Published online by Cambridge University Press: 03 March 2004
We characterized 14 trypanosome isolates from sylvatic mammals (9 from primates, 1 from sloth, 2 from anteaters and 2 from opossum) plus 2 human isolates of Brazilian Amazon. These isolates were proven to be Trypanosoma rangeli by detection of metacyclic trypomastigotes in the salivary glands of triatomines and by a specific PCR assay. Polymorphism determined by randomly amplified polymorphic DNA (RAPD) revealed that most (12) of the Brazilian T. rangeli isolates from the Amazon differed from those of other geographical regions, thus constituting a new group of T. rangeli. Four Brazilian isolates clustered together with a previously described group (A) that was described as being composed of being isolates from Colombia and Venezuela. Isolates from Panama and El Salvador form another group. The isolate from Southern Brazil did not cluster to any of the above-mentioned groups. This is the first study that assesses the genetic relationship of a large number of isolates from wild mammals, especially from non-human primates. A randomly-amplified DNA fragment (Tra625) exclusive to T. rangeli was used to develop a PCR assay able to detect all T. rangeli groups.
Trypanosoma rangeli lacks mammalian host specificity being found in humans, domestic and wild mammals. It is widely distributed in Central, Northern and Western South America, where it is sympatric with Trypanosoma cruzi, sharing vertebrate and invertebrate (triatomine bugs) hosts. This species has several unique characteristics regarding development on both vertebrate and invertebrate hosts, being considered non-pathogenic for mammalian hosts whereas damaging to insect vectors. Different from T. cruzi, intracellular stages (amastigote) were not detected in vertebrate hosts of T. rangeli (D'Alessandro, 1976; D'Alessandro & Saravia, 1992, 1999; Guhl & Vallejo, 2003).
Although transmission of T. rangeli is mainly by the inoculation of triatomine saliva containing metacyclic trypomastigotes during bug feeding, which is typical of salivarian trypanosomes, it can also be transmitted via vector faeces, a feature of stercorarian trypanosomes (D'Alessandro & Saraiva, 1999). This species was classified within the subgenus Herpetosoma of the Stercoraria Section (Hoare, 1972). However, due to this unusual transmission, its relationship with T. brucei, a typical salivarian species, has been questioned (Ãnez, 1982). Phylogenetic studies based on small subunit of ribosomal RNA gene (SSU rRNA) sequences tightly positioned T. rangeli within Stercoraria, close to T. cruzi and distant from all the salivarian species (Stevens et al. 1999, 2001). Populations of T. rangeli exhibited a significant degree of genetic polymorphism that permitted their partition in two main groups, apparently related to geographical origin, based on kDNA (Vallejo et al. 2002); isoenzymes and RAPD patterns (Steindel et al. 1994) and mini-exon gene sequences (Grisard, Campbell & Romanha, 1999).
T. rangeli has been sporadically reported in Brazil, especially in wild mammals and triatomines of the Amazon region (Deane et al. 1972; Shaw, 1985; Miles et al. 1983; Ziccardi & Oliveira, 1998; Ziccardi et al. 2000). Few confirmed human T. rangeli cases have also been reported in this region (Coura et al. 1996). In Brazil, outside Amazon, confirmed T. rangeli was related in triatomines, wild rodents and opossums in Southern and Southeast Regions (Steindel et al. 1991; Ramirez et al. 1998, 2002).
While the taxonomic position of isolates from human and triatomines is well defined, the status of T. rangeli-like trypanosomes from wild mammals has not been investigated. T. rangeli has been reported in non-human primates, edentates, marsupials, carnivores and rodents (Hoare, 1972; D'Alessandro, 1976; Miles et al. 1983; D'Alessandro & Saravia, 1992, 1999; Marinkelle, 1976; Dereure et al. 2001). There is a high prevalence of trypanosomes in neotropical monkeys (Marinkelle, 1976), with several reports from Brazilian Amazon (Deane et al. 1972; Ziccardi & Oliveira, 1998; Ziccardi et al. 2000), Colombia (Marinkelle, 1976; D'Alessandro & Saravia, 1992); Panama (Sousa, Rossan & Baerg, 1974; Sousa & Dawson, 1976) and French Guyana (de Thoisy et al. 2001).
