Introduction
Ethiopian mustard (Brassica carinata A. Braun) originated from the natural hybridization between black mustard [Brassica nigra (L.) W.D.J. Koch.] and wild cabbage (Brassica oleracea L.). Ethiopian mustard has been recently introduced as a nonedible winter oilseed crop in the southeastern United States for biofuel production (Cardone et al. Reference Cardone, Mazzoncini, Menini, Rocco, Senatore, Seggiani and Vitolo2003; Seepaul et al. Reference Seepaul, Small, Mulvaney, George, Leon, Paula-Moraes, Geller, Marois and Wright2019). Approximately 100,000 ha of land are suitable for producing Ethiopian mustard in Florida, which can help to meet the current demand for renewable energy (Alam and Dwivedi Reference Alam and Dwivedi2019). Besides their potential for oil production, Brassicaceae species have been considered good options for crop rotation because of their ecological services: a deep taproot to break up compacted soils and scavenge nutrients, a wide canopy to suppress weeds, and attractive flowers for pollinators (Alcántara et al. Reference Alcántara, Sánchez, Pujadas and Saavedra2009; Brown Reference Brown1997; Díaz et al. Reference Díaz, Martínez, Piñera, Alarcón and Plasencia2013; Haramoto and Gallandt Reference Haramoto and Gallandt2004; Manning and Wallis Reference Manning and Wallis2005; Rahman et al. Reference Rahman, Khatun, Liu and Barkla2018).
Because Ethiopian mustard is a new crop in the U.S. southeastern region, research is ongoing to evaluate basic agronomic practices, including planting strategies, nutrient management, and identification of genotypes suitable to the region (Kumar et al. Reference Kumar, Seepaul, Mulvaney, Colvin, George, Marois, Bennett, Leon, Wright and Small2020; Mulvaney et al. Reference Mulvaney, Leon, Seepaul, Wright and Hoffman2019). Regarding weed management, herbicides registered for other crops have been evaluated for Ethiopian mustard tolerance, but this research has only identified a few herbicides that can be safely used in this crop (Leon et al. Reference Leon, Ferrell and Mulvaney2017). Therefore, greater understanding about winter weed behavior in Ethiopian mustard will aid in the selection and implementation of options/strategies for integrated weed management.
As demonstrated by widespread infestations of herbicide-resistant weeds in summer crops, overreliance on a single technology (i.e., herbicides) not only threatens economic viability, but also ecological sustainability of agroecosystems (Busi et al. Reference Busi, Vila-Aiub, Beckie, Gaines, Goggin, Kaundun, Lacoste, Neve, Nissen, Norsworthy, Renton, Shaner, Tranel, Wright, Yu and Powles2013). The use of integrated strategies that incorporate several tools, including crop rotation systems, is imperative for sustainable weed management (Tiwari et al. Reference Tiwari, Reinhardt Piskáčková, Devkota, Mulvaney, Ferrell and Leon2021). Thus, growers might benefit from growing Ethiopian mustard during winter months to complement existing crop rotations. Although crop rotation is known to be an effective strategy for weed control, no research has been conducted to study how integrating winter Ethiopian mustard production in a diversified crop rotation might affect winter and summer weed population dynamics and emergence patterns. Recent research conducted on Ethiopian mustard demonstrated this crop’s weed-suppressive ability (Tiwari et al. Reference Tiwari, Reinhardt Piskáčková, Devkota, Mulvaney, Ferrell and Leon2021). Moreover, growers might be more willing to grow Ethiopian mustard if its addition to the existing crop rotation can contribute to overall weed seedbank management for subsequent crop growing seasons.
Weed management decisions could be improved by predicting weed emergence timing and increasing the efficiency of weed control tactics, particularly during the vulnerable crop stages (Forcella et al. Reference Forcella, Arnold, Sanchez and Ghersa2000; Leon et al. Reference Leon, Wright and Marois2015; Myers et al. Reference Myers, Curran, VanGessel, Calvin, Mortensen, Majek, Karsten and Roth2004; Reinhardt Piskackova et al. Reference Reinhardt Piskackova, Reberg-Horton, Richardson, Jennings and Leon2020a, 2020b). Soil environmental conditions such as soil temperature and moisture can influence weed seedling emergence intensity and timing; thus these factors are critical components for planning and successful implementation of integrated weed management strategies (Calado et al. Reference Calado, Basch and de Carvalho2009; Deen et al. Reference Deen, Swanton and Hunt2001; Hartzler et al. Reference Hartzler, Buhler and Stoltenberg1999; Shaner and Beckie Reference Shaner and Beckie2014). Currently, no research has reported the emergence patterns of winter and summer annual weeds in Ethiopian mustard cropping systems in the southeastern region of the United States.
In the present study, we hypothesized that crop history and weed management treatments affect the emergence patterns of winter and summer annual weed species in Ethiopian mustard. Therefore, the objectives of this research were: (1) to determine the effect of previous summer crops on the emergence patterns of winter weed species; and (2) to evaluate the effect of planting Ethiopian mustard in the winter and its influence on the emergence patterns of summer weed species in the subsequent season.
Materials and Methods
Site Description
Field research was conducted at the University of Florida/IFAS West Florida Research and Education Center, Jay, FL (30.777°N 87.139°W) from May 2018 to September 2019 (2018 to 2019) and May 2019 to July 2020 (2019 to 2020). Soils at the 2018 to 2019 site were a Dothan loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudults) with pH 6.3 and an Orangeburg loamy sand (fine-loamy, kaolinitic, thermic Typic Kandiudults) with pH 6.0. Soils at the 2019 to 2020 location were a mosaic of Orangeburg loamy sand with pH 6.0 and Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudults) with pH 5.8.
Experimental Design
The experiment was arranged as a randomized complete block split-plot design with seven and eight replications in the 2018 to 2019 and 2019 to 2020 seasons, respectively. The main plot was the crop planted in the preceding summer growing season (i.e., crop history): (1) cotton (Gossypium hirsutum L., ‘DP1646’); (2) peanut (Arachis hypogea L., ‘Georgia-06G’); and (3) weed-free fallow. The subplot was weed management during the Ethiopian mustard growing season: (1) Ethiopian mustard (‘Avanza 641’) with PRE-applied S-metolachlor at 1,420 g ai ha−1 (Dual Magnum®, Syngenta Crop Protection, Greensboro, NC, USA); (2) Ethiopian mustard without S-metolachlor; and (3) weedy winter fallow. The main plots were 11-m long by 22-m wide, and subplots were 11-m long by 7-m wide. Seeds of sicklepod [Senna obtusifolia (L.) Irwin & Barneby] were collected from natural populations at the West Florida Research and Education Center in 2018 and were obtained from Azlin Seed Service (Leland, MS) for the 2019 to 2020 season. Smooth pigweed (Amaranthus hybridus L.) seeds were obtained from Azlin Seed Service for both years. Three 1-m2 quadrats were randomly placed within each subplot before planting of Ethiopian mustard. Senna obtusifolia seeds were spread at 2,000 seeds m−2 in November 2018 and 3,500 seeds m−2 in November 2019 in one of the quadrats. Likewise, A. hybridus seeds were spread at 25,000 seeds m−2 for both years in another quadrat, while the third quadrat was left for tracking the emergence of natural seedbank of the winter weeds henbit (Lamium amplexicaule L.), common chickweed [Stellaria media (L.) Vill.], and cutleaf evening-primrose (Oenothera laciniata Hill).
Field Maintenance
The site was maintained during