INTRODUCTION
The free-living amoeba and human pathogen Naegleria fowleri occurs worldwide in soil and water. It has been identified as the aetiological agent of primary amoebic meningoencephalitis (PAM). PAM is a rare but fulminating disease of the central nervous system (CNS), and is fatal in the great majority of the cases (Barnett et al. 1996; Martinez and Visvesvara, 1997). Infection occurs via the nasal cavity upon inhalation or aspiration of contaminated water. In the course of the disease, the pathogen reaches the CNS via migration from the nasal submucosa along the olfactory nerves inducing a massive inflammatory response, associated with haemorrhage and tissue necrosis (Kuroki et al. 1998; Jarolim et al. 2000; Jarolim, McCosh and Howard, 2002).
Both in vivo and in vitro models have been used to study the pathogenesis of PAM due to infection with N. fowleri. Since rodents and humans are suggested to share a common anatomical or physiological determinant that makes them susceptible to infection with N. fowleri, in vivo models used thus far have been standardly based on mice infected by intranasal inoculation (John and Hoppe, 1990; Rojas-Hernandez et al. 2004). A broad range of pathogen-related aspects such as migration pathways (Jarolim et al. 2000; Rojas-Hernandez et al. 2004), changes in morphology (Ryu, Soh and Im, 1984), and changes in pathogenicity and virulence that depend on the environment (John and Howard, 1993; John and John, 1994; Toney and Marciano-Cabral, 1994; Bradley et al. 1996) and on the host immunity (Im and Lee, 1985) have been investigated in vivo. However, animal models depend on the availability of specific infrastructural facilities, are time-consuming and costly. Thus, more simple alternatives may offer some advantages, especially for first or preliminary experimental approaches.
In vitro models aimed at studying N. fowleri pathogenicity are based on a broad variety of target cell types including rat neuroblastoma (Fulford and Marciano-Cabral, 1986), Jurkat T cells (Herbst et al. 2002) and Chinese hamster ovary (CHO) cells (Cho et al. 2003). Although in vitro models provide a useful tool to elucidate selected aspects of pathogen-induced cytotoxicity, they lack the complexity of an organ. In the context of CNS pathogenesis, vulnerability to particular forms of cell death has often been shown to be limited to defined regions or certain cells within a specific region, which is the case in e.g. status epilepticus (Becker et al. 1999), excitotoxic lesions (Chen and Strickland, 1997), ischaemia (Nitatori et al. 1995), or in bacterial meningitis (Bifrare et al. 2003; Gianinazzi et al. 2004b). For this reason, in vitro models that retain organ-specific features (e.g. microarchitecture, cell-specific connections) can – for certain questions – offer similar or even higher experimental accuracy than highly complex in vivo models.
In the present study, we report for the first time infection of organotypic slice cultures from rat brain with N. fowleri trophozoites. The culture system has been validated to mirror the situation in vivo, including the development of infection and the induction of tissue damage as criteria. The in vivo rat model of PAM itself was compared to the standard experimental infection in the mouse, with respect to the outcome of disease and brain damage, and was found to yield comparable findings.
MATERIALS AND METHODS
Organotypic slice cultures
The culturing technique used in this study is a modification of the procedure originally published by Stoppini, Buchs and Müller (1991), and the detailed methods have been described elsewhere (Gianinazzi et al. 2004a). Seven-day-old Wistar rat pups were sacrificed by an overdose of pentobarbital (100 mg/kg i.p. Nembutal, Abbott Laboratories, North Chicago, IL, USA). The brain was carefully removed, and a piece of cortical tissue of approximately 7×5 mm was dissected from each brain hemisphere by coronal cuts using a scalpel. The tissue was horizontally aligned on the Teflon plate of a McIlwain tissue chopper (Mickle Laboratory, Guildford, UK), and cut into 400 μm-thick slices. Five slices were then transferred onto the porous (0·4 μm) membrane of one Transwell® insert (Corning Inc., Corning, NY, USA), and the inserts placed into the tissue culture 6-well plate with equilibrated serum-free Neurobasal™ medium (Life Technologies, Basel, Switzerland) supplemented with B27-Supplement (Life Technologies, Basel, Switzerland). The cultures were kept at 37 °C in 5% CO2-enriched atmosphere. On days 1, 4, 7, and 9 the culture medium was replaced with fresh equilibrated medium. Infections were performed on day 12.
