, Bastian, Hily, Boucher and Chauvaud2009). Fluxes of CO2 (μmol CO2 h−1) were corrected for the net volume of the system and incubation time. The aerial temperature was maintained at that of the seawater during aquatic incubations.
Data analysis
SHELL COMMUNITY CONTRIBUTION AND INDIVIDUAL BIOMASS
Calcification, aquatic and aerial respiration measurements were repeated on empty shells of the same individuals. Shells were washed, cleared of any flesh and carefully dabbed inside with a paper towel damped with 70% ethanol. The measured parameters for the shell community were then subtracted to the total calcification and respiration rates. The parameter values presented in this study are the shell community corrected values. The contribution of the dissolution of the inner shell part was considered as negligible (maximum empty shell calcification rate of 0.17 µmol CaCO3 h−1 g−1 in September, and a minimal flux of −0.03 µmol CaCO3 h−1 g−1 in January).
All the studied parameters were further normalized to biomass as described below. Individual biomass was estimated as ash-free dry weight (AFDW) through loss on ignition (4 h combustion at 450°C of 60°C dried individuals until constant weight) and expressed in g AFDW−1.
STATISTICAL ANALYSIS
The relationship between the biomass (AFDW) and the physiological aquatic parameters were assessed using the allometric equation Y = a × W b (Marsden et al., Reference Marsden, Shumway and Padilla2012) where a corresponds to the physiological rate per gram AFDW, and b shows the rate at which the physiological parameter evolves with the biomass.
Normality and homogeneity of variances of the data distributions of calcification, aquatic and aerial respiration, and ammonium excretion were investigated using the Shapiro–Wilk and Bartlett tests, respectively. An ANOVA was performed to study the seasonal variability. When normal distributions of the data were not verified, the Kruskal–Wallis test was used. When the assumption of homogeneity of variance was not verified, a Welch's ANOVA test was performed as recommended by Day & Quinn (Reference Day and Quinn1989). Subsequent non-parametric post hoc analyses were conducted with the Tukey and Kramer (Nemenyi) test (Pohlert, Reference Pohlert2014) to distinguish differences between seasons. The Wilcoxon Signed-Ranks test was run to compare aquatic and aerial respiration at each season.
The relationships between the aquatic respiration, calcification and excretion rates, and the seawater temperature experienced at each season were investigated using the Arrhenius equation after logarithmic transformation as a function of T −1 as follows:
where Flux is respiration (ΔDIC aquatic or ΔCO2 in the air, μmol g−1 h−1), a is a normalization constant, EA is the activation energy (J mole−1), K is Boltzmann's constant (8.31 J K−1 mol−1), and T is the absolute temperature (°K).
Comparison of calcification, aquatic respiration and ammonium excretion between day- and night-time were conducted with the Wilcoxon Signed-Rank test.
As the data were normally distributed (P > 0.05), hourly variations in aerial respiration over 6 h emersion at different ambient temperatures (10, 14 and 18°C) were assessed using repeated measures ANOVA with a Greenhouse–Geisser Correction when the data violated the assumption of sphericity (Mauchly's test of sphericity). Post hoc tests incorporating the Bonferroni correction were done to distinguish differences in aerial respiration at different times of emersion. Also, ANOVA tests were conducted to compare abalone respiration rates between different temperatures (10, 14 and 18°C) at each hour of emersion. When significant, a subsequent post hoc test (Tukey's HSD test) was run to distinguish which group(s) significantly differed.
RESULTS
Seasonal variations
A seasonal effect was observed on aquatic respiration (Welch test, F 3,32 = 19.25, P < 0.001) and aerial respiration (ANOVA test, F 3,60 = 3.63, P < 0.05). Aquatic respiration was significantly lower in autumn (8.41 ± 1.34 µmol DIC g AFDW−1 h−1) and winter (6.34 ± 1.93 µmol DIC g AFDW−1 h−1) than in summer (13.17 ± 3.66 µmol DIC g AFDW−1 h−1, Figure 2). Aerial respiration was significantly lower in winter (1.49 ± 0.66 µmol CO2 g AFDW−1 h−1) than in summer (2.21 ± 0.83 µmol CO2 g AFDW−1 h−1, Figure 2).