Sylvatic mammals described as natural hosts of T. rangeli can be infected by other Herpetosoma spp., by T. cruzi, and by species of subgenera Megatrypanum. On the basis of their resemblance to T. rangeli in blood and/or in culture, mammalian host restriction and different behaviour in distinct triatomine species, some trypanosomes from non-human primates, sloths, anteaters and opossums have been identified as T. rangeli-like or allied species: T. cebus; T. diasi; T. saimiri; T. myrmecophage; T. preguici; T. mesnilbrimonti; T. mycetae; T. leewennhoeki, etc. (Hoare, 1972; D'Alessandro, 1976; Marinkelle, 1976; Shaw, 1985; D'Alessandro & Saravia, 1992). However, there are controversies about those species and their taxonomic positions remain to be revised. We do not known if there are trypanosomes genetically closer to T. rangeli which deserve specific status within the subgenus Herpetosoma or if the so-called T. rangeli-like trypanosomes are indeed different organisms worthy of specific names.
In this study, we isolated T. rangeli from sylvatic mammals of Brazilian Amazon, especially from non-human primates, and characterized them by comparing morphology and behaviour in triatomine bugs and mice. These isolates were also analysed by the RAPD method with the following purposes: (i) to evaluate the polymorphism among isolates from wild mammals and from man of Brazilian Amazon and their relatedness with those from other geographical origins; (ii) to investigate if the populations from sylvatic animals differed according to their host-species and/or geographical origin; (iii) to define the relationships of T. rangeli with T. rangeli-like and allied trypanosomes; (iv) to identify taxonomic markers for T. rangeli.
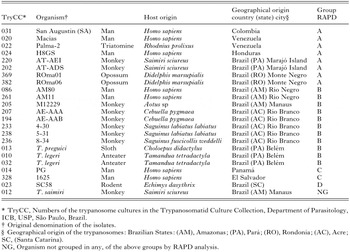
Trypanosomes were obtained by culture of the peripheral blood of wild mammals (Table 1) from Pará, Amazonas, Rondonia and Acre States of Brazilian Amazon (Fig. 1). Sylvatic animals were manipulated with authorization and according to the Brazilian Institute of Environment (IBAMA – Instituto Brasileiro de Meio Ambiente) recommendations. Isolates from anteaters and sloth had been previously obtained (Shaw, 1985), while those from monkeys and opossums were recently isolated. Isolation was done using BAB-LIT medium, consisting of Blood Agar Base (BAB-DIFCO) containing 15% sheep blood as a solid phase with an overlay of LIT medium supplemented with 15% foetal bovine serum (FBS), with incubation at 28 °C. Selected isolates were also co-cultivated with monolayers of LLCMK2 and Hela cells in DMEM medium (GIBCO) containing 10% FSB. All T. rangeli isolates and related species were grown in BAB-LIT, by incubation at 28 °C. The other trypanosome species and Blastocrithidia culicis were grown in LIT medium with 10% FBS, at 28 °C. All trypanosomatids are cryopreserved in the Trypanosomatid Culture Collection (TCC) of the Department of Parasitology, University of São Paulo, Brazil.
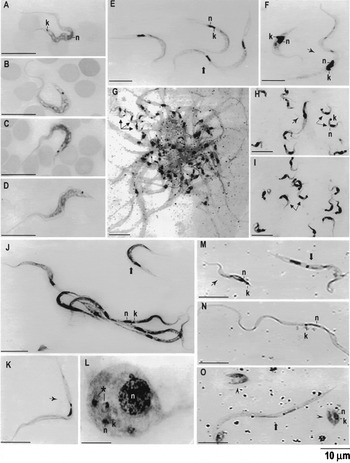
Fig. 1. Light microscopy of Giemsa-stained blood and culture smears of Trypanosoma rangeli and related species. Bloodstream trypomastigotes of (A) naturally infected opossum and (B) mouse experimentally infected with culture forms of isolate 369 obtained from the same opossum. Bloodstream trypomastigotes on blood smears of mouse experimentally infected with T. preguici (C) and T. legeri (D). Culture smears showing epimastigotes (E) and trypomastigotes (F) of isolate 194 (from monkey). Mass of epimastigotes and metacyclic trypomastigotes in the salivary glands of R. prolixus infected with T. legeri (G). Metacyclic trypomastigotes in the salivary glands of R. neglectus infected with monkey isolate 205 (H) or with human isolate AM80 (I). Haemolymph of R. neglectus infected with isolate 205 showing (J) epimastigotes, (K) trypomastigotes and (L) ‘amastigote’ and spheromastigote inside haemocytes. Digestive tube smears of Rhodnius prolixus infected with isolate 194 showing trypo- and epimastigote (M,N), spheromastigotes and epimastigotes (O). Spheromastigote (); metacyclic trypomastigote (); ‘amastigote’ (); trypomastigote (); epimastigote ().