Naegleria fowleri cultures
Trophozoites of the pathogenic N. fowleri strain 30863 (ATCC, Manassas, VA, USA) were isolated from the brain of an experimentally infected mouse suffering from PAM, and cultured for 4 days in buffered PYNFH medium containing 1% Bacto Peptone, 1% yeast extract, 0·1% ribonucleic acid, and 15 mg/l folic acid and 1 mg hemin/l medium. In order to inhibit bacterial contaminations, penicillin and streptomycin (100 μg/ml) was added. For infection in vivo and in vitro, the trophozoites were transferred to L929 cell cultures, co-cultured for 4 days at 37 °C, washed in phosphate-buffered saline (PBS), pelleted, and resuspended to the desired concentration.
L929 cultures
The murine fibroblast cell line L929 was grown to confluency in MEM Earle's medium supplemented with 1% L-glutamine, 5% fetal calf serum, 1% non-essential amino acids, and 1% of penicillin and streptomycin to inhibit bacterial contaminations. The cultures were maintained in a volume of 20 ml of MEM Earle's medium (refreshed every other day) at 37 °C in a 5% CO2-enriched atmosphere.
In vitro infection
Three μl of MEM Earle's medium containing 100 or 1000 trophozoites, respectively, were gently pipetted onto the surface of 1 organotypic slice culture each. The cultures were regularly monitored every 12 h by inverse microscopy, and 12, 24 and 72 h after infection, the cultures were gently washed 3× in PBS to remove trophozoites that had not attached to or entered the tissue, fixed in 4% formaldehyde (in PBS) for 1·5 h at 4 °C, and cryo-protected in an 18% sucrose solution (in PBS) for 4 h at 4 °C. Each culture was cut into 14 or 20 μm-thick cryosections using a 1800 cryostat (Leica Microsystems, Glattbrugg, Switzerland) as previously described in detail (Gianinazzi et al. 2004b). The cryosections were then transferred onto chrome-alum-gelatin-coated glass slides, dried for 15 min, and evaluated by immunohistochemistry and Nissl stain. For each concentration and time-point (12 and 72 h, respectively), 1 culture was frozen at −20 °C, and used for polymerase chain reaction (PCR) (see below).
In vivo infection
Two 3-week-old Wistar rats were briefly anaesthetized by inhalation (Isoflurane), and intranasally infected by gently pipetting 5 μl of PBS containing 5×105 trophozoites into each nasal cavity. The suspension was naturally taken into the nasal cavity by capillary action, diffusion and inhalation processes. The animals were placed back into the cage with access to water and food ad libitum. Six and 7 days after infection, respectively, the animals were sacrificed by CO2-inhalation. Animals were sacrificed at the time when clinical signs of infections became apparent (obtundation, ataxy, shivering, ophistotonus). Following necropsy, the brains were carefully removed. One hemisphere of each brain was used for PCR analysis, the other hemisphere was fixed in 4% formaldehyde (in PBS) for 4 days. The fixed tissue was used for sagittal cryosections (20 μm) as described in detail previously (Gianinazzi et al. 2004b). The sections were then transferred onto chrome-alum-gelatin-coated glass slides, dried for 15 min, and evaluated by immunohistochemistry, Nissl and Haematoxylin-Eosin stain, respectively. In order to comparatively confirm suitability of the rat model, a 6-week-old C57/BL6 mouse was infected by using identical conditions as for the rat. The mouse was sacrificed 3 days after infection upon onset of clinical signs. Following necropsy, one sagittal half of the head was processed for histological investigation as described above.
Immunohistochemistry
Sections from organotypic cultures and rat brain sections were incubated with a monoclonal antibody (MAb) directed against N. fowleri trophozoites (Indicia Biotechnology, Oullins, France) at a dilution of 1[ratio ]100 in PBS containing 1·5% bovine serum albumin (BSA) for 1 h at 37 °C. After washing, the sections were incubated with an FITC-labelled secondary goat anti-mouse IgG antibody (1[ratio ]100) (Sigma, Buchs, Switzerland) for 30 min, and the glass slides mounted using Vectashield® mounting medium containing Dapi (Vector Laboratories, Inc., Burlingame, CA). For a negative control, an irrelevant MAb was included in the same procedure as described above.
