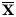Introduction
Soil salinity is increasing over time and has been a global environmental challenge to sustainable agriculture and food security. Salinization has negative impacts on agricultural production, the living conditions of farmers, the economy at different levels and the balance of ecosystems including the quality of natural resources (FAO, 2019). It is therefore imperative to have a detailed understanding of the response of crops towards soil salinity at the morpho-agronomical, physiological and molecular levels for its effective management.
Rice is considered sensitive to salinity, particularly during early vegetative and later at reproductive stages specifically at panicle initiation and pollination stage (Singh et al., Reference Singh, Gregorio and Ismail2008). Mukta et al. (Reference Mukta, Hossain, Nasiruddin and Islam2017) and Yichie et al. (Reference Yichie, Brien, Berger, Roberts and Atwell2018) cited that modern rice varieties are highly sensitive to salinity consequently reducing rice production. Thus, it is essential to develop and employ technologies that will reduce the spread of salinization, reduce salinity levels in crop fields or increase salt tolerance of crops.
Assessment of genetic diversity could provide valuable information for genetic improvement of salt-tolerant rice. It has long been recognized that salinity can cause sterility in rice, particularly if imposed during pollen development and fertilization; hence, high-yielding salt-tolerant rice varieties must possess reproductive-stage tolerance. Screening for salinity tolerance in rice germplasm is therefore essential to establish the true worth of plant genetic resources that are necessary for the development of salinity tolerant varieties. Germplasm screening for salinity tolerance at the seedling stage is readily acceptable as it is based on simple criterion as well as quick assessment, but screening at reproductive stage to determine physiological mechanism that contributes to salinity tolerance is limited (Hossain et al., Reference Hossain, Rahman, Alam and Singh2014; Ahmadizadeh et al., Reference Ahmadizadeh, Vispo, Palao, Pangaan, Dela Viña and Singh2016). One of the reasons is probably the difficulty in screening during reproductive stage compared to screening at seedling stage due to its complex genetic basis, lack of reliable stage-specific phenotyping techniques (Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013) and labour intensive nature. In addition, tolerance during the seedling and reproductive stage seems to be poorly correlated, suggesting that different sets of salt tolerance mechanisms are involved (Moradi et al., Reference Moradi, Ismail, Egdane and Gregorio2003; Singh et al., Reference Singh, Redoña, Refuerzo, Pareek, Sopory, Bohnert and Govindjee2010).
Improving the salt tolerance and evaluation of salinity tolerance in rice germplasm requires access to efficient screening techniques for the identification of salt-tolerant genotypes. Salinity screening in different crops particularly in rice has been well documented. Different screening protocols were used for this purpose but screening under controlled condition such as screen/glasshouse is the most common method used. Reddy et al. (Reference Reddy, Kim, Yoon, Kim and Kwon2017) pointed out that reliable and repeatable screening techniques are the mainstay of any successful breeding programs and they should be rapid, reproducible, easy and affordable.
This study was conducted to screen rice germplasm for salinity tolerance at the reproductive stage using different screening methods namely: gravel-based hydroponics, soil, field and controlled mini-field. We also determined the genotypic variability of rice germplasm in response to salinity stress using morpho-agronomic and physiological traits. This experiment is necessary for deciding which screening method is appropriate to identify salinity tolerant rice varieties/cultivars at reproductive stage that can be used as donor parents in future breeding programs.
Materials and method
Preparation of planting materials
The experimental material composed of 50 rice genotypes with diverse genetic background. Some of them are landraces originated from different Asian countries while some are varieties already registered in the National Seed Industry Council (NSIC) in the Philippines (online Supplementary Table S1). The seeds were obtained from the Genetic Resources Centre, International Rice Research Institute (GRC-IRRI).
The seeds were incubated at 50 °C for 3–5 days in a convection oven to break dormancy and allowed to pre-germinate in a moistened paper towel. To remove the possible contaminants, the seeds were soaked in 1% zonrox for about 5 min then rinsed in running water until the odour was gone. The seeds were incubated again in a convection oven at 37 °C to continue the process of germination for 48 h.
After pre-germination, the germinated seeds designated for gravel-based hydroponics method were placed in styrofoam floats punched with 15 mm holes (10 rows × 10 columns). The holes were in a grid pattern separated 30 mm between holes in a row and 20 mm within a column. Once the seedlings developed primary root ~2–3 cm long in the paper towel, they were carefully transferred into the hole. Two seedlings were transplanted per hole. After all the seeds were transplanted, the styrofoam floats were placed in 8 litre plastic trays with modified Yoshida's solution (Singh et al., Reference Singh, Redoña, Refuerzo, Pareek, Sopory, Bohnert and Govindjee2010; Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013). The modified culture solution has KH2PO4 and K2HPO4 in place of NaH2PO4 as sodium salt within the culture solution may increase the Na ion. The pH of the solution was maintained at 5.0 ± 0.1 and the water level was monitored daily. The seedlings were allowed to grow in the solution for about 14–21 days before transplanting. On the other hand, the pre-germinated seeds designated for soil-based, controlled mini-field and field methods were sown in plastic trays filled with soil. Similarly, the seedlings were allowed to grow for about 14–21 days before transplanting.
Glasshouse screening using gravel-based hydroponics
The test entries were grown both in saline and nonsaline conditions to determine the effect of salinity on the reproductive growth of rice genotypes. The experiment was laid in randomized complete block design (RCBD) with two replications.