Fifth instar nymphs of triatomines (Rhodnius neglectus, R. prolixus and Triatoma infestans) were inoculated intracoelomically with stationary-phase cultures of T. rangeli (Hecker, Schwarzenbach & Rudin, 1990). The infected triatomines (~30 for each culture) were fed on normal mice every 15 days, and 20, 30, and 60 days p.i., haemolymph (H), digestive tube (DT) and salivary glands (SG) were smeared on glass sides, fixed with methanol and Giemsa stained. Balb/c mice were infected by inoculation (i.p.) of stationary-phase cultures containing metacyclic forms or metacyclics from SGs of triatomines (~106 cells/mouse) and also by the bite of infected bugs. Mice blood samples were examined weekly from 5 to 90 days p.i. by the microhaematocrit method. R. neglectus was used for the xenodiagnosis of experimentally infected mice.
Thin blood smears made from infected mice, naturally infected opossums, H, DT and SG contents of triatomines, and smears of logarithmic and stationary-phase cultures were fixed in methanol and Giemsa-stained for light microscopy.
DNA was extracted from cultured trypanosomes using the phenol/chloroform method. Crude preparations of DNA templates from smears of triatomines (SG, DT and H) on glass-slides were obtained as described (Serrano et al. 1999), except for the use of 0·5% Tween 20 instead of 0·02% SDS. We initially tested 15 primers to amplify DNA from 4 T. rangeli isolates and then we selected the 625 (CCGCTGGAGC), 601 (CCGCCCACTG), 606 (CGGTCGGCCA) and 639 (ATCGAGCACC), that yielded the most discriminating RAPD patterns to analyse all isolates. Amplifications were performed in 50 μl reaction volumes using 50 ng of DNA, 2.5 U of Taq DNA polymerase, 0·2 mM each dNTP and 200 pM of each primer, for 34 cycles as follows: 1 min at 95 °C, 2 min at 37 °C and 2 min at 72 °C. The amplified products were separated on 2·0% agarose gels and stained with ethidium bromide. The molecular sizes of DNA fragments were determined using GeneRuler DNA Ladder Mix (MBI Fermentas).
Digitized gel images were analysed using the RFLPscan Plus software (version 3.0, Scanalytics CSP Inc., Billerica, MA, USA). The discrete character matrix was analysed by RAPDistance software (version 1.03) for the calculation of genetic distances, using the Jaccard similarity coefficients, to produce a pairwise matrix, which was used to construct dendrograms based on the UPGMA and Neighbour-joining (NJ) methods as before (Serrano, Camargo & Teixeira, 1999). Bootstrap values (100 replicates) were calculated with the PHYLIP package SEQBOOT program.
Amplified fragments generated by primer 625 were separated in 2% agarose gels and transferred to Nylon membrane (Hybond-N, Amersham Pharmacia). Slot blots of genomic DNA (2·0 μg) were prepared as described (Ventura et al. 2000). A RAPD-derived DNA fragment generated by primer 625 (Tra625) from T. rangeli (Choachi) DNA was purified (Spin-X, Costar), labelled by random primed synthesis with [α-32P] dCTP (Ready to Go kit, Amersham Pharmacia) and used as probe. Slot blots were pre-hybridized for 1 h and hybridized with Tra625 probe, at 37 °C, for 16–18 h in 2×SSC, 2% SDS, 4·0 mM sodium pyrophosphate, and 40 mg/ml of salmon sperm DNA. Membranes were washed 3 times for 15 min each in 1×SSC, 2% SDS and 4 mM sodium pyrophosphate at 55 °C.
Tra625 DNA fragments from selected trypanosomes were excised and purified from agarose gels, cloned (pGEM kit, Promega) and the nucleotide sequences of 2 clones from each isolate were determined by automated sequencing. The primers Tra625a and Tra625b (Fig. 5A) were designed based on the sequence of the Tra625 fragment for PCR amplification of a 300 bp DNA fragment (PCR-Tra625. Amplifications were done in 25 μl reaction volumes using 50 ng of DNA, 2.5 U of Taq DNA polymerase, 0·2 mM each dNTP and 8 μM of each primer through 30 cycles as follows: 1 min at 95 °C, 1 min at 66 °C and 1 min at 72 °C, with an initial cycle of 3 min at 95 °C, and a final extension cycle of 10 min at 72 °C.
In the present study trypanosomes were isolated by haemoculture of several sylvatic mammals from different regions of the Brazilian Amazon (Table 1; Fig. 1). No culture forms were obtained that were compatible with members of the subgenus Megatrypanum (Hoare, 1972). Haemocultures showing mixed infections of trypanosomes resembling T. rangeli and T. cruzi were inoculated in triatomines to recover T. rangeli from SG, thus separating them from T. cruzi. All new cultures were analysed regarding their development in triatomine and mouse to distinguish isolates of T. rangeli or related species. The PCR described by Vargas et al. (2000) were used for molecular diagnosis of T. rangeli (data not shown).