Fig. 2. Haliotis tuberculata aquatic and aerial respiration, calcification and ammonium excretion over four seasons with regard to seawater temperature (white dots: summer; dark grey dots: autumn; black dots: winter; light grey dots: spring.) in 2014–2015. Values are means and error bars are standard deviations. N = 16 in summer, N = 15 in autumn, N = 17 in winter and in spring. Significant differences (P < 0.05) between seasons from Tukey and Kramer post hoc tests are indicated by letters.
Aerial respiration was significantly lower than aquatic respiration over the four seasons with abalone respiring between 5 and 6 times more underwater than during emersion (Table 1; Figure 2).
Table 1. Comparison of aquatic (UW) and aerial (A) respiration in Haliotis tuberculata over four seasons (Wilcoxon Signed Ranks Test).

Calcification rates of abalone (net CaCO3 fluxes) had a mean annual value of 0.55 ± 0.53 µmol CaCO3 g AFDW−1 h−1. A seasonal effect was observed (Welch test, F 3,27.5 = 26.78, P < 0.001) with CaCO3 fluxes significantly lower in winter (Figure 2) but with still 53% of individuals exhibiting shell calcification (G > 0). CaCO3 fluxes were positive in 88% of individuals in spring, and 100% of individuals in summer and autumn.
Ammonium fluxes were on average 0.29 ± 0.26 µmol NH4+ AFDW−1 h−1 over the year. A seasonal effect was found (Welch test, F 3,27.4 = 5.88, P < 0.01) with lower values measured in spring (0.17 ± 0.20 µmol NH4+ AFDW−1 h−1) and winter (0.19 ± 0.07 µmol NH4+ AFDW−1 h−1) than in summer (0.36 ± 0.22 µmol NH4+ AFDW−1 h−1) and/or autumn (0.47 ± 0.36 µmol NH4+ AFDW−1 h−1, Figure 2).
A good relationship was observed between aquatic respiration and calcification, and temperature (respectively, R 2 = 0.88 and R 2 = 0.70, Table 2). The relationship between aerial respiration and temperature was rather high but lower than that of aquatic respiration (R 2 = 0.68, Table 2). A poor relationship was observed between ammonium excretion and temperature (R 2 = 0.35, Table 2).
Table 2. Relationships between metabolic parameters and temperature described by Arrhenius equation lnFlux = lna − (E A/K) × (1/T). Standard errors are in parentheses.

Day-night variations (in autumn only)
Day- and night-time aquatic respiration rates were 8.41 ± 1.34 and 9.10 ± 3.74 µmol DIC g AFDW−1 h−1, respectively, and did not differ significantly (Wilcoxon signed rank test, P = 0.64).
CaCO3 fluxes were significantly higher (Wilcoxon signed rank test, P < 0.01) during the night than during the day (1.19 ± 0.72, and 0.71 ± 0.47 µmol CaCO3 g AFDW−1 h−1, respectively).
Likewise, NH4+ fluxes were significantly greater (Wilcoxon signed rank test, P < 0.05) during the night than during the day (0.50 ± 0.17, and 0.47 ± 0.36 µmol NH4+ AFDW−1 h−1, respectively).
Individual size effect upon aquatic physiological parameters
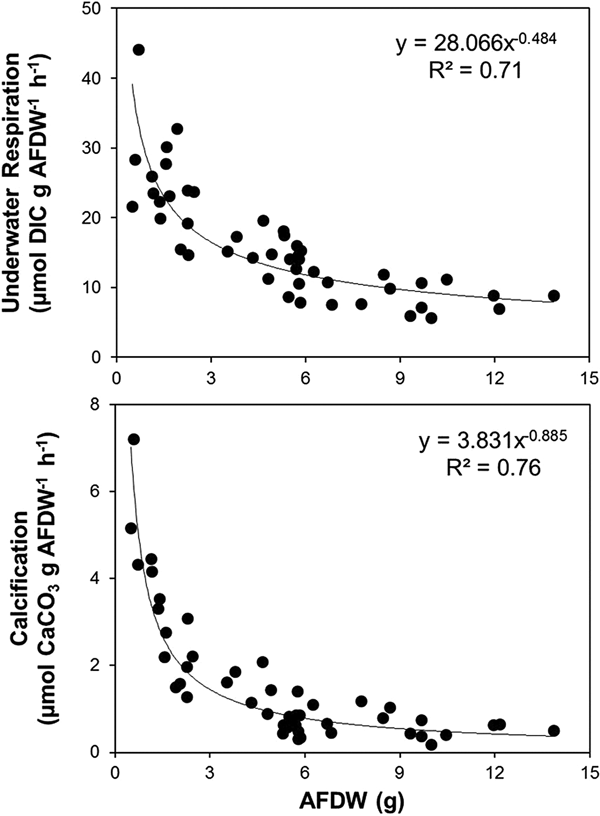
Both aquatic respiration and calcification rates per unit biomass decreased as the individual biomass increased (aquatic respiration: y = 28.066 × x −0.484, R 2 = 0.71; calcification: y = 3.831 × x −0.885, R 2 = 0.76; Figure 3). No distinguishable pattern was found between the individual biomass and ammonium excretion rate (R 2 = 0.004).

