Introduction
In the last decades with further evolution in IVF methods, it became standard practice to grow embryos in vitro up to D5, at which point they have reached the blastocyst stage. A better pregnancy outcome of fresh blastocysts compared with cleavage-stage embryos has been described in several studies (Gardner et al., Reference Gardner, Schoolcraft, Wagley, Schlenker, Stevens and Hesla1998; Rienzi et al., Reference Rienzi, Gracia, Maggiulli, LaBarbera, Kaser, Ubaldi, Vanderpoel and Racowsky2017). Additionally, Assisted Conception Units are increasingly moving towards a policy of elective single embryo transfer (eSET), to reduce the incidence of multiple pregnancies (Vilska et al., Reference Vilska, Tiitinen, Hyden-Granskog and Hovatta1999; Gerris et al., Reference Gerris, de Neubourg, Mangelschots, van Royen, Vercruyssen, Barudy-Vasquez, Valkenburg and Ryckaert2002; Adashi et al., Reference Adashi, Barri, Berkowitz, Braude, Bryan, Carr, Cohen, Collins, Devroey, Frydman, Gardner, Germond, Gerris, Gianaroli, Hamberger, Howles, Jones, Lunenfeld, Pope, Reynolds, Rosenwaks, Shieve, Serour, Shenfield, Templeton, van Steirteghem, Veeck and Wennerholm2003).
Single embryo transfer remains the most effective way to decrease the rate of multiple pregnancies, and it has presented a challenge to embryologists who must try to optimise embryo culture and selection to maintain an adequate success rate, while reducing the overall number of embryos transferred. Indeed, vitrification has become a highly important step of the assisted reproductive technology (ART) treatment for several reasons: first, to store supernumerary embryos for future use; second, in preimplantation genetic screening (PGS) cycles, trophectoderm biopsy is a growing method of choice. In that context, cryopreservation plays a crucial role by overcoming time restraints and allowing the embryologist team to perform biopsies at different times, vitrify the blastocyst directly afterwards, followed by warming and transfer of euploid embryos in a later menstrual cycle (McArthur et al., Reference McArthur, Leigh, Marshall, de Boer and Jansen2005; Cohen et al., Reference Cohen, Wells and Munne2007). Finally a freeze-all concept has become attractive and recommended by specialists, especially in patients at risk to develop ovarian hyperstimulation syndrome (OHSS) when fresh embryo transfer cannot be performed (Roque et al., Reference Roque, Lattes, Serra, Solà, Geber, Carreras and Checa2013).
The ability to cryopreserve human embryos has also improved significantly in the last decade (Liebermann and Tucker, Reference Liebermann and Tucker2006; Stanger et al., Reference Stanger, Wong, Conceicao and Yovich2012; Rienzi et al., Reference Rienzi, Gracia, Maggiulli, LaBarbera, Kaser, Ubaldi, Vanderpoel and Racowsky2017). There is now sufficient evidence showing that results from vitrification are superior to those achieved with the slow-freezing protocols (Loutradi et al., Reference Loutradi, Kolibianakis, Venetis, Papanikolaou, Pados, Bontis and Tarlatzis2008; AbdelHafez et al., Reference AbdelHafez, Desai, Abou-Setta, Falcone and Goldfarb2010; Li et al., Reference Li, Wang, Ledger, Edgar and Sullivan2014). However, it is important to determine whether a good quality D6 blastocyst has a decreased pregnancy outcome compared with a D5 blastocyst in a vitrified-warmed transfer. Conflicting results still persist in the published literature, some studies have reported higher clinical pregnancy after D5 vitrified-warmed transfer compared with D6 transfer (Desai et al., Reference Desai, Ploskonka, Goodman, Attaran, Goldberg, Austin and Falcone2016; Haas et al., Reference Haas, Meriano, Laskin, Bentov, Barzilay, Casper and Cadesky2016). Others have reported similar outcomes for D5 and D6 (Behr et al., Reference Behr, Gebhardt, Lyon and Milki2002; El-Toukhy et al., Reference El-Toukhy, Wharf, Walavalkar, Singh, Bolton, Khalaf and Braude2011; Wang et al., Reference Wang, Zhen, Sun, Yu, Deng, Zhou, Wang and He2016; Kaye et al., Reference Kaye, Will, Bartolucci, Nulsen, Benadiva and Engmann2017).