Blood forms were rarely observed in the natural hosts of the isolates. In anteaters, large blood forms compatible with Megatrypanum species were seen (Shaw, 1985), whereas blood forms typical of Herpetosoma were seen in opossums (Fig. 1A). Culture forms could be detected after 5–20 days of haemoculture, with growth and morphological features of isolates being essentially identical. The incapacity of the isolates to multiply within mammalian cells was demonstrated by co-cultivation with monolayers of LLCMK2 and Hela cells at 37 °C, using T. cruzi (G) as a control of cell infection.
To illustrate the morphological features of isolates from wild mammals we selected isolates from different host species representative of distinct genetic groups: T. rangeli from monkeys (194, 205 and 220) and from opossum (369), T. legeri, T. saimiri and T. preguici (Table 1; Fig. 1). For comparative purposes, the human T. rangeli isolate (AM80) (Coura et al. 1996) was included (Fig. 1). Light microscopy of Giemsa-stained smears from recently obtained cultures revealed highly pleomorphic epi- (Fig. 1E) and trypomastigotes (Fig. 1F). After 20–30 days, logarithmic-phase cultures showed mostly long and slender epimastigotes (Fig. 1E) whereas small metacyclics typical of T. rangeli were observed in stationary-phase cultures (data not shown). Length and shape of body, size and position of kinetoplast, and undulant membrane of all isolates are compatible with those described for T. rangeli (Hoare, 1972; D'Alessandro & Saraiva, 1992).
All new T. rangeli isolates developed in DT, H and SG of experimentally infected R. neglectus, especially when parasites were inoculated into the hemocoel (~90% of SG infection) rather than feeding on infected mice (xenodiagnosis). After about 30 days p.i., many insects died, confirming the pathogenicity of T. rangeli for its vector. DT of the triatomines showed large epi- and trypomastigotes, spheromastigotes and few short trypomastigotes, quite different from those of the SG (Fig. 1M–O). After the 10th day, long and slender epimastigotes, sometimes in the form of huge masses, short epimastigotes and trypomastigotes were seen free in the H (Fig. 1J,K) whereas ‘amastigotes’ or spheromastigotes were found inside haemocytes (Fig. 1L). After the 20th day p.i., few long epi- and trypomastigotes were present in the SG, in addition to a great number of metacyclic trypomastigotes with a large subterminal kinetoplast (Fig. 1G–I). Thus, triatomine infections developed as typically described for T. rangeli (Hecker, Schwarzenbach & Rudin, 1990).
Although the infection rate and the ability to produce metacyclic trypomastigotes in the SG was higher in R. neglectus, our new isolates of groups A and B also reach the SG's of R. prolixus and T. infestans. The behaviour of T. saimiri in triatomine bugs was identical to T. rangeli. Exceptions were the old cultures of T. legeri and T. preguici that, despite flagellates, could be frequently observed in the H, showed few metacyclics in the SG of only ~5% of the infected bugs, and exclusively in R. neglectus.
Metacyclics from both stationary cultures and from SG of triatomines infected with all new isolates infected Balb/c mice. The levels of parasitaemia were always very low, detectable by microhaematocrit from 3 to 15 days p.i. and lasting in general less than 1 month. The morphology of trypomastigotes seen in mouse blood (Fig. 1B, C, D) was typical of T. rangeli, and identical to forms present in the blood of naturally infected animals (Fig. 1A). Infection in mice was assessed by haemocultures (BAB/LIT) and by xenodiagnosis (R. neglectus).
The polymorphism among T. rangeli populations from sylvatic mammals compared with isolates from humans and triatomines of different geographical origin was assessed by RAPD patterns. The branching patterns of UPGMA (Fig. 2B) and Neighbour-joining (data not shown) dendrograms were identical and supported by significant bootstrap values. T. rangeli isolates and related species were segregated into 2 major groups (A and B), besides 2 minor groups (C and D). Group A, which was composed of Northwest South American (Colombia and Venezuela) and Central American (Honduras) isolates, and from Brazilian isolates from Marajó Island, Pará (202 and 220), and Rondônia (369 and 382). Group B was composed exclusively of isolates from Acre, Amazônia and Pará States of the Brazilian Amazon. T. saimiri, although closer to group B was not included in this group due to the small percentage of RAPD bands shared with members of this group (Table 2). Isolates of group C (from Panama and El Salvador) and the isolate SC58 (South Brazil) constantly showed different RAPD patterns with all primers investigated (Fig. 3).