PCR
DNA was extracted from tissue of organotypic cultures and the cortex of the infected rat was sacrificed on day 6 using the DNAeasy kit (Qiagen, Basel, Switzerland) according to the manufacturer's instructions. For molecular identification of N. fowleri, the protocol published by McLaughlin and co-workers (McLaughlin, Vodkin and Huizinga, 1991) was followed using the following primers (as described by the authors): forward primer (NAEGF1): 5′CGTATCTAGTAGATAGAACA; reverse primer (NAEGF2): 5′CGTAACGACACAAACCTACAGA. The following 3-step cycle was used: 1 min at 94 °C, 1 min at 47 °C, 3 min 72 °C. DNA was amplified for 33 cycles; in the final cycle polymerization occurred for twice the time (6 min). The PCR amplification products were electrophoresed through a 2% agarose gel containing ethidium bromide, and visualized under a UV lamp. This PCR (McLaughlin et al. 1991) had previously been assessed by Tsvetkova and co-workers (Tsvetkova et al. 2004) to demonstrate suitable methodical sensitivity and specificity.
RESULTS
Organotypic slice cultures
After 12 days in culture, non-infected control slices were found to flatten from 400 μm (day 0 in culture) to approximately 160 μm, as assessed by the number of 20 μm-thick cryosections generated per slice culture. The tissue morphology of organotypic slice cultures (Fig. 1B) corresponded well with that of in vivo cortex tissue from uninfected rats (Fig. 1A).

Fig. 1. Documentation of experimental Naegleria fowleri infection in rats (in vivo) and in organotypic cultures slice cultures from rat cortex (in vitro), respectively. Nissl stain (A, B; G–J), a widely used technique to stain neuronal tissue sections, and immunohistochemistry combined with Dapi, a dye used to specifically stain cell nuclei in fluorescent techniques, (D–F; green fluorescence: immunostaining for N. fowleri, blue fluorescence: Dapi;) were used. (A) 20 μm-thick cortex slice of rat brain; (B) Corresponding organotypic slice culture (20 μm) from the same region (after 12 days in culture). The organ-specific features are well preserved in the organotypic tissue cultured in vitro as shown by organization and density of the neuronal cells. The inset in (B) documents a clear preservation of shape and size of the neuronal cells, comparable to those seen in vivo (inset A). Scale bar (A, B): 400 μm. (C) Six days after infection, only a small number of trophozoites (green) was detected within the cortex of the infected rat. (D) Analogously, in organotypic cultures, only single trophozoites were detectable within the cortical tissue at 24 h p.i., exclusively located in proximity of the edge of the slice cultures (arrowheads). (E) In the rat, the trophozoites were present in great abundance on day 7 p.i., suggesting strong parasite multiplication in this region of the brain within the last 24 h. (F) 72 h after infection, the trophozoites had migrated further into the organotypic brain tissue, and were predominantly found in the centre of the slice cultures, where formation of clusters (arrowheads) suggested multiplication of the amoebae analogously to the situation in vivo. Scale bar (C, D, E, F): 400 μm. Negative controls including an irrelevant MAb provided no signal (data not shown). (G) On day 7 p.i. inflammation in the rat brain appeared particularly strong in the anterior region of the olfactory bulb as shown by massive recruitment of neutrophils (arrowheads) into the brain parenchyma. (H) In slice cultures challenged with 1000 trophozoites for 72 h, the brain tissue was completely invaded by the parasite, and only few cells within the tissue could be identified as intact neurons. Scale bar (G, H): 400 μm. I: Typically, within the brain parenchyma, the trophozoites (white arrowheads) were found as clusters, surrounded by infiltrating neutrophils (asterisk) which were recognizable by the a dark blue staining and the small size (black arrowheads). (J) N. fowleri trophozoites (arrowheads) in the process of attaching to and invading an organotypic slice culture. Scale bar (I, J): 100 μm.
Infection of organotypic slice cultures
Infection of 12-day-old organotypic cortex cultures from rat with N. fowleri resulted in colonization of the tissue, followed by trophozoite multiplication within this tissue, which finally resulted in a severe damage of the cultures. Trophozoites were detected by PCR within the tissue as soon as 12 h after infection (Fig. 2), and were, at that time-point, predominantly localized at the edges of the slice cultures (Fig. 1D). During the course of infection, the trophozoites were found to increasingly migrate further into the centre of the cultures where, at 72 h, they were mainly present in the form of clusters, thus strongly suggesting multiplication within the tissue (Fig. 1F). In mock-infected control-cultures, no trophozoites were detected throughout the time-course of infection (data not shown). Follow-up investigation of the live cultures for the time-course of infection by inverse microscopy showed increasing motility of the trophozoites starting from 60 h of infection. At 72 h after infection, trophozoites found within the brain tissue were characterized by a clear motility (visible under the light microscope), especially in cultures infected with the high inoculum dose (1000 trophozoites).