Results from a meta-analysis in 2010 suggested that slower developing blastocysts cryopreserved on D6, but at the same stage of development as those on D5, had similar clinical pregnancy and live-birth rates following transfer (Sunkara et al., Reference Sunkara, Siozos, Bolton, Khalaf, Braude and El-Toukhy2010). The question of when to cryopreserve blastocysts and which blastocysts to cryopreserve was largely unresolved at the start of our study. We have therefore used our results to investigate whether blastocysts that formed and were vitrified on D6 have a similar implantation potential to those that formed and were cryopreserved on D5. Therefore, the goal of the present study was to compare the pregnancy and implantation rates between good quality blastocysts vitrified/warmed on D5 versus those on D6 in programmed warmed single blastocyst transfer (WSBT).
Materials and methods
Study
This was a retrospective cohort study carried out at Edinburgh Assisted Conception Programme, EFREC, Royal Infirmary of Edinburgh in Scotland from January 2011 to May 2018. In total, 2080 vitrified-warmed blastocyst were analyzed and included in the study. After fresh embryo transfer, supernumerary good quality blastocysts ≥ 2, according to Gardner’s score (Gardner and Schoolcraft, Reference Gardner and Schoolcraft1999), excluding those graded CC, BC or CB, were vitrified on D5 or D6 after fertilization. A small percentage of patients (8%) did not have fresh embryo transfer due to the risk of ovarian hyperstimulation syndrome (OHSS), therefore all embryo were frozen on D5 or D6. Details of ovarian stimulation and egg retrieval have been described previously by Sciorio et al. (Reference Sciorio, Thong and Pickering2018).
Programmed warmed cycle
Most frozen embryo transfers (ETs) were artificial cycles (around 95%), if patients had regular menstrual cycles (25–35 days) they underwent downregulation using a gonadotrophin releasing hormone (GnRH) agonist triptorelin acetate (Decapeptyl 3 mg), which was administered on day 21 of their menstrual cycle. Women were advised to take 6 mg oestradiol valerate (Progynova) daily orally from day 2 or day 3 of commencement of their periods after administration of triptorelin acetate. A transvaginal scan was carried out after administration of oestradiol valerate around 14 days to measure their endometrial thickness. If endometrial thickness was more than 6 mm (the optimal endometrial thickness is preferably 8 mm or more), embryo transfer was arranged after a full 5 days of a progesterone pessary, Cyclogest 400 mg, twice daily per vaginal administration. The day of embryo transfer was arranged after a transvaginal scan to confirm that optimal thickness was reached. All warmed blastocysts, both vitrified on D5 or D6 were replaced in the D5 endometrium, after a full 5 days of progesterone pessary administration. For patients who had regular menstrual cycles, as defined above, and who wished a natural cycle had baseline estradiol (E2) and luteinizing hormone (LH) taken between days 2–5 of their menstrual cycles. Patients were then advised to have blood tests for LH/E2 from day 10 onwards either daily or every 2 days depending on their LH/E2 result. Blastocyst embryo transfer was arranged 4 days from the detected LH peak. A pregnancy test was arranged 9 days after embryo transfer.