Fig. 2. (A) Geographical origin of Trypanosoma rangeli isolates and allied species employed in this study. Countries are indicated within circles: SV, El Salvador; HN, Honduras; PA, Panama; VE, Venezuela; CO, Colombia; BR, Brazil. Amazonas; Pará; Rondônia and Acre are states from Brazilian Amazon. (B) Genetic distance dendrogram based on RAPD patterns constructed using the UPGMA method. The numbers in parentheses refer to the bootstrap values of the clusters in 100 replicates. The organisms were segregated into groups A ([bull ]), B ([squf ]), C ([lozf ]), and D ([starf ]). T. saimiri ([utrif ]) was not grouped.
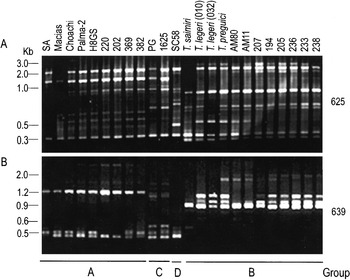
Fig. 3. Agarose gels (2%) stained with ethidium bromide showing RAPD patterns generated from DNA of Trypanosoma rangeli isolates and allied species using primers 625 and 639, selected to illustrate the genetic polymorphism (A) and grouping pattern (B) among the organisms.
Table 2. Percentage similarity of Randomly Amplified Polymorphic DNA (RAPD) bands shared within and among groups of Trypanosoma rangeli isolates and allied species (A–D correspond to branches observed in the dendrogram of the Fig. 3.)
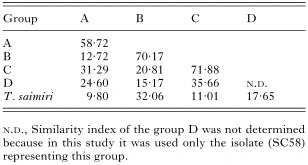
Topology of dendrogram (Fig. 2B) and similarity indexes determined by number of shared RAPD fragments indicated a close genetic relationship among populations within the groups contrasting with the low similarity between groups. The average similarity index of ~58% and ~64% were detected within groups A and B, respectively, whereas the similarity between these groups was only ~13% (Table 2). The dendrogram revealed significant variability within groups A and B, especially in the last group which clearly demonstrated subclusters of organisms that could also be grouped by 639-RAPD patterns (Fig. 3).
Isolates from Panamá and El Salvador had a high similarity (72%) and formed the group C that was always separated from the others by significant divergences (21–36%). Similarly, isolate SC58, from a rodent of Southern Brazil was always distinct from the others and ascribed into group D (Fig. 2B, Table 2).
The patterns generated by primer 625 revealed a DNA fragment (Tra625 of ~0·3 kb) shared by all T. rangeli isolates (Fig. 3A) but not by 7 other trypanosome species (Fig. 4A). To investigate whether the same-sized Tra625 fragment generated from DNA of all T. rangeli isolates and allied species consists of a conserved species-specific DNA sequence, the fragment taken from the isolate SA was used as probe. There was no cross-hybridization with any species except T. rangeli and allied species using both Southern Blot of amplified fragments generated by primer 625 (Fig. 4B) and slot blots of genomic DNA (Fig. 4C). Sequences of Tra625 fragments from 5 isolates representing the distinct groups (Choachi, SC58, T. saimiri, 220 and PG) were identical (Fig. 5A) and when submitted to Blast search of NCBI showed no significant similarity with any known sequences. The sequence data from Tra625 fragments have been submitted to GenBank and assigned to the following Accession numbers: Choachi (AY362188); SC58 (AY362190); T. saimiri (AY362192), 220 (AY362191) and PG (AY362189).

Fig. 4. (A) Agarose gels (2%) stained with ethidium bromide showing RAPD patterns generated by primer 625 using DNA of Trypanosoma rangeli isolates, related species (T. saimiri, T. preguici and T. legeri) and other trypanosomes. (B) Southern blot hybridization of the same gel (A) with Tra625 probe. (C) Slot blot of genomic DNA of trypanosomes hybridized with Tra625 probe and subsequently with SSU rDNA probe for DNA amount control. Tra=T. rangeli. 1a, Tra SA; 2a, Tra Macias; 3a, Tra H8GS; 4a, Tra 205; 5a, Tra PG; 6a, Tra Choachi; 7a, Tra Palma-2; 8a, Tra SC58; 9a, T. saimiri; 10a, T. legeri; 11a, T. preguici; 1b, Tra AM80; 2b, T. lewisi; 3b, T. blanchardi; 4b, T. rabinowitschae; 5b, T. dionisii; 6b, T. conorhini; 7b, T. freitasi; 8b, T. cruzi G; 9b, T. cruzi Y; 10b, DNA of Tra625 fragment from T. rangeli (SA) used as positive control; 11b, control without DNA.