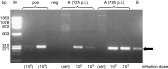
Fig. 2. PCR analysis of in vitro organotypic cortical tissue and in vivo brain tissue, respectively. In organotypic slice cultures (A), a positive signal specific for Naegleria fowleri DNA (arrow) was detected as soon as 12 h p.i. after culture, for both infection doses (1×102 and 1×103 trophozoites, respectively). Brain tissue, recovered 6 days p.i from an infected rat, was found to be positive for N. fowleri-DNA (B). The positive control (pos) included either 1×102 or 1×103N. fowleri trophozoites, respectively, the negative control (neg) an appropriate volume of distilled water. Ctrl: No-infection control. M: Molecular size marker.
In cultures infected with the low inoculum dose (100 trophozoites), the number of parasites found by immunohistochemistry and their motility were lower at all time-points when compared with cultures infected with the high inoculum. Macroscopically, the tissue of cultures challenged with the low inoculum dose remained intact during the entire incubation time. In brain slices challenged with the high inoculum dose, however, areas of decreased thickness of the tissue became macro- and microscopically apparent, predominantly at the edges, starting at 60 h after infection, and subsequently extending into the entire tissue slice by 72 h. Additionally, 2 out of 5 cultures had been completely damaged to the point that only fragments of the edges could still be recognized on the membrane. Generally, the level of tissue damage found at 72 h after infection (high inoculum dose) did not allow further re-sectioning of the remaining tissue pieces, and thus these pieces were stained unsectioned. Typically, the tissue was completely invaded by parasites (Fig. 1H), and only very few host tissue cells could be identified as intact neurons within the tissue. The majority of the host tissue cells displayed typical necrotic hallmarks such as pyknotic nuclei and swollen cytoplasm.
In vivo
The infected rats did not show any symptoms of disease until day 5 post-infection (p.i.), when they started to lose weight (−9·8±0·3 g/24 h; average increase in weight from day 1 to day 5 p.i.: +5·6±1·8 g/24 h), but they did not show obvious behavioural abnormalities. On day 6 p.i., the animals became severely ill and showed marked lethargy and ambulatory problems, which was even more accentuated in the terminally ill animal sacrificed on day 7 p.i., when animals developed obvious signs of disease including obtundation, ataxy, shivering and ophistotonus. Histopathologically, the marked presence of neutrophil inflammatory cells in the posterior area of the olfactory bulb was indicative of a severe inflammatory response in both animals. Inflammation of the cortex was more pronounced in the terminally ill animal sacrificed on day 7 p.i. (Fig. 1G). The parasite was organized in clusters, indicating multiplication within the tissue. Characteristically, the trophozoites were surrounded by inflammatory cells (Fig. 1I). In the cortex of the animal sacrificed on day 7 p.i., a remarkably high number of trophozoites was detected (Fig. 1E), while in the cortex of the rat sacrificed on day 6 p.i., trophozoites were much less in number (Fig. 1C). Tissue damage was especially evident in the anterior region of the olfactory bulb in both animals, and in the cortex of the terminally ill animal only, as no damage became apparent in the cortex of the animal sacrificed on day 6 p.i.. PCR analysis of cortical tissue of the animal sacrificed on day 6 p.i. revealed an N. fowleri-specific signal (Fig. 2). The mouse infected for comparative purposes exhibited the first clinical signs on day 3 p.i.. The histological assessment of the gross brain lesions showed striking similarities between the frontal brain damage from both animal species (Fig. 3).

Fig. 3. Gross histological comparison of mouse (A) and rat brain (B) following experimental infection with Naegleria fowleri (Haematoxylin-Eosin stain) demonstrates similarities in the spread of the parasite and induction of brain damage. In both animal species, the front line (arrows) of the amoebic trophozoite invasion from the olfactory bulb (ob) into the brain parenchyma is clearly visible. Reduced staining intensity is indicative of parenchymal damage (arrowheads). The stars indicate the site of entry of the trophozoites into the olfactory bulb.
For both in vivo and in vitro tissues, no cyst forms of N. fowleri were detectable.