Fig. 3. Allometric relation (aAFDWb) between individual abalone biomass (AFDW) and aquatic respiration (top graph), and calcification (bottom graph) in summer 2014.
Hourly variations in aerial respiration rates
At 10°C, aerial respiration rates were lower at 1 h of emersion than at 3, 5 and 6 h of aerial exposure (Repeated measure ANOVA test, F 5,35 = 13.98, P < 0.001, Figure 4). At 14°C, abalone significantly respired less after 1 h of emersion than at 4 and 6 h of emersion (repeated measures ANOVA test, F 3.1,21.6 = 5.43, P < 0.01, Figure 4). No difference in respiration rate was observed over the 6 h emersion at 18°C (F 2.1,6.7 = 1.13, Figure 4).

Fig. 4. Haliotis tuberculata aerial respiration during emersion at 10°C, 14°C and 18°C. Values are means and error bars are standard deviations (N = 8 at 10°C and 14°C; 6 < N < 8 at 18°C).
Significant differences in aerial respiration rates between temperatures were obtained at 1 h (ANOVA test, F 2,21 = 5.46, P < 0.05), 2 h (ANOVA test, F 2,21 = 5.25, P < 0.05), 4 h (ANOVA test, F 2,20 = 3.94, P < 0.05) and 6 h (ANOVA test, F 2,20 = 5.54, P < 0.05) of emersion : abalone significantly displayed lower respiration rates at 10°C than at 18°C after 1 h of emersion (Tukey's HSD test, P < 0.01, Figure 4). At 2 and 4 h of emersion, abalone displayed lower rates at 10° than 14°C (Tukey's HSD test, P < 0.05, Figure 4). At 6 h of emersion, abalone displayed lower rates at 18° than 14°C (Tukey's HSD test, P < 0.05, Figure 4).
DISCUSSION
Effect of Haliotis tuberculata biomass upon its physiological rate
Body size has been identified as a good proxy for metabolic rates of animals (Newell, Reference Newell1973). Abalone respiration rates increase as weight increases (Gaty & Wilson, Reference Gaty and Wilson1986; Basuyaux et al., Reference Basuyaux, Mathieu and Richard2001; Cunningham et al., Reference Cunningham, Smith and Lamare2016). An allometric relationship between aquatic respiration and calcification rates per unit biomass was observed with negative b values much less than unity which indicates that respiration and calcification do not decrease in direct proportion to biomass (Marsden et al., Reference Marsden, Shumway and Padilla2012). Our results, i.e. higher aquatic respiration and calcification rates per unit biomass in smaller H. tuberculata individuals, corroborate the evidence that metabolic demand and growth decrease with age (at least during the first years of growth) like in other molluscs such as Tectus niloticus (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015), Crepidula fornicata (Martin et al., Reference Martin, Thouzeau, Chauvaud, Jean, Guérin and Clavier2006), Mytilus edulis and M. galloprovincialis (Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud, Koken and Grall2012). Previous findings in H. tuberculata have also found that growth rate, underpinned by metabolism and energy budgets, decreases progressively as its size increases (Hayashi, Reference Hayashi1980b; Clavier & Richard, Reference Clavier and Richard1986; Roussel et al., Reference Roussel, Huchette, Clavier and Chauvaud2011). However, because a number of the small abalone were from a farmed origin, resulting from systematic mating between wild and farmed broodstock, it cannot be excluded that acclimation to the farm may have slightly modified physiological rates compared with the wild abalone. In addition, because respiration was tested in still water conditions, both respiration and calcification values may be greater in animals in moving water in farm conditions or in the wild (Taylor & Ragg, Reference Taylor and Ragg2005).