Fig. 5. (A) Nucleotide sequence of Tra625 fragment and localization of primers Tra625a and Tra625b (italicized and underlined, respectively). (B) Agarose gel (2%) stained with ethidium bromide (EtBr) showing DNA fragments generated by Tra625-PCR using genomic DNA from Trypanosoma rangeli isolates and related species and absence of DNA bands using DNA from T. cruzi isolates or from Blastocrithidia culicis. Sensitivity of the Tra625-PCR using purified DNA of T. rangeli stained with EtBr and after hybridization with Tra625 probe. (D) DNA bands generated by fTra625-PCR using as template crude preparations of DNA from smears of Rhodnius prolixus experimentally infected with T. saimiri (1) or R. neglectus infected with isolate 369 (2) in agarose gels (2%) stained with EtBr and hybridized with Tra625 probe.
Of the patterns generated by all primers, those obtained using primer 639 showed the clearest division of the isolates into the 4 groups (Fig. 3B), in a total concordance with the dendrogram (Fig. 2B), enabling T. rangeli isolates to be ascribed into their respective groups, without using other markers. Moreover, the small heterogeneity of 639-RAPD patterns within group B could be correlated with subclusters within this group also revealed by dendrogram branching pattern (Fig. 2B).
To avoid the RAPD shortcomings with respect to reproducibility, sensitivity and requirement for purified and well-preserved DNA templates, we developed a conventional PCR assay based on the Tra625 sequence for the diagnosis of T. rangeli (Fig. 5A). PCR-Tra625 amplified DNA from culture forms of all T. rangeli isolates and the related species (Fig. 5B). There was no amplification using DNA of the other trypanosome species previously tested by RAPD (Fig. 4 and data not shown) or B. culicis, a species of the genus Blastocrithidia, which is commonly harboured by triatomines (Fig. 5B).
The sensitivity of PCR-Tra625 was shown to be ~10 pg of DNA and could be enhanced to ~5·0 pg by post-hybridization of the amplified fragments with Tra625 probe (Fig. 5C), which correspond to ~50 cells. This assay is not sensitive enough for diagnosis of T. rangeli on blood of mammals. However, its suitability for field surveys of vectors was proved using as templates crude preparations from smears of DT, SG and H of triatomines infected with T. rangeli (Fig. 5D) or mixed-infected with both T. rangeli and T. cruzi (data not shown). Uninfected R. prolixus and R. neglectus DNA, mouse and human DNA and a tube without DNA were included as negative controls of PCR reactions. Identical results were obtained using either Tra625-PCR or the PCR method as described by Vargas et al. (2000) (data not shown).
Host and geographical ranges, genetic diversity, and the precise identification of the trypanosome species found in sylvatic animals is a fundamental problem in the epidemiology of American trypanosomiasis. In this study we have focused on elucidating the genetic diversity of T. rangeli populations from neotropical monkeys, opossums, sloth and anteaters of the Brazilian Amazon. Besides behaviours in mice and triatomines, all new isolates investigated in this study also showed morphological features compatible with T. rangeli. According to traditional taxonomical criteria, all new isolates from monkeys and opossums, and the previously classified T. saimiri, T. preguici and T. legeri can be classified as T. rangeli.
Cultures of isolates from wild mammals do not necessarily correspond to trypanosomes seen in blood, as these hosts can be infected by more than one species and only one can be isolated in culture. This seems to be the case of the isolates classified as T. (Megatrypanum) legeri based on morphology of large blood trypomastigotes on anteaters, from which cultured flagellates were all revealed to be T. rangeli.
Traditionally, identifying trypanosomes as T. rangeli requires isolation by haemocultures, detection of metacyclic trypomastigotes in the SG of triatomines, and mice infection by bite or inoculation of metacyclic forms. Feeding directly on infected mammals may not be always successful for triatomine infection and even when inoculated directly into the haemocoel, infection of SG may not occur. This may be either due to specificity for sympatric vector species or caused by long periods in culture (D'Alessandro & Saravia, 1992). In addition, the ability to infect mice depends on the existence of large numbers of metacyclic forms, which is achieved only by recent cultures. Thus, identification of T. rangeli and descriptions of new species using exclusively this criteria must be avoided or done very carefully to avoid misclassification.
Based on RAPD analysis, all Brazilian populations from the Amazon segregated into two main groups. In Group A, originally described with isolates from Colombia and Venezuela (Grisard et al. 1999), we added Brazilian isolates from Pará (Marajó Island) and Rondônia. Group B, however, is a new assemblage of T. rangeli isolates composed exclusively of Brazilian isolates from Acre, Amazonas and Pará (Belém) States. The Panamanian and El Salvadorian isolates grouped together (Group C) and showed high genetic distances from all other isolates. The isolate from Southern Brazil (Group D), did not group with any other isolates examined either in this work or in a study based on mini-exon gene (Grisard et al. 1999). However, this isolate clustered with others from the same geographical origin, from the same rodent species and from Panstrongylus megistus, all sharing RAPD and isoenzyme patterns, thus constituting a homogeneous group from Southern Brazil (Steindel et al. 1994).