Embryo culture and blastocyst vitrification
Cumulus–oocyte complexes (COC) were isolated from follicular fluid, rinsed in G-IVF™ medium (VitroLife, Sweden) transferred to 0.5 ml G-IVF™ medium and returned to an incubator equilibrated at 37°C and 6.0% CO2 in atmospheric air. Oocytes were examined for the presence of two pronuclei approximately 16–19 h after insemination or microinjection. Normally fertilized oocytes were placed in an EmbryoScope™ time-lapse incubator for culture using a 12-well EmbryoSlide™ (VitroLife, Sweden). Embryo culture was performed with 6% CO2, 5% oxygen and nitrogen balance at 37°C, in time-lapse single step medium (G-TL™, VitroLife, Sweden). All embryos were scored for cell number, regularity of cleavage and the degree of fragmentation, according with the scoring system described by Cutting et al. (Reference Cutting, Morroll, Roberts, Pickering and Rutherford2008). On the morning of D5, one blastocyst with the best morphology quality was transferred, any remaining good quality blastocysts were cryopreserved. A small percentage of patients (8%) was freeze-all cycle, due to risk of ovarian hyperstimulation syndrome (OHSS).
On D5, embryos at the morula or early blastocyst stage were left in culture for 1 day more, reassessed on the morning of D6 and, if considered good quality, were cryopreserved. Blastocysts were classified using Gardner’s score according to blastocyst size, morphology of the inner cell mass (ICM) and trophectoderm (TE) (Gardner and Schoolcraft, Reference Gardner and Schoolcraft1999). Blastocysts with a score ≥ 2, excluding those with grades CC, BC or CB, were selected on D5 and D6 for vitrification. Irvine Scientific Freeze Kit (Irvine, USA), together with Rapid-i closed device (VitroLife, Sweden) that can prevent the direct contact between embryos and liquid nitrogen and reduces to zero the risk of cross-contamination, was used for vitrification. The procedure was always performed using one blastocyst for each single device. If the expansion of the blastocyst was grade 3, or more an artificial shrinkage (AS), using a laser pulse was performed before vitrification.
The ICM was positioned at a distance from the focus of the laser beam (Saturn 5 Active™ laser system RI-Research Instrument, CooperSurgical Fertility Companies, Malov, Denmark) before being subjected to a minimum setting (200 ms) laser pulse to generate a small hole at the junction of two trophectoderm cells and resulting in the release of fluid from the blastocoel cavity. Blastocyst shrinkage occurred within 1 or 2 min. The blastocyst was then moved at room temperature (22–25°C) to ES medium (equilibration solution: 7.5% v/v of each dimethyl sulfoxide (DMSO) and ethylene glycol). After 6–8 min, the blastocyst was quickly washed in VS drop (Vitrification Solution: 15% v/v of each DMSO and ethylene glycol, 0.5 M sucrose), for 45–60 s and transferred onto the Rapid-i device using a micropipette. The smallest possible volume of VS containing the embryo was loaded into the hole of the straw, which was inserted into an external straw immersed vertically into liquid nitrogen (LN2). The external straw could be sealed and transferred to the tank for long storage in LN2.
Blastocyst warming
An Irvine Scientific Thaw Kit (Irvine, USA) was used for warming. In a Nunc 35 × 10 mm culture dish, 1 ml of TS (thawing solution: 1 M sucrose) was warmed at 37°C for 20–30 min in an incubator and then placed on a stage warmer. The straw containing the Rapid-i was opened while still submerged in LN2. The carrier containing the embryo was removed from the straw and placed as quickly possible into the dish containing the thawing medium (thawing solution) preheated. The blastocysts immediately fell from the device and could be easily identified in the medium. After 1 min, blastocysts were transferred to the DS medium (dilution solution: 0.5 M sucrose) for 4 min at room temperature 22–25°C. In the last step, blastocysts were placed for 4 min, twice, in the WS medium (washing solution: HEPES-buffered solution of Medium-199 containing gentamicin sulphate, 35 μg/ml HEPES and 20% DSS). The embryo was then returned to time-lapse single step medium (G-TL™, VitroLife, Sweden) supplemented with 20% HSA solution™ (human serum albumin, VitroLife, Sweden) for culture until transfer. At this stage, an assessment was performed on an inverted microscope to establish if the embryo survived based on morphological integrity of the ICM and trophectoderm. After 1 or 2 h of culture the embryo was reassessed again and often the re-expansion of the blastocoel was reported; this indicated that the embryo physiologically survived the warming procedure. Embryo transfer was normally performed within 2 or 3 h. If there were any instances in which the same patient had both D5 and D6 blastocysts stored, the best quality embryo was warmed first. If the embryo did not survive, another embryo was warmed if the patient had any in storage, otherwise the transfer was cancelled. All programmed warmed cycles, both at D5 and D6, were transferred in D5 endometrium.