Perforated plastic pots (20 cm in diameter) were prepared and drilled with 3–4 mm holes in four rows down its sides with 2 cm intervals between each hole. Fourteen (14)-d-old seedlings were transplanted in plastic pots filled with volcanic gravel and kept in trays with a simple nutrient addition program (SNAP) solution. The working solution was prepared by mixing SNAP-A and SNAP-B which are buffer-based nutrient solutions developed by the Plant Physiology Laboratory, Institute of Plant Breeding, Crop Science Cluster, University of the Philippines Los Baños (Santos and Ocampo, Reference Santos and Ocampo2005). The stock solutions were as 100× concentrations. For every litre of working solution, 980 ml of reverse osmosis (RO) water was prepared and added with 10 ml of each SNAP-A and SNAP-B stock solutions. Ferrous sulphate ~2.5 g/100 litre was added to the solution. The solution was replaced every 2 weeks and the pH was monitored and maintained every 2 days at 5.0 ± 0.1.
Glasshouse screening using soil method
The same plastic pots used in the gravel-based hydroponics method were prepared. Nylon mesh was placed inside the pots and then filled with sterilized soil ~1 cm above the topmost row of holes. Fourteen (14)-d-old seedlings were replanted on the plastic pots and kept on trays filled with tap water. The water volume of the trays was reconstituted daily to compensate for the water loss due to evaporation and transpiration. The experiment was laid out in RCBD with two replications.
Field screening
The seeds were sown in soil germination trays. After 21 days, the seedlings were transplanted in nonsalinized and artificially salinized blocks paddy. The screening was laid out in RCBD with two replications.
Controlled mini-field screening
Twenty-one (21)-d-old seedlings were transplanted in two concrete blocks (one for nonsaline and one for saline set-up). The blocks were lined with pipes at the bottom for the passageway of both saline and nonsaline water. There were two water tanks (saline and potable) which served as the source of saline and nonsaline water, respectively. In each tank, there were valves which allowed the passageway of water underneath the concrete plant bed when opened. The test entries were laid out in RCBD.
Salinity treatments
For all the screening methods, salt stress was imposed when the test entries were at the panicle initiation stage, the time when the plants were at the booting stage.
For the glasshouse set-ups, the plants were initially grown in a nonsaline condition. The pots were placed in a tray filled with tap water or SNAP solution depending on the type of screening method until the appearance of the flag leaf. Once the flag leaf emerged, all other leaves were trimmed except for the penultimate and flag leaf prior to transfer to saline solution (Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013). Salt was applied by dissolving analytical grade NaCl in water to a final electrical conductivity (EC) of 10 dS/m. The EC was maintained and checked every day. After 10 days in the salt solution, spikelets were collected and preserved in 70% ethanol (Sarhadi et al., Reference Sarhadi, Bazargani, Sajise, Abdolahi, Vispo, Arceta, Nejad, Singh and Salekdeh2012). The samples were stored in a refrigerator at 4 °C until analysis. The plants were transferred again to nonsaline condition 10 days after spikelet collection (Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013). The same procedure was done for the nonsaline set-up except that they were transferred in tap water or nonsaline SNAP solution.
On the other hand, the salt required for a particular EC reading was applied to the field set-up. The imposition of stress was gradually starting from EC 4 dS/m then, EC 6 dS/m up to EC 10 dS/m without definite time interval. The salinity was imposed for 20 days starting from the emergence of the flag leaf.
To salinize the test entries in the controlled mini-field set-up, salt was put in a saline tank filled with water. The motor was turned on and the appropriate valve was opened to mix the solution. The water sample was obtained from the tank using a built-in faucet and the EC was read. Once an EC reading of 10 dS/m was attained, a combination of valves was opened to allow the saline water to flow into the plant bed. The EC in the plant bed was also measured to ensure that proper salinity is received by the test entries. The salinity was imposed for 20 days starting the emergence of the flag leaf.
Phenotyping and physiological techniques for salinity tolerance
Different morpho-agronomic and physiological characters were determined to assess the effect of salinity stress in rice genotypes. A novel phenotyping technique for salinity tolerance at the reproductive stage (Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013) with some modifications was used to assess the test entries.
Pollen fertility was analyzed only in the glasshouse set-ups. Spikelets were harvested from individual plants at heading stage after 10 days of salinization and preserved in 70% ethyl alcohol. During analysis, the anthers were extracted from the florets and squashed carefully on a microscope slide to release the pollens. A drop of 1% I2KI solution was added and covered with coverslip. Pollen grains were observed under the microscope at 100× magnification and then classified based on its staining capacity and morphology. Unstained withered, unstained spherical and partially round pollen grains were scored as sterile while darkly stained and round pollens were scored as fertile. Four microscope fields of view with a minimum of 300 pollen grains were considered for the analysis. The average pollen fertility was determined by dividing the fertile pollens with the total number of pollen counted.
Grain yield components and spikelet fertility were determined by selecting five plants. The gathered data were reported as average. Plant height (m) was measured from the soil surface up to the tip of longest rice panicle during harvest while panicle length (cm) was measured starting from the pulvinus up to the topmost grain using a ruler. On the other hand, spikelet fertility was determined by separating unfilled grains from filled grains using a seed separator. The number of filled and unfilled spikelets was recorded and the percentage of spikelet fertility was computed by dividing the number of filled spikelets with the total number of spikelets counted.
After harvest, the panicle was threshed, cleaned, oven-dried, and weighed. The moisture content of the grains was determined and the grain weight was adjusted to 14% moisture. Grain yield was reported in terms of grams/plant for the glasshouse set-ups and grams/plot for the field and controlled mini-field set-ups.
To determine the sodium (Na+): potassium (K+) ratio, three flag leaves were collected from the test entries in the glasshouse after 20 days of salinization (Ahmadizadeh et al., Reference Ahmadizadeh, Vispo, Palao, Pangaan, Dela Viña and Singh2016). The leaves were placed separately in paper envelopes and stored in a refrigerator. During analysis, the leaves were divided into three sections. Small portions of the leaf blade (~13 mm) from the base, middle lamina and the tip were placed in screw-capped tubes and digested by adding ~10 ml acetic acid. The samples were placed in a water bath at 90°C for 2 h. After digestion, the flag leaf samples were oven-dried for 3 days at 37°C and weighed to estimate the sodium and potassium concentration. To determine the ion concentration, the samples were diluted 10−1 and the absorbance was read in flame spectrophotometer. The ion concentration was determined using the modified formula given by Palao et al. (Reference Palao, Dela Viña, Gregorio and Singh2013): concentration of Na+ or K+ (mmol/g dry weight) = {(flame photometer reading × dilution factor)/molecular weight of Na or K}/dry weight of the sample.