The high consistency of the two T. rangeli populations from Brazilian Amazon was ascertained by the highest genetic distances separating two groups of T. rangeli. Isolates from the same or neighbouring regions were distributed in both groups. Organisms from Pará (Belém), Amazonas and Acre exhibited marked similarity. However, other isolates from Pará (from Marajó Island, which is very close to Belém) and from Rondônia (close to Acre) were ascribed to group A, together with populations from Colombia and Venezuela. The partition of Brazilian Amazon isolates in this group had previously been demonstrated for a few isolates using SSU and ITS rDNA and mini-exon gene sequences (Silva et al. 1999), and is now being validated for all isolates (Maia da Silva et al., manuscript in preparation).
There are several T. rangeli-specific PCR assays based on several genes/sequences (Valejjo et al. 1999; Souto, Vargas & Zingales, 1999; Grisard et al. 1999; Vargas et al. 2000; Morales et al. 2002). However, most methods achieved remarkable separation of T. rangeli from T. cruzi ignoring other trypanosome species harboured by sylvatic mammals. Besides evaluation of few samples, not all genetic groups were tested by these methods, thus avoiding evaluation of suitability of these assays, despite the high intraspecific variability of T. rangeli. Because of the genetic complexity of T. rangeli populations, we decided to develop a PCR based on a RAPD-derived sequence. Besides high specificity to identify isolates from all genetic groups, suitability for testing crude DNA templates makes this method a reliable tool for detection of T. rangeli vectors.
According to our results, there are neither reliable differences in morphology or behaviour in mice and triatomines, nor sufficient degree of genetic polymorphism to classify any trypanosome from wild mammals examined in this study as T. rangeli-like. Moreover, we have no data to justify the status of separated species of Herpetosoma sp. allied to T. rangeli. Although T. saimiri differed from all isolates suggesting that this species perhaps deserves a separate name. T. rangeli isolates from South Brazilian and Central American sources were much more divergent. Moreover, it is believed that T. saimiri is restricted to the gut of triatomines and our T. saimiri behaviour in bugs was identical to T. rangeli. Host-restriction (Deane & Damasceno, 1961) was also not observed. This isolate infected mice as all T. rangeli, and two isolates from the same monkey species (Saimiri sciureus) differed from T. saimiri and clustered with typical T. rangeli. Morphology and triatomine behaviour of other T. saimiri isolates also indicated that this species is a synonym of T. rangeli (Ziccardi & Oliveira, 1998). Probably, the isolate here classified as T. saimiri belongs to an additional group of T. rangeli.
T. rangeli is a complex of isolates presenting particular genetic characteristics that permitted us to distribute the isolates into at least 4 distinct phylogenetic groups. The distribution of T. rangeli isolates in groups is independent of their host species and is not only determined by geographical isolation, despite some geographical segregation pattern. The same grouping defined by different and independent molecular markers suggested clonal evolution of T. rangeli populations. A recent study supported either clonal evolution or speciation of T. rangeli populations in their triatomine vectors (Valejjo et al. 2003). The isolate PG from Panama (Group C) can be distinguished by their inability to develop in vectors other than the sympatric species R. pallescens (Sousa & Dawson, 1976). Several studies on triatomine behaviours revealed vector restriction of some T. rangeli isolates to their local vector species (D'Alessandro, 1976; Machado et al. 2001; Guhl & Valejjo, 2003).
This is the first study to demonstrate that isolates from sylvatic mammals and man are highly genetically related, confirming the lack of host restriction of T. rangeli and suggesting that the same population can circulate among sylvatic mammals, humans and triatomines. We demonstrated that T. rangeli is more complex than previously described, with at least 4 genetic groups. More isolates from mammals and vectors of several geographical regions and new molecular markers must be investigated to make definitive statements concerning the determinant factors of this segregation as well as to ascribe some taxonomic status for each group.
We are particularly indebted to many colleagues, who kindly provided several isolates, and also to many students for inestimable help during the fieldwork. Work in Rondônia was done at the laboratory of the ICBV, USP. We are grateful to J. M. Barata who kindly provided the triatomines employed in this study, Rodrigo Fernandez for initial studies of T. saimiri and R. V. Milder for critical comments on the manuscript. This work was supported by grants from the Brazilian agency FAPESP. F.M.S. and A.C.R. are FAPESP graduate student fellows.