Clinical outcome
Chemical pregnancy was assessed based on serum beta human chorionic gonadotrophin (β-hCG) levels at 10 days after the embryo transfer. At 7 weeks, a scan was performed to confirm the presence of fetal heart activity or a gestational sac formation. The implantation rate (IR) was defined as the number of gestational sacs at the 7 weeks’ scan. A clinical pregnancy (CP) was defined as a pregnancy with a fetal heart.
Statistical analysis
A chi-squared test was performed to examine the relationship between blastocysts frozen on D5 and D6 in terms of survival rate, implantation and CP. Differences were considered statistically significant at the level of P-value < 0.05.
Results
In total, 2080 vitrified-warmed blastocyst were included in the study and analyzed. The mean age of the women was not different, 33.8 years in the D5 vitrified-warmed group and 34.3 years in the D6 group. In spite of similar survival rates after warming for D5 [95.3% (1489/1563)] and D6 [94.2% (487/517)] blastocyst (Table 1), the overall CPR was better for D5 blastocysts group (Table 2). There was a significant difference in outcome following WSBT of D5 compared with D6. As shown in Table 2, implantation and CP rates per transfer occurring in the D5 group were 49.4% and 42.6% respectively, values that were significantly different (P < 0.05) compared with the D6 group (37.4% and 32.2%; respectively). Multiple pregnancies were similar in both groups.
Table 1. Correlation of retrospective data from the blastocyst vitrification programme at the Edinburgh Assisted Conception Programme of D5 and D6 blastocysts (January 2011 to May 2018)
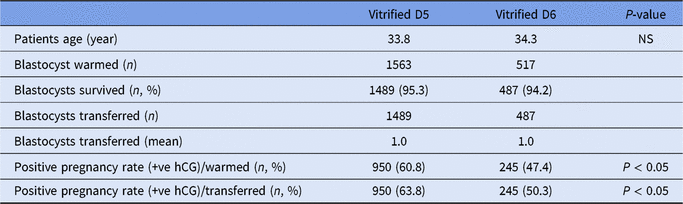
hCG, human chorionic gonadotrophin; n, numbers; NS, not significant.
Table 2. Implantation, clinical pregnancy and multiple pregnancy rates after D5 and D6 vitrified-warmed single blastocyst transfer at Edinburgh Assisted Conception Programme (January 2011 to May 2018)

n, numbers; NS, not significant.
Table 3 shows the mean age and clinical outcome of patients who completed the vitrified/warming programme. The mean age of the women was 34.05. In total, 2080 vitrified blastocysts (D5 plus D6) were warmed, of which 1976 survived warming (95.0%). All surviving blastocysts were replaced in 1976 patients, in a policy of single blastocyst transfer. Overall, the positive pregnancy rate (+ve hCG), implantation and CP rates per transfer were 60.5, 46.4 and 40.1%, respectively. Total multiple pregnancy reported was 1.32%.
Table 3. Overall data from the blastocyst vitrification program at Edinburgh Assisted Conception Programme of D5 plus D6 blastocysts (January 2011 to May 2018)

hCG, human chorionic gonadotrophin.
Table 4 shows the total number of fresh eSET at blastocyst stage performed over the same time period (January 2011 to May 2018). The total number of oocyte pick ups (OPU) was 2478. The mean female age was 34.8 years. The proportion of elective single transfer at blastocyst stage (D5) was 49%. The positive pregnancy rate (+ve hCG), implantation and CPRs per eSET at blastocyst stage (D5) were 65, 59 and 53%, respectively (Table 4).
Table 4. Data from fresh cycles at Edinburgh Assisted Conception Programme (January 2011 to May 2018)

CP, clinical pregnancy rate; eSET, elective single embryo transfer; hCG, human chorionic gonadotrophin; OPU, oocyte pick up; PN, pronuclei; Trf, transfer.