Single nucleotide polymorphisms (SNP) genotyping of rice genotypes
Genomic DNA was extracted from the composite samples of young green leaf tissues from 3 to 5 plants of each genotype. DNA was extracted following the mini-preparation modified cetyl trimethyl ammonium bromide method. The young leaf samples were freeze-dried for 3 days, cut into small pieces using a sterilized scissors and placed in 2.0 ml microcentrifuge tubes. The samples were homogenized by adding 800 µl of pre-heated extraction buffer, mixed well by vortexing and incubated in a convection oven (65°C) for 1 h. After incubation, 800 µl of chloroform:isoamyl alcohol mix (24:1) was added and then mixed by putting the tubes in a shaker for 10 min. The samples were centrifuged for 10 min at 10,000 rpm. After centrifugation, 500 µl of the upper aqueous layer was transferred into a 1.5 ml microcentrifuge tube. Cold isopropanol was added in 1:1 volume ratio and then mixed by inverting the tubes. The tubes were left overnight in a freezer at −20°C. The samples were centrifuged for 10 min at 10,000 rpm and the isopropanol was taken out using a pipettor. Ethanol (70%) was added to wash the pellet and then centrifuged for 5 min at 10,000 rpm. The ethanol was removed and the pellet was dried by inverting the tube on a paper towel. The pellet was eluted by adding 100–200 µL of 1× TE, 1 µl of RNase was added and then incubated at 37°C for 30 min. The DNA samples were stored in a refrigerator at 4°C until further use. The quality of the isolated DNA was checked by running on 1% agarose gel while the concentration was determined using Nanodrop. The DNA samples were sent to IRRI-Genotyping Services Laboratory (GSL) for SNP genotyping using 6 K infinium chip.
Salinity stress indices and statistical analysis
Different tolerance indices were used to evaluate the capacity of each screening method to differentiate tolerant and susceptible rice genotypes. These indices include stress susceptibility index (SSI) (Fischer and Maurer, Reference Fischer and Maurer1978), stress tolerance (TOL) (Fernandez, Reference Fernandez and Kuo1993), mean productivity (MP) (Rosielle and Hamblin, Reference Rosielle and Hamblin1981), geometric mean productivity (GMP) (Rosielle and Hamblin, Reference Rosielle and Hamblin1981), stress tolerance index (STI) (Fernandez, Reference Fernandez and Kuo1993; Kristin et al., Reference Kristin, Serna, Perez, Enriquez and Gallegos1997), yield index (YI) (Bouslama and Schapaugh, Reference Bouslama and Schapaugh1984) and yield stability index (YSI) (Gavuzzi et al., Reference Gavuzzi, Rizza, Palumbo, Campanile, Ricciardi and Borghi1997). For each genotype, these indices were calculated manually using Microsoft Excel.
Where appropriate, all of the results were tested statistically for differences or correlations. The response of the rice genotypes in nonsaline versus saline treatments was compared in terms of per cent reduction. Principal component analysis (PCA) was used to detect underlying sources of variability using tolerance indices values while cluster analysis was used to investigate the groupings of rice genotypes based on SNP and yield data. All the statistical parameters were analyzed using STAR Version 2.0.1, PBTools Version 1.4 and SPSS Version 16.0.
Genetic diversity analysis
The resulting SNP calls were re-formatted for subsequent data analysis for SNP visualization using Flapjack graphical genotyping software. Map and genotype data files were imported in the software to create a distance matrix for cluster analysis. The map file provides details on the chromosomes and markers while the genotype file contains the list of rice genotypes with allele data per marker. On the other hand, the heterozygosity, gene diversity, major allele frequency and polymorphic information content were analyzed using PowerMarker Version 3.25 using the default settings. Moreover, DARwin software Version 6 was used to make dendrograms for each screening method using the yield data.
Results
Morpho-physiological response of rice genotypes to salinity
Generally, the effect of salinity to the morphology of rice genotype was leaf tip burning, chaffy panicles, sterile spikelets, drying of leaves and eventually death of some genotypes. Fig. 1 shows the morphology of some rice genotypes after salinization. For plant height, the general response of rice genotypes to salinity is stunted growth and reduced plant vigour. A significant variation in plant height was observed among different genotypes in response to salinity using different screening methods. However, the difference in plant height between nonsaline and saline conditions in controlled mini-field, field and gravel-based hydroponics methods is not statistically significant (P > 0.05) but statistically significant in soil method (P = 0.0294). In the gravel-based hydroponics method, the highest per cent reduction (59.1) was recorded in TKM6 while the lowest (0.2) was recorded in Mushkan 41 (online Supplementary Table S2). On the other hand, Soc Nau and PR30244-AC-V19 showed the highest (37.2) and lowest (0.7) per cent reduction in the soil method, respectively (online Supplementary Table S3). The genotypes Hasawi (16817) and Cheriviruppu showed the highest (21.9) and lowest (0) percent reduction in controlled mini-field (online Supplementary Table S4) while in the field method, <5% reduction was recorded in genotypes Damodar, IR 107321-1-141-3-120, IR 29 (Salinity selection), IR 4630-22-2-5-1-3, IR 84675-58-4-1-B-B, IRRI 170 and Mushkan 41 (online Supplementary Table S5). Analysis of variance showed that the response of rice genotypes in different screening methods under nonsaline condition was statistically significant (P = 0.0012) but not under saline condition (P = 0.0922). However, regardless of whether the plants are grown in nonsaline or saline condition, there was a significant interaction (P < 0.01) between the screening method and genotype (Table 1 and 2).