No relationship was observed between H. tuberculata ammonium excretion rate and biomass as in the trochus T. niloticus (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015). This may indicate a similar excretion rate per unit biomass for adults and juveniles. The rate of excretion per unit of biomass diminishes in mussels as individuals grow larger (Vaughn & Hakenkamp, Reference Vaughn and Hakenkamp2001; Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud, Koken and Grall2012). A positive correlation however has been reported between body size and ammonium excretion rates per individual in Haliotis discus discus, H. gigantea and H. madaka (Ahmed et al., Reference Ahmed, Segawa, Yokota and Watanabe2008).
Seasonal variations in H. tuberculata physiological parameters
Haliotis tuberculata aquatic respiration showed a two-fold seasonal difference with minimal rates in winter and maximal rates in summer. Seasonal variations in aquatic respiration have been observed in many other benthic molluscs. From 1.6 up to five-fold differences in aquatic respiration rates between winter and summer have been observed in Patella vulgata (Tagliarolo et al., Reference Tagliarolo, Grall, Chauvaud and Clavier2013b), C. fornicata community (Martin et al., Reference Martin, Thouzeau, Richard, Chauvaud, Jean and Clavier2007), Crassostrea gigas (Lejart et al., Reference Lejart, Clavier, Chauvaud and Hily2012), the trochus T. niloticus (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015) and the abalone H. tuberculata (Gaty & Wilson, Reference Gaty and Wilson1986).
In our experiment, aerial respiration was minimal in winter (1.49 ± 0.66 µmol CO2 g AFDW−1 h−1) and maximal in summer (2.21 ± 0.83 µmol CO2 g AFDW−1 h−1) with an average five-fold higher for aquatic respiration compared with aerial respiration. Seasonal aerial respiration has not been extensively studied in molluscs. However, this experiment showed that season has similar effects on underwater and aerial respiration. This can have direct consequences on live transport procedures. As respiration rates and energy demand are higher in summer, it might be more appropriate to transport live abalone during winter when energy needs are lower and when the recovery time post-air exposure may be less.
CaCO3 accretion showed that 53% of H. tuberculata individuals did not stop their growth over winter in Britanny temperature conditions. The seasonal growth pattern reported in this study is consistent with previous observations in H. tuberculata: abalone growth rate was found to drop in winter whilst 70% of annual growth occurred between May and November (Clavier & Richard, Reference Clavier and Richard1986). Similarly, more recent studies using stable oxygen isotopes techniques have found that H. tuberculata does not stop its growth in winter (Roussel et al., Reference Roussel, Huchette, Clavier and Chauvaud2011; Jolivet et al., Reference Jolivet, Chauvaud, Huchette, Legoff, Thébault, Nasreddine, Schöne and Clavier2015). CaCO3 accretion in H. tuberculata was greater in warmer months when the temperature and the development of the gonad maturation are near its maximum (Hayashi, Reference Hayashi1980a) and decreased over winter leading to a net calcification rate over the year (0.55 ± 0.53 µmol CaCO3 g AFDW−1 h−1). In contrast, the abalone Haliotis discus hannai showed retardation in growth during gonad maturation and spawning (Sakai, Reference Sakai1960). Seasonal variation in CaCO3 accretion is common in molluscs; higher CaCO3 fluxes have been found in warmer months (Martin et al., Reference Martin, Thouzeau, Chauvaud, Jean, Guérin and Clavier2006; Lejart et al., Reference Lejart, Clavier, Chauvaud and Hily2012) whilst CaCO3 accretion decreases or stops in winter (Lejart et al., Reference Lejart, Clavier, Chauvaud and Hily2012; Tagliarolo et al., Reference Tagliarolo, Grall, Chauvaud and Clavier2013b).
Finally, ammonium excretion rates in H. tuberculata also varied seasonally; lower average values were measured in spring and winter (0.17–0.19 µmol NH4+ AFDW−1 h−1, respectively) whilst maximal average values were found in summer and autumn (0.37–0.47 µmol NH4+ AFDW−1 h−1, respectively). These results are consistent with the variations in excretion rates reported for the limpet P. vulgata; low shore individuals had fluxes ranging from 0.5 to 0.7 µmol NH4+ AFDW−1 h−1 in winter and in summer, respectively (Tagliarolo et al., Reference Tagliarolo, Grall, Chauvaud and Clavier2013b). In contrast, other mollusc species showed maximum excretion rates in spring (Martin et al., Reference Martin, Thouzeau, Chauvaud, Jean, Guérin and Clavier2006) and minimum rates in winter (Bayne & Scullard, Reference Bayne and Scullard1977; Martin et al., Reference Martin, Thouzeau, Chauvaud, Jean, Guérin and Clavier2006).