Discussion
The current study assessed the clinical implications of vitrification on D5, as compared with delayed D6 blastocysts. Results demonstrated vitrification on D5 to be an independent predictor of implantation and clinical pregnancy. In fresh cycles, superior clinical results for D5 versus D6 transfers have been already demonstrated (Shapiro et al., Reference Shapiro, Richter, Harris and Daneshm2001). Barrenetxea et al. (Reference Barrenetxea, López-de Larruzea, Ganzabal, Jiménez, Carbonero and Mandiola2005) have shown in fresh cycles significantly increased pregnancy rate when transferring embryos on D5 after fertilization compared with blastocysts on D6: the pregnancy rate was extremely low in the D6 group (11%).
Delayed embryo growth, as well as a displaced window of implantation, might explain these results. Additional studies came to the conclusion that embryos that are expanding on D5 have a higher implantation potential than those that do not reach this stage until D6. A two-fold difference in implantation rates between D5 and D6 transfers (Khorram et al., Reference Khorram, Shapiro and Jones2000; Shoukir et al., Reference Shoukir, Chardonnens, Campana, Bischof and Sakkas1998, Shapiro et al., Reference Shapiro, Richter, Harris and Daneshm2001) has been reported. These reports suggested that embryos that are a day behind the normal rate of development are almost half as likely to be viable compared with blastocysts that expand on D5.
Hashimoto et al. (Reference Hashimoto, Amo, Hama, Ohsumi, Nakaoka and Morimoto2013) also reported a lower pregnancy rate of slow-growing embryos compared with normally developing embryos. The study noted that the incidence of abnormal spindles in the group of slow developing embryos (D6) was significantly higher compared with normal growing embryos (D5). However, in a frozen cycle, it is still the object of debate whether a vitrified good quality D5 blastocyst has a superior pregnancy outcome compared with a vitrified blastocyst on D6 (Behr et al., Reference Behr, Gebhardt, Lyon and Milki2002; Richter et al., Reference Richter, Shipley, McVearry, Tucker and Widra2006; Shapiro et al., Reference Shapiro, Daneshmand, Garner, Aguirre and Ross2008). A meta-analysis based on both slow-freezing and vitrification methods of cryopreservation and including 15 studies (Sunkara et al., Reference Sunkara, Siozos, Bolton, Khalaf, Braude and El-Toukhy2010) reported significantly higher CPRs and ongoing pregnancy rates for D5 versus D6 transfers. When the stage of embryo development was taken into account (four studies), this advantage vanished and the authors concluded that more well designed trials are needed before conclusions can be made.
Subsequently, a large study by Kovalevsky et al. (Reference Kovalevsky, Carney, Morrison, Boylan, Neithardt and Feinberg2013) that included both slow-freezing and vitrification cycles showed that implantation, clinical and ongoing pregnancy rates were significantly increased for D5 versus D6 transfers. Similar outcomes were reported by Desai et al. (Reference Desai, Ploskonka, Goodman, Attaran, Goldberg, Austin and Falcone2016) whose multivariate regression analysis demonstrated clinical pregnancy and live-birth rates to be three times higher after the transfer of a D5 blastocyst compared with those vitrified on D6.