Fig. 1. Morphological changes in rice genotypes after salinization. (a) leaf tip burning/drying of leaves (b) sterile spikelets and (c) papery florets.
Table 1. Analysis of variance of morpho-agronomic and physiological traits among 50 rice genotypes, Oryza sativa L. grown in nonsaline condition using different screening methods

*Significant at 5% level (P < 0.05) and **highly significant at 1% level (P < 0.01).
Table 2. Analysis of variance of morpho-agronomic and physiological traits among 50 rice genotypes, Oryza sativa L. grown in saline condition using different screening methods

*Significant at 5% level (P < 0.05) and **highly significant at 1% level (P < 0.01).
The pollen fertility of rice genotypes was reduced significantly by salinity both for the soil and gravel-based hydroponics methods (P < 0.01). Furthermore, salinity reduced the percentage of fertile pollen grains among rice genotypes from 0.6 to 45.0% in the gravel-based hydroponics method (online Supplementary Table S2) and 1.3–56.2% for the soil method (online Supplementary Table S3). In the soil method, the highest per cent reduction (56.2) in pollen fertility was recorded in BPI RI 2 while the lowest (1.3) was recorded in genotype IR10G107. On the other hand, the genotypes IR 29 and Swarna showed the highest (45.0) and lowest (0.6) per cent reduction in the gravel-based hydroponics method, respectively. For both screening methods, majority of the rice genotypes exhibited higher percentage of pollen fertility relative to the sensitive genotype IR 29. Analysis of variance further supports a significant difference between the screening method and pollen fertility under saline condition (P = 0.0277) but not in nonsaline condition (P = 0.3374). There was a significant interaction (P = 0.0001) observed between genotype and screening method in the nonsaline condition (Table 1) while no interaction (P = 0.1410) under saline condition.
The Na+ and K+ ion concentrations were highly variable with the intensity being highly dependent on genotypes. Comparison of means between nonsaline and saline condition showed that the Na+ concentration increased significantly because of salinity (P < 0.01) both in soil and gravel-based hydroponics methods. In contrast, the K+ ion concentration was not significantly different between nonsaline and saline condition both for the gravel-based hydroponics (P = 0.1589) and soil (P = 0.0969) methods. For the gravel-based hydroponics method, the proven salt-tolerant landrace Nona Bokra showed the lowest Na+:K+ ratio in nonsaline (0.068) and saline (0.080) conditions (online Supplementary Table S2). On the other hand, the genotype Mushkan 41 was able to maintain the lowest Na+:K+ ratio both in nonsaline (0.079) and saline (0.262) conditions for the soil method (online Supplementary Table S3). In addition, the high value of Na+:K+ ratio in IR 29 reconfirmed its proven sensitivity to salinity stress. Analysis of variance showed that the difference in Na+ and K+ ion concentrations under nonsaline and saline conditions both in the gravel-based hydroponics and soil methods were not statistically significant (P > 0.05). Analysis of variance further showed that Na+ ion concentration exhibited no significant interaction (P = 0.2697) between screening method and genotype under nonsaline condition while there is a significant interaction (P = 0.0360) under saline condition. No significant interaction was observed for K+ concentration between genotype and screening method both for nonsaline (P = 0.9309) and saline (P = 0.3016) conditions (Table 1 and 2).
The average number of filled grains was significantly reduced by salinity (P < 0.01) while the average number of unfilled grains was increased significantly (P < 0.01) both in soil and gravel-based hydroponics methods. On the other hand, the difference in the unfilled grains was not statistically significant (P = 0.4754) between saline and nonsaline conditions in the field method. In the gravel-based hydroponics method, the genotype Damodar showed the lowest per cent reduction (7.2) in spikelet fertility while the highest per cent reduction (99.6) was recorded in genotype Nona Bokra (online Supplementary Table S2). In the soil method, the genotypes PR28377-AC97-54 and PR28378-AC96-36 exhibited low reduction in spikelet fertility at a minimum of 10% (online Supplementary Table S3). The highest and the lowest per cent reduction was observed in genotype FL449 (100%) and IRRI 165 (6.1%), respectively. In the controlled mini-field set-up, the genotype IR 4630-22-2-5-1-3 exhibited the lowest per cent reduction (0.6) in spikelet fertility while Nona Bokra exhibited the highest (77.7). Moreover, majority of the genotypes exhibited percent reduction at a minimum of 20% (online Supplementary Table S4). In the field set-up, the genotypes that reached the reproductive stage showed 21–100% reduction in spikelet fertility. The genotypes Cheriviruppu, IRRI 147, IRRI 170 and PR28377-AC97-54 showed per cent reduction of <50% (online Supplementary Table S5).
Under the nonsaline gravel-based hydroponics method, the highest grain yield (44.0) was recorded in Hasawi (121913) while the lowest (0.28) was recorded in Kajalsail. However, under saline condition, the highest yield (22.84) was recorded in Bhura Rata 4-10 while the lowest (0.09) was recorded in Kinandang Patong (online Supplementary Table S2). The lowest per cent reduction was observed in Damodar (9.5). On the other hand, the genotype Jumbo jet and BPI RI 2 exhibited the highest (43.08) and lowest (0.42) grain yield in the soil method under nonsaline condition, respectively. For the saline condition, PR30244-AC-V19 showed the highest grain yield (8.73) while Dharga Sail showed the lowest (0.01) (online Supplementary Table S3). The lowest per cent reduction was recorded in FL478 (11.2%) while the highest was recorded in Dharga Sail.