This seasonal pattern in H. tuberculata physiological rates can be explained by both environmental stressors (e.g. temperature), and biogenic factors (e.g. reproductive needs, resource abundance, distribution and availability).
Seasonal variation in temperature was most likely the primary driver of the observed seasonal variations in carbon fluxes in this study. This hypothesis is consistent with the strong relationships observed between temperature and the studied physiological rates (except ammonium rates); lower rates occurred in winter when the temperature was 11°C and maximal rates were recorded in summer when the temperature reached 18.3°C. Temperature is known to impact all physiological rates in ectotherms (Somero, Reference Somero2002) and it has been shown to influence the aquatic respiration of many mollusc species (Lejart et al., Reference Lejart, Clavier, Chauvaud and Hily2012; Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud and Grall2013a, Reference Tagliarolo, Grall, Chauvaud and Clavierb). As temperature increases, the oxygen demand increases as more energy is required to fulfil physiological requirements. This has already been reported in H. tuberculata, which exhibited lower oxygen consumption rates at 8°C compared with 16°C or 18°C (Gaty & Wilson, Reference Gaty and Wilson1986).
Biotic factors such as food abundance can contribute to respiration rates. Abalone are herbivorous (Stephenson, Reference Stephenson1924) and mostly sedentary (Clavier & Richard, Reference Clavier and Richard1982). Abalone increase their feeding activity in summer (Allen et al., Reference Allen, Marsden, Ragg and Gieseg2006) when their resources i.e. drifting seaweed (Clavier & Chardy, Reference Clavier and Chardy1989) are more abundant following summer macroalgal blooms on the Atlantic coast (Dion & Le Bozec, Reference Dion, Le Bozec, Schramm and Nienhuis1996). Fresh algae were provided ad libitum before the experiment in order to reduce variability due to starvation since at the same temperature, oxygen consumption rate is 30% lower in 2-weeks starved abalone compared with fed H. tuberculata (Gaty & Wilson, Reference Gaty and Wilson1986). Because H. tuberculata are more inclined to forage in summer (Roussel, pers. comm.), higher aquatic and aerial respiration rates in summer may be related to higher metabolic demands as more energy is required for digestion, absorption and assimilation processes (Widdows & Shick, Reference Widdows and Shick1985). Higher respiration rates may also be related to the production of large gonads. Indeed, the spawning period has been associated with increased metabolic demand as energy is required to produce gametes (Hayashi, Reference Hayashi1983). Finally, the low growth and the decrease in excretion rates may be associated with less abundant resources over winter (Allen et al., Reference Allen, Marsden, Ragg and Gieseg2006).
Day-night variations in H. tuberculata physiological parameters
Haliotis tuberculata is characterized like other abalone species (Momma & Sato, Reference Momma and Sato1970; Barkai & Griffiths, Reference Barkai and Griffiths1987) by nocturnal behaviour with movements that are initiated an hour after sunset (Werner et al., Reference Werner, Flothmann and Burnell1995). However, unlike other gastropods such as the abalone H. discus hannai (Uki & Kikuchi, Reference Uki and Kikuchi1975), and the trochus T. niloticus (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015) which display higher metabolic rates whilst being active overnight, no circadian rhythm in H. tuberculata aquatic respiration was observed. Movement of the animal could not be controlled in most of the experiment design. The apparatus used in this experiment allowed movement of the abalone inside the bottle, and no movement restraint was used. However, no abalone movements were observed during visual observation of the bottle, even if punctual movement cannot be completely excluded. This result probably indicates that aquatic respiration is similar during night and day periods. However, we cannot exclude that aquatic respiration would be on average higher at night when abalone is crawling to get food.
Intrinsic higher metabolic rates overnight may also explain the counterintuitive greater ammonium fluxes at night than during the day, even though the difference was small (0.03 µmol NH4+ AFDW−1 h−1). Indeed since H. tuberculata has a nocturnal feeding habit like the abalone H. midae, ammonium excretion was expected to be higher during the diurnal elimination phase (Barkai & Griffiths, Reference Barkai and Griffiths1987). Further research is required to determine the factors driving day-night patterns of ammonium fluxes in H. tuberculata.