This finding was not confirmed in single embryo transfer cycles by Kaye et al. (Reference Kaye, Will, Bartolucci, Nulsen, Benadiva and Engmann2017) who observed no differences with respect to clinical pregnancy rate, ongoing pregnancy rate, live-birth and miscarriage rates between D5 and D6 vitrified and frozen embryos. Similar to our study, Haas et al. (Reference Haas, Meriano, Laskin, Bentov, Barzilay, Casper and Cadesky2016) observed that clinical pregnancy rate (44.7 vs. 33%, P = 0.002) and ongoing pregnancy rate (41.1 vs. 28.3%, p < 0.001) were higher in the group in which blastocysts were vitrified on D5 compared with D6. However they reported a similar IR in both groups (30% vs. 24.3%, P = 0.02). In the mentioned study embryos vitrified on D5 were warmed on D5 of progesterone treatment and transferred after 20–24 h. Embryos vitrified on D6 were warmed on D6 of progesterone treatment and transferred after 2–4 h. In both groups, the embryos were transferred on D6 of progesterone treatment (Haas et al., Reference Haas, Meriano, Laskin, Bentov, Barzilay, Casper and Cadesky2016). However, there was an important difference compared with our study, in which all embryos vitrified on D5 or D6 were warmed on D5 progesterone and transferred after 2 or 3 h. There is limited information on the reasons why blastocysts with delayed blastulation exert a lower clinical outcome. In addition to metabolic imbalances and bioenergetics issues, higher aneuploidy rates in slow growth embryos have been proposed as a possible explanation (Capalbo et al., Reference Capalbo, Rienzi, Cimadomo, Maggiulli, Elliott, Wright and Ubaldi2014; Yin et al., Reference Yin, Jiang, He, Wang, Zhu and Luan2015). Taylor et al. (Reference Taylor, Patrick, Gitlin, Wilson, Crain and Griffin2014) calculated that blastocysts that formed and were vitrified on D6 (slow developing embryos) had a 10% increase in aneuploidy rates compared with those that formed on D5, and this difference might be the reason for their lower pregnancy outcome after vitrified-warmed transfer. In the present study we did not tested blastocyst euploidy, therefore this retrospective study could not investigate whether the differences between the D5 and D6 groups was related to a different rate of chromosomal abnormalities as proposed above. However, a study by Kroener et al. (Reference Kroener, Ambartsumyan, Briton-Jones, Dumesic, Surrey, Munné and Hill2012) reported no differences in aneuploidy rates according to the day of blastulation, although the absence of blastulation was correlated with increased aneuploidy rates.
Additional studies have found euploidy rates to be similar between the D5 and D6 groups (Alfarawati et al., Reference Alfarawati, Fragouli, Colls, Stevens, Gutierrez-Mateo, Schoolcraft and Wells2011; Fragouli et al., Reference Fragouli, Alfarawati, Spath and Wells2014). Finally Yang et al. (Reference Yang, Yang, Dai, Li, Jin, Yao and Sun2016) described better pregnancy outcomes for D5 versus D6 blastocysts, but this was due to better blastocyst quality with no differences in aneuploidy rates. This points to a worsened metabolic/bioenergetic balance in slow developing embryos. However, it remains unclear whether these issues are an intrinsic weakness of some embryos or depend on variation in laboratory procedures, endometrial preparation, culture medium, vitrification and warming procedures. Our study carried the limitation of its retrospective design and could not contribute to understanding the causes of low performance in delayed growth embryos. However, we added further evidence of clinical advantage of D5 vitrification within a closed device and single embryo transfer setting. However, we also noted that D6 blastocyst transfers have resulted in clinical pregnancies and remain therefore a viable option, at least when good quality D6 blastocysts are available. Future studies will have to address other open questions including live-birth rates, and long-term post natal outcomes after transfer of slowly developed and late vitrified blastocyst.
Authors contributions
Conception and design of the study, acquisition and interpretation of data, and writing the article (Romualdo Sciorio), statistical analysis and interpretation of data (K.J. Thong), manuscript revision for important intellectual content (S.J. Pickering).
Conflict of interest statement
None of the authors has any financial or other potential conflict of interest related to this manuscript.
Acknowledgements
We would like to acknowledge our embryology group for the assistance and thank Maurizio Dattilo for the constructive comments on the manuscript.
Compliance with ethical standards
Human and animal rights. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and with the 1964 Declaration of Helsinki and its later amendments. For this type of study, formal consent is not required.
Financial support
Project support was provided by the Edinburgh Assisted Conception Programme, EFREC, Royal Infirmary of Edinburgh, 51 Little France Crescent, Old Dalkeith Road, Edinburgh, Scotland, EH16 4SA, UK.