Under nonsaline controlled mini-field set-up, the highest grain yield was recorded in Kajalsail (197.55) while the lowest was in Kinandang Patong (3.52). Under the saline condition, the highest yield (136.5) was recorded in Bhura Rata 4-10 and the lowest (0.45) was recorded in Nonabokra (online Supplementary Table S4). However, the lowest per cent reduction was recorded in Damodar (0.06%). On the other hand, the genotypes PR25997-B-B-B and Kinandang Patong showed the highest (96.59) and lowest (0.04) grain yield, respectively, in the field set-up under nonsaline condition. While the genotype PR30244-AC-V19 showed the highest grain yield (23.41) under saline condition (online Supplementary Table S5). The lowest per cent reduction was recorded in Cheriviruppu (7.99).
Salinity reduced the total yield of rice genotypes regardless of the screening method used (P < 0.01). However, analysis of variance showed that the difference in yield under nonsaline condition (Table 1) between different screening methods is not statistically significant (P = 0.9765) but statistically significant (P = 0.0207) under saline condition (Table 2). A statistically significant interaction was also observed between screening method and genotype under nonsaline (P = 0.0028) and saline (P < 0.01) conditions. In terms of filled grains per panicle, a statistically significant difference was observed among rice genotypes under nonsaline condition using different screening methods (P = 0.0007). Analysis of variance also showed a statistically significant interaction between genotype and screening method (P < 0.01). Variable response in terms of average unfilled grains per panicle was observed among genotypes using different screening methods under nonsaline condition (P = 0.0005) while no significant difference was observed in saline condition (P = 0.0702). Statistical analysis further showed a significant interaction between genotypes and screening method under nonsaline (P < 0.01) and saline (P = 0.0010) conditions (Table 1 and 2).
In terms of panicle length, a significant reduction was observed in most of the genotypes using different screening techniques except for the field method (P = 0.1983). In the gravel-based hydroponics method, the notable genotypes that exhibited <10% reduction in panicle length include the landraces Bhura Rata 4-10, Kajalsail, Kalarata 1–24, Kinandang Patong, Pokkali (108921), Sadri and TKM 6 (online Supplementary Table S2). Majority of the landraces in the soil set-up such as Anbarloo Sadri, Damodar, Dharga Sail, Hasawi (16817), Kalarata 1–24 and Samba Mahsuri showed <10% reduction (online Supplementary Table S3). In the controlled mini-field set-up, majority of the rice genotypes showed <10% reduction in panicle length except for the genotypes Hasawi (16817), Damodar, Kajalsail, Hasawi (121913), FL478 and IRRI 170 which showed per cent reduction ranging from 10.7 to 14.5% (online Supplementary Table S4). Contrastingly, no definite trend was observed among genotypes in the field set-up. Analysis of variance showed that the response of rice genotypes was statistically significant (P = 0.0482) under nonsaline condition but not significant in saline condition (P = 0.5123). However, a significant interaction between genotype and screening method was observed both in nonsaline (P = 0.0002) and saline (P = 0.0004) conditions (Table 1 and 2).
Cluster analysis using yield and SNP data
The yield data under saline condition were used to compare the response of rice genotypes to salinity. For each screening method, different clustering of rice genotypes was observed which indicate their relatively different performance under saline condition. This observation further indicates that each screening method has the capacity to distinguish tolerant and sensitive genotypes but they do not do so similarly. The clustering of the genotypes using SNP data indicates a significant relatedness among rice genotypes and the correspondence between their genetic make-up and their response to salinity. For example, the majority of the salt-tolerant landraces such as Cheriviruppu and Pokkali are found on the same cluster. The same trend was observed for salt-sensitive genotypes such as IR 29 and IR 29 (Salinity selection). The maximum inter-genotypic similarity (0.999) was observed between Pokkali (117275) and Pokkali (108921) and PR25997-B-B-B and PR30244-AC-V2 rice genotypes while the minimum similarity (0.314) was observed between Mushkan 41 and IR 64. The 50 rice genotypes were grouped into four distinct clusters at a minimum distance of 1.0 and consisted of several sub-clusters. The number of rice genotypes in these four clusters were 17, 25, 4 and 4 for clusters I, II, III, and IV, respectively (Fig. 2). Interestingly, clusters III and IV emerged as the most genotypically distinct cultivars since they formed a separate cluster apart from the rest.

Fig. 2. Cluster dendrogram of the 50 genotypes of rice, Oryza sativa generated by centroid hierarchical clustering. The similarity matrix was obtained from SNP data using Euclidean distance.
Genetic diversity and heritability estimate of salinity tolerance
From the original 4606 SNP markers, 2970 were informative (polymorphic). The result showed that the average number of alleles ranged from two to three with a mean value of 2.5148. Analysis of SNPs among 50 rice genotypes indicates that the population has moderate genetic diversity as indicated by polymorphism information content (PIC) value (![]() ${\bar{\rm x}}^\circ $ = 0.2960) and gene diversity value (
${\bar{\rm x}}^\circ $ = 0.2960) and gene diversity value (![]() ${\bar{\rm x}}^\circ $ = 0.3548) (Table 3). The most informative markers to evaluate genetic differences in the rice germplasm are 18852690 and 7825479 with PIC values 0.5923 and 0.5906, respectively. Moreover, the results indicate that the rice germplasm we evaluated has low heterozygosity (
${\bar{\rm x}}^\circ $ = 0.3548) (Table 3). The most informative markers to evaluate genetic differences in the rice germplasm are 18852690 and 7825479 with PIC values 0.5923 and 0.5906, respectively. Moreover, the results indicate that the rice germplasm we evaluated has low heterozygosity (![]() ${\bar{\rm x}}^\circ $ = 0.0310) and high frequency of major allele (
${\bar{\rm x}}^\circ $ = 0.0310) and high frequency of major allele (![]() ${\bar{\rm x}}^\circ $ = 0.7416).
${\bar{\rm x}}^\circ $ = 0.7416).
Table 3. Summary of the results of genetic diversity analysis among 50 genotypes of rice, Oryza sativa L. using SNP markers

a >0.5-high; 0.25–0.5-moderate; <0.25-low.