Haliotis tuberculata calcifies 1.7-fold more at night than during the day. Diurnal growth ridge formation in other gastropods like the limpet Acmaea antillarum have been related to the light-dark cycle (Kenny, Reference Kenny1977). Since incubations at both day- and night-times were conducted in dark conditions, the calcification process in H. tuberculata may be intrinsic and be related to its nocturnal behaviour and related higher metabolic activity in the same way as the trochus T. niloticus (Lorrain et al., Reference Lorrain, Clavier, Thébault, Tremblay-Boyer, Houlbrèque, Amice, Le Goff and Chauvaud2015). More research is required to examine the day/night pattern in calcification rates in shelled gastropods, which has to date been poorly documented.
Haliotis tuberculata aerial respiration during 6 h exposure
Haliotis tuberculata aerial carbon respiration represents ~20% of aquatic carbon respiration, which is in the range of what has been observed in intertidal mussels (19–23%, Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud, Koken and Grall2012) but lower than other intertidal gastropod species (40–230%) (Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud and Grall2013a). The low emersion/immersion ratio, i.e. 0.2, may be an energy-saving strategy for emerged H. tuberculata individuals which potentially implies a switch to anaerobic processes (Widdows & Shick, Reference Widdows and Shick1985). Another explanation of this lower aerial respiration would be the collapse of the gill in the air, so that the respiration would be limited. This indicates that H. tuberculata can physiologically adapt to emersion like other intertidal gastropods. However, regardless of how carefully abalone is handled, it cannot be excluded that an acute stress response due to detachment modified, at least partly, aerial respiration at the beginning of the measurement period. Further research is required to better understand aerobic and anaerobic pathways during emersion in H. tuberculata.
Some intertidal gastropods have been shown to either decrease aerial respiration over emersion or to maintain stable rates (Tagliarolo et al., Reference Tagliarolo, Clavier, Chauvaud and Grall2013a, Reference Tagliarolo, Grall, Chauvaud and Clavierb). Here, abalone increased their aerial respiration over time at 10°C and 14°C. At 18°C, aerial respiration was already high in the first hour of aerial exposure and no further increase was observed over time. Similarly, mussels (M. edulis) showed an increase in aerial energy expenditure when exposed to the air for longer than under natural conditions (Widdows & Shick, Reference Widdows and Shick1985). Abalone are mostly found in subtidal areas or at the low shore level of intertidal rocky shores, therefore intertidal abalone are emerged for short periods of time in nature i.e. less than 3 h, rather than the imposed 6 h of emersion of this study. Respiration rates in H. tuberculata started to increase after 3 h of emersion at the anticipated time of re-immersion. This result showed that H. tuberculata can handle emersion during 3 h with probably very limited energetic cost. However, after 3 h of emersion, abalone increased their respiration rate (at 10°C and 14°C) which suggests a higher metabolic cost of longer emersion period at these temperatures (up to 6 h in this study) and potential physiological impacts on the recovery time (and higher post-emersion mortality). Respiration rates at 10°C were lower than at greater temperatures after 1, 2 and 4 h of emersion. This suggests that transporting live ormers at low temperature (10°C) using ice packs for a short time period may minimize the metabolic cost of emersion and hence may improve survival rates during the post-emersion recovery period (Buen-Ursua & Ludevese, Reference Buen-Ursua and Ludevese2011).
CONCLUSION
This study has underscored that (i) aquatic carbon respiration and calcification rates per unit biomass are higher in smaller ormers; (ii) rates of physiological parameters are lower in winter than in summer, in particular, ormers do not stop calcifying in winter; (iii) calcification and excretion rates are higher at night than during the day; (iv) aerial carbon respiration corresponds to 20% of aquatic respiration which indicates that H. tuberculata is adapted to periods of emersion. The clear patterns of variation in H. tuberculata physiological rates reported in the present study may constitute important information to understand the relationship between growth, survival and metabolism during farming procedures.
ACKNOWLEDGEMENTS
We thank SOMLIT group for providing temperature and nutrient data, and for gas-analyser calibration (http://somlit.epoc.u-bordeaux1.fr/fr/). We thank the anonymous reviewers for their comments, suggestions and inputs which greatly improved an earlier version of this work.
FINANCIAL SUPPORT
This work was supported by the ‘Laboratoire d'Excellence’ LabexMER (ANR-10-LABX-19) and co-funded by a grant from the French government under the program ‘Investissements d'Avenir’, and by a grant from the Regional Council of Brittany (SAD programme). C. Chapperon was also supported by a grant from the Departmental council of Finistère. This project received the support of the PIA-ANR IDEALG BTBR-10-04.