Fig. 1. Light microscopy of Giemsa-stained blood and culture smears of Trypanosoma rangeli and related species. Bloodstream trypomastigotes of (A) naturally infected opossum and (B) mouse experimentally infected with culture forms of isolate 369 obtained from the same opossum. Bloodstream trypomastigotes on blood smears of mouse experimentally infected with T. preguici (C) and T. legeri (D). Culture smears showing epimastigotes (E) and trypomastigotes (F) of isolate 194 (from monkey). Mass of epimastigotes and metacyclic trypomastigotes in the salivary glands of R. prolixus infected with T. legeri (G). Metacyclic trypomastigotes in the salivary glands of R. neglectus infected with monkey isolate 205 (H) or with human isolate AM80 (I). Haemolymph of R. neglectus infected with isolate 205 showing (J) epimastigotes, (K) trypomastigotes and (L) ‘amastigote’ and spheromastigote inside haemocytes. Digestive tube smears of Rhodnius prolixus infected with isolate 194 showing trypo- and epimastigote (M,N), spheromastigotes and epimastigotes (O). Spheromastigote (); metacyclic trypomastigote (); ‘amastigote’ (); trypomastigote (); epimastigote ().

Table 1. Trypanosoma rangeli isolates and allied trypanosomes used in this study, geographical and host species of origin, and distribution in groups based on the RAPD-derived patterns and dendrogram (Fig. 3)
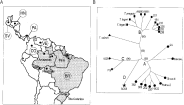
Fig. 2. (A) Geographical origin of Trypanosoma rangeli isolates and allied species employed in this study. Countries are indicated within circles: SV, El Salvador; HN, Honduras; PA, Panama; VE, Venezuela; CO, Colombia; BR, Brazil. Amazonas; Pará; Rondônia and Acre are states from Brazilian Amazon. (B) Genetic distance dendrogram based on RAPD patterns constructed using the UPGMA method. The numbers in parentheses refer to the bootstrap values of the clusters in 100 replicates. The organisms were segregated into groups A ([bull ]), B ([squf ]), C ([lozf ]), and D ([starf ]). T. saimiri ([utrif ]) was not grouped.

Fig. 3. Agarose gels (2%) stained with ethidium bromide showing RAPD patterns generated from DNA of Trypanosoma rangeli isolates and allied species using primers 625 and 639, selected to illustrate the genetic polymorphism (A) and grouping pattern (B) among the organisms.

Table 2. Percentage similarity of Randomly Amplified Polymorphic DNA (RAPD) bands shared within and among groups of Trypanosoma rangeli isolates and allied species

Fig. 4. (A) Agarose gels (2%) stained with ethidium bromide showing RAPD patterns generated by primer 625 using DNA of Trypanosoma rangeli isolates, related species (T. saimiri, T. preguici and T. legeri) and other trypanosomes. (B) Southern blot hybridization of the same gel (A) with Tra625 probe. (C) Slot blot of genomic DNA of trypanosomes hybridized with Tra625 probe and subsequently with SSU rDNA probe for DNA amount control. Tra=T. rangeli. 1a, Tra SA; 2a, Tra Macias; 3a, Tra H8GS; 4a, Tra 205; 5a, Tra PG; 6a, Tra Choachi; 7a, Tra Palma-2; 8a, Tra SC58; 9a, T. saimiri; 10a, T. legeri; 11a, T. preguici; 1b, Tra AM80; 2b, T. lewisi; 3b, T. blanchardi; 4b, T. rabinowitschae; 5b, T. dionisii; 6b, T. conorhini; 7b, T. freitasi; 8b, T. cruzi G; 9b, T. cruzi Y; 10b, DNA of Tra625 fragment from T. rangeli (SA) used as positive control; 11b, control without DNA.

Fig. 5. (A) Nucleotide sequence of Tra625 fragment and localization of primers Tra625a and Tra625b (italicized and underlined, respectively). (B) Agarose gel (2%) stained with ethidium bromide (EtBr) showing DNA fragments generated by Tra625-PCR using genomic DNA from Trypanosoma rangeli isolates and related species and absence of DNA bands using DNA from T. cruzi isolates or from Blastocrithidia culicis. Sensitivity of the Tra625-PCR using purified DNA of T. rangeli stained with EtBr and after hybridization with Tra625 probe. (D) DNA bands generated by fTra625-PCR using as template crude preparations of DNA from smears of Rhodnius prolixus experimentally infected with T. saimiri (1) or R. neglectus infected with isolate 369 (2) in agarose gels (2%) stained with EtBr and hybridized with Tra625 probe.