The heritability of the studied traits was estimated using PBTools software which estimates the broad sense heritability. The heritability estimates ranged from 0 to 0.97 under the nonsaline condition and 0.23–0.95 under saline condition which indicate low to high heritability (Table 4). The heritability of K+ and Na+ ion concentrations increased under saline condition regardless of the screening method used. In contrast, decreased in heritability estimate for filled grain, pollen fertility, plant height, panicle length, unfilled grains and yield were observed under saline condition regardless of the screening method used except for the panicle length and yield in the gravel-based hydroponics method and filled grain in the field method. Under saline condition, filled grain exhibited the highest value (0.50) which indicates that the trait is moderately heritable. Interestingly, the trait K+ ion concentration has low heritability under nonsaline condition but moderate heritability under saline condition. Na+ ion concentration showed low to moderate heritability in the nonsaline condition of soil (0) and gravel-based hydroponics (0.36) methods but high heritability in saline condition. The traits plant height, panicle length, unfilled grains and yield showed high heritability under nonsaline condition regardless of the screening method used. Similarly, these traits also exhibited high heritability in saline condition except for panicle length and unfilled grains in the field method and yield in the soil method.
Table 4. Heritability estimate of morpho-agronomic and physiological characters among 50 genotypes of rice, Oryza sativa L. grown under nonsaline and saline conditions using different screening methods

a Not determined.
b Not determined because of missing entries/values.
>0.50-high; 0.20–0.50-moderate; <0.20-low.
Salinity tolerance indices
We used several tolerance indices to determine the susceptibility and tolerance of rice genotypes to salinity. To compute for these tolerance indices, the yield both in the saline (Ys) and nonsaline (Yp) conditions is needed. Some genotypes died before reaching maturity while some have problems in panicle exertion. Low tolerance index (TOL) and SSI and high MP, GMP, STI, YI and YSI indicate salinity tolerance.
In the gravel-based hydroponics method (online Supplementary Table S6), the genotype Bhura Rata 4-10 consistently showed the highest value for MP (25.928), GMP (25.744) and STI (3.552). On the other hand, the genotypes Damodar (0.144) and IR 29 (Salinity selection) (0.145) showed the lowest value for SSI and the highest value (0.905) for YSI. The tolerant genotype Pokkali (26869) showed the highest value (38.345) for TOL while the genotype Kajalsail consistently showed the lowest value for TOL (0.316), MP (0.208), GMP (0.197) and STI (0). For YI, the genotype FL478 showed the highest value (4.920) while the lowest was recorded in Kinandang Patong (0.020). Lastly, the lowest value for YSI was recorded in Nonabokra (0.024).
A different trend was observed for the soil method (online Supplementary Table S6). For example, the genotype Dharga Sail showed the highest (1.200) and lowest (0.006) value for SSI and YI, respectively, while the genotype Jumbo jet showed the highest value for TOL (42.083) and MP (22.034). The genotype FL478 showed the lowest (0.134) and highest (0.888) value for SSI and YSI, respectively. While Samba Mahsuri consistently showed the lowest value for MP (0.655), GMP (0.297) and STI (0). It is also interesting to note that the improved cultivar PR30244-AC-V19 showed the highest values for GMP (11.503), STI (0.726) and YI (3.861) while IRRI 169 (0.189) and FL449 (0.001) showed the lowest value for TOL and YSI, respectively.
For the controlled mini-field set-up (online Supplementary Table S7), the genotype Damodar showed the lowest value for SSI (0.001) and TOL (0.071) while the highest value for YSI (0.999). On the other hand, the genotype Bhura Rata 4-10 consistently showed the highest value for MP (142.300), GMP (142.181), STI (3.081) and YI (3.242). The landrace Nonabokra showed the lowest value for YI (0.011) and YSI (0.002) while the highest value for SSI (2.078). The genotype Kinandang Patong consistently showed the lowest value for MP (3.192), GMP (3.175) and STI (0.002) while Kajalsail showed the highest value for TOL (196.283).
The field set-up showed highly variable values for the salinity tolerance indices (online Supplementary Table S7). Negative values were also recorded which indicate that some of the entries in the saline condition performed better than the nonsaline condition in terms of the total yield. This result is postulated to be due to heterogeneity of the environment in the field set-up. Nevertheless, the genotype TKM6 showed the lowest value (0.148) in SSI while the genotypes Hasawi (16817), Kinandang Patong and A 69-1 showed the highest value (1.232). Moreover, Kinandang Patong showed the lowest value for TOL (0.040) and MP (0.020) while PR25997-B-B-B showed the highest value for TOL (83.035) and MP (55.076). For GMP, the highest (44.071) and lowest value (0.000) was recorded in genotypes PR30244-AC-V19 and Kinandang Patong, respectively. The genotypes A69-1, Bhura Rata 4-10, BPI RI 2, Hasawi (16817), Kinandang Patong and TKM 6 showed the lowest value (0) for STI while the genotype PR30244-AC-V19 showed the highest value (3.656). For YI, the lowest value (0) was recorded in A 69-1, Hasawi (16817) and Kinandang Patong while the highest value (5.393) was recorded in PR30244-AC-V19. The genotypes A 69-1, Hasawi (16817) and Kinandang Patong also showed the lowest value (0) for YSI while Mushkan 41 showed the highest value (17.738).
Correlation coefficients between different salinity tolerance indices indicate a significant relationship (P < 0.01) between SSI and YSI, TOL and MP, MP and GMP, GMP and STI, GMP and YI; and STI and YI regardless of screening method used. Furthermore, yield under nonsaline condition (Yp) exhibited a significant positive relationship with TOL and MP while yield under saline condition (Ys) exhibited a significant positive relationship with GMP, STI and YI (P < 0.01). Similarly, significant positive relationships between SSI and TOL and YI and YSI while significant negative relationships between SSI and YI and TOL and YSI were observed in all screening techniques except for the field method. Lastly, SSI and YSI exhibited perfect negative correlation (r = −1.0000) for all the screening methods while GMP and YI exhibited a strong positive relationship (r = 0.9572).