DISCUSSION
The present work documents the feasibility of infecting organotypic slice cultures from rat brain with N. fowleri, the causative agent of PAM. During the course of infection, the trophozoites were found to enter the brain cultures and to propagate within the tissue, analogously to infection in vivo in the rat. Depending on the inoculum size used, the tissue assessed 72 h after infection was either found to be intact, or it displayed the presence of severe damage, particularly necrosis including swelling of the cells and pyknotic nuclei. In both experimental infection of animals (May and John, 1982; Kuroki et al. 1998; Rojas-Hernandez et al. 2004) and in clinical cases (Barnett et al. 1996; Okuda and Coons, 2003; Stephany, Pearl and Gonzalez, 2004), the brain was shown to undergo significant tissue damage. The olfactory bulb and the frontal and temporal lobe of the cerebrum are the most affected regions in PAM, and the tissue damage shows morphological features of necrosis.
Noteworthy, the tissue maintained in vitro remained undamaged until late in the infection, when, the damage occurred rapidly within 12 h. This observation is in agreement with the findings in the complementary in vivo rat model of PAM where a marked parasite proliferation, the clinical signs of disease and the development of brain injury were observed between day 6 and 7 p.i.. The comparative gross histological assessment of the brain lesions showed striking similarities between the rat and the infected mouse. Conclusively, the rat model used in the present work appears equally suitable as the conventional mouse model.
Organotypic slice cultures of rat brain tissue appear as an appropriate model for PAM, not only with regard to the time-course of proliferation of the trophozoites, but also with regard to the development of direct, parasite-induced tissue damages. Furthermore, in both organotypic cultures and the brain, amoebic trophozoites appeared to be organized in clusters, strongly suggestive of a marked local multiplication of the parasite population while invading the tissue. A major difference to the in vivo infection exists in respect to the inflammatory response. While the organotypic culture system excludes inflammatory cells, the inflammatory reaction was considerable in the in vivo system, which is in line with observations made by others in similar mouse models for PAM (May and John, 1982; Okuda and Coons, 2003; Rojas-Hernandez et al. 2004). In the cortex, the number of inflammatory cells differed greatly in both animals, and was very pronounced in the terminally ill animal with a high number of intracerebral amoebae, suggesting a burst of the inflammatory response within this region during the terminal stage of the disease. Typically, the inflammatory cells surrounding the trophozoites presented similarly to those already described in experimentally infected mice (Rojas-Hernandez et al. 2004). Nevertheless, the contribution of the inflammatory response to brain damage in experimental PAM has not been addressed yet. In other infectious diseases of the CNS that induce severe inflammation e.g. bacterial meningitis (Leib et al. 2001), the inflammatory reaction has been shown to play a central role in the pathogenesis of brain damage. This observation raises the question as to whether the massive recruitment of inflammatory cells may play a similar role in PAM. The unique possibility to add or omit peripheral leukocytes to the system in a controlled fashion makes the organotypic slice culture system particularly well suited to delineate the contribution of invading inflammatory cells to the pathogenesis of brain damage associated with PAM (Meli, Christen and Leib, 2003).
Organotypic slice cultures from rat brain tissue have successfully been used so far for experimental infection in vitro with Neospora caninum (Müller et al. 2002; Vonlaufen et al. 2002), Toxoplasma gondii (Scheidegger et al. 2005), Trypanosoma brucei (Stoppini et al. 2000), and bacterial meningitis (Meli et al. 2003; Gianinazzi et al. 2004a). Culturing rat tissue in vitro offers the advantage of preservation of tissue morphology and of local neuronal circuits compared to primary cultures that lack these features (Strasser and Fischer, 1995). Thus, infection in organotypic cultures more closely reflects the situation in vivo. Moreover, in contrast to in vivo experiments, this approach enables manipulations to be carried out in a controlled environment under defined conditions. The system allows control of parameters that may contribute to the pathogenesis of diseases in vivo, specifically, temperature, oxygen tension and leukocyte invasion, to name a few. Changes in tissue morphology in the course of the infection can readily be followed using inverted microscopy, and the inoculum size can be precisely adjusted according to the desired end-point such as tissue damage and extent of infection within the culture.
In summary, we propose a new in vitro approach to study CNS infection with N. fowleri. In the rat organotypic slice culture model, the development of infection and tissue damage correspond well with the findings in a complementary in vivo rat model of PAM. The organotypic culture thus represents an attractive tool to delineate the pathophysiology of PAM and provides a platform to study pathogenetic mechanisms under defined conditions.
The work was supported by the Swiss National Science Foundation (SCOPES No. 7IP062584 and 632-66057.01) and by the Swiss Defense Procurement Agency (Project No. 4500303638). We are indebted to Dr Nadia Schürch and Dr Martin Schütz from the Spiez Laboratory for their valuable support and logistic contribution to the work.