PCA of salinity tolerance indices
For all the screening methods, PCA grouped the rice germplasm into two main components using the tolerance indices values (online Supplementary Fig. S1). For the controlled mini-field method, the first two components with eigenvalues >1 accounted for 95.51% of the total variation in grain yield. The first principal component (PC1) explained >50% of the total variation while the second principal component (PC2) explained nearly one-third of the total variation. The first two components with eigenvalues >1 accounted for about 89.19% of the total variation in grain yield in the field set-up. The PC1 explained 66.6% of the total variation while PC2 explained 22.58% of the total variation. The total variation in grain yield for the gravel-based hydroponics method can be explained by two components with eigenvalues >1. The two components contributed to nearly 95% of the total variation. The PC1 and PC2 explained 64.16% and 30.70% of the total variation, respectively. Lastly, the two PCs with eigenvalues >1 explained 92.49% of the total variation in grain yield for the soil method. Moreover, the first component explained 53.22% of the total variation while the second component explained 39.27%.
Comparison of different screening methods
The results of this study suggest a strong genotype × screening method interaction as indicated by highly variable responses of rice genotypes to different screening methods. Different performance among rice genotypes were observed in terms of yield, yield contributing characters and morpho-physiological characters. However, it is notable that the rice genotypes exhibited maximum yield potential when grown in controlled mini-field set-up either in saline or nonsaline conditions. In terms of ease of use, the gravel-based hydroponics method is the most labour intensive and costly. Contrastingly, the soil method is not costly because it does not require nutrient solution to grow the plants. The controlled mini-field set-up is costly at first because of the construction of the concrete plant beds and water tanks while the field method is the least expensive among the four methods. The soil and gravel-based hydroponics method have the highest reproducibility. Because of other factors in the environment, the controlled mini-field and the field methods have low reproducibility.
Some problems were encountered during the conduct of the experiment. For example, the plants in the glasshouse were repeatedly infested by insects starting from flag leaf stage up to harvest. The plants in the gravel-based hydroponics method showed symptoms of iron deficiency at the early vegetative stage. Moreover, some of the landraces showed problems in panicle exertion and photoperiod sensitivity.
Discussion
Salinity causes certain morphological changes in rice. The result of this study is consistent with the published literature (Singh et al., Reference Singh, Redoña, Refuerzo, Pareek, Sopory, Bohnert and Govindjee2010; Palao et al., Reference Palao, Dela Viña, Gregorio and Singh2013). In terms of plant height, salinity caused the rice genotypes to become stunted. According to Munns et al. (Reference Munns, Greenway, Delane and Gibbs2002), salt stress decreases growth in most plants which limits its ability to produce their maximum biomass. As a result, plants tend to be stunted because of disturbance in the uptake of water and nutrients. Rubel et al. (Reference Rubel, Hassan, Islam, Robin and Alam2014) also observed that salinity significantly reduced plant height from 4.3 to 20.6%. Pollen fertility was reduced by salinity, particularly in sensitive genotypes. Salinity hampers the synthesis of starch in the pollen grains as indicated by the lightly stained pollen grains. Reduction in pollen viability and stigma receptivity are highly correlated to increased Na+ concentration in floral parts (Khatun and Flowers, Reference Khatun and Flowers1995). Maintaining ion homeostasis by uptake and compartmentalization is not only crucial for normal plant growth but also an essential process for growth during salt stress (Hasegawa, Reference Hasegawa2013). Salinity stress increased the Na+ and K+ ion concentrations in the flag leaf which led to elevated Na+:K+ ratio. Abdullah et al. (Reference Abdullah, Khan and Flowers2001) argued that the Na+:K+ ratio under high salt concentration tends to increase in the floral parts and leaves of rice plant at the reproductive stage. However, K+ ion concentration is not significantly different between nonsaline and saline conditions. This observation suggests that K+ sequestration is somehow not much affected by salinity as Na+ uptake. In rice, Na+ uptake is mechanistically different from K+ uptake (Garcia et al., Reference Garcia, Rizzo, Ud-Din, Barto, Senadhira, Flowers and Yeo1997). The ability of the genotypes Mushkan 41 and Nona Bokra to maintain low Na+:K+ ratio is significant in finding donor cultivars that can be used for improvement of salinity tolerance through low Na+:K+ ratio. Joseph et al. (Reference Joseph, Jini and Sujatha2010) emphasized that breeding for low ion accumulation could be a simple way to improve salt tolerance. Grain yield and yield contributing characters are very important parameters at the reproductive stage. Reduction in different yield and yield parameters were also observed in rice genotypes grown in saline condition regardless of screening method used. Salinity affects the ability of rice genotypes to produce fertile spikelet which often results to reduced number of filled grains. Lower grain yield is the result of the inability of the pollen tube to germinate during fertilization. Salinity affects this process biochemically by hampering the translocation of soluble carbohydrates into the spikelet due to reduced starch condensing enzyme activity (Abdullah et al., Reference Abdullah, Khan and Flowers2001; Gul and Ahmad, Reference Gul and Ahmad2006). Yield contributing characters, e.g. panicle length was also reduced by salinity. Panicle length of rice is adversely affected by both saline and saline-sodic soils (Aslam et al., Reference Aslam, QureshI and Ahmed2003). Some of the landraces even though known to exhibit salinity tolerance at the seedling stage exhibited susceptibility at the reproductive stage. Tolerance during the seedling and reproductive stages seems to be poorly correlated, suggesting that different sets of salt tolerance mechanisms are involved (Moradi et al., Reference Moradi, Ismail, Egdane and Gregorio2003; Singh et al., Reference Singh, Redoña, Refuerzo, Pareek, Sopory, Bohnert and Govindjee2010).
The hierarchical clustering of rice genotypes using SNP data proved to be useful in showing internal (within cluster) homogeneity and external (between clusters) heterogeneity of the genetic make-up among cultivars. However, this clustering does not indicate the overall performance of the rice genotypes to salinity as indicated by their response to different parameters evaluated. For example, BPI RI2 although known to exhibit a very high tolerance to salinity at seedling stage (Platten et al., Reference Platten, Egdane and Ismail2013) is found to be salt-sensitive due to high per cent reduction both in pollen viability and spikelet fertility. Furthermore, cluster analysis using yield and SNP data indicates a highly different clustering among rice genotypes. This clustering at some point signifies a non-correspondence between the genetic make-up of the rice genotypes and their performance to salinity stress. The same observation was deduced by De Leon et al. (Reference De Leon, Linscombe, Gregorio and Subudh2015) in an attempt to determine the genetic relationship among 49 rice genotypes based on salinity stress responses. They reported that the grouping of tolerant Pokkali and susceptible IR29 in one cluster indicated that genetic profiling based on the SSR markers spanning the 12 chromosomes of rice cannot explain the varietal grouping based on salinity responses. Genetic diversity reflects the genetic variability amongst individuals or populations within a variety or species (Razak et al., Reference Razak, Ismai, Jaafar, Yusof, Kamaruzaman, Rahman, Nasir and Abdullah2016). The analyses of genetic diversity and structure are helpful for management, research and utilization of plant germplasm. The reported average PIC value in this study is similar to Singh et al. (Reference Singh, Choudhury, Singh, Kumar and Srinivasan2013) wherein a PIC value of 0.25 was reported using SNP markers in 375 Indian rice varieties. Rice is primarily a self-pollinated species but also exhibit outcrossing, therefore, low heterozygosity value is expected. In an attempt to develop a core rice germplasm set, Choudhury et al. (Reference Choudhury, Singh, Singh, Kumar, Srinivasan, Tyagi, Altaf Ahma, Singh and Singh2014) analyzed the genetic diversity of 6984 rice accessions originating from North-eastern region of India. They reported 0.26–0.34 for average gene diversity, 0.07–0.16 for average heterozygosity and 0.74–0.8 for average major allele frequency. The result of this study is within the range of what they have reported. Heritability estimates and genetic advance are significantly important to breeders for selection of salinity tolerance related traits that are highly heritable. High variability in the heritability estimates of the traits we examined using different screening methods conformed to the results of Betrán et al. (Reference Betrán, Moreno-González, Romagosa, Ceccarelli, Guimarães and Weltzien2009) who reported that heritability of a particular trait depends on several factors which include: environment, the reference population, and the sample of genotypes evaluated. Nevertheless, the result of the experiment is valuable in future breeding program involving salinity tolerance.
The over-all results clearly indicate that tolerance indices are indeed good measure to discriminate the tolerance and sensitivity of rice genotypes to salinity stress. Among these indices, GMP, STI and YI showed the consistent result in different screening methods suggesting that they are better predictors of salinity tolerance compared to others. The controlled mini-field method is able to discriminate tolerant and susceptible genotypes compared to other methods. This is supported by a negative or positive significant relationship observed for all the tolerance indices with the yield under saline and nonsaline conditions.
The variability in the performance of the rice genotypes in different screening methods indicates a genotype × screening method interaction. Screening methods do exert the selection pressure on rice genotypes to distinguish between tolerant and sensitive but they do not do so similarly. This is the reason why the genotypes exhibited variation in performance from one screening method to another. However, the screening methods could make a distinction between the proven tolerant and proven sensitive genotypes more or less uniformly. Each method has its own advantages and disadvantages which imposed limitation for its utilization. Though screening techniques vary with crop species, growth stage and type of stress imposed, ideally it should be rapid, reproducible, easy and affordable (Amaranatha et al., Reference Amaranatha, Francies, Nabi Rasool and Prakash Reddy2014; Reddy et al., Reference Reddy, Kim, Yoon, Kim and Kwon2017). Glass house screening is significant because it accounts for the correct timing of imposition of stress while it may not be uniform in the controlled mini-field and field set-ups due to differences in the timing of flowering. However, some of the landraces grown in the glasshouse set-up exhibited photosensitivity and problems with panicle exertion. Das et al. (Reference Das, Nutan, Singla-Pareek and Pareek2015) proposed that the wild types or the landraces are connected with a host of innate difficulties of reduced agronomic characters like photo-sensitivity, tallness, low yield and poor grain quality. Soil method is advantageous over the gravel-based hydroponics method because it is easy to use and less expensive. The least effective method is the field method primarily because of environmental heterogeneity. In the field, different types of stresses aside from the imposed salinity stress occurred simultaneously. Screening in the field is also difficult because of significant influence of environmental factors such as temperature relative humidity and solar radiation (Ali et al., Reference Ali, Aslam, Awan, Hussain and Cheema2004). Nevertheless, the controlled mini-field set-up is relatively advantageous among the screening methods. However, the reproducibility and the correct timing of salinization should be optimized if this method will be adapted in the future.
Among the rice genotypes, the majority of the landraces showed promising results which open new interest for future breeding programs. The genotypes Mushkan 41 and Nona Bokra are significant for developing salt-tolerant varieties through low Na+:K+ ratio. However, it was observed that these genotypes exhibit strong photosensitivity, problems in panicle exertion and are poor yielders. On the other hand, the genotypes Damodar and Bhura Rata 4-10 are valuable for breeding salt-tolerant cultivars with high yield potentials.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262119000364
Acknowledgements
This work was conducted under the research grant from the International Rice Research Institute (IRRI), Agreement No. A-2012-180/33-233-11957. The concerned staffs and researchers in IRRI-Salinity Group are also gratefully acknowledged for their help and support during data collection. The major author is also grateful to the support from Southern Luzon State University-Faculty and Staff and Development Program.
Conflict of interest
The authors declared no conflict of interest or any financial interest or benefit that has arisen from the direct applications of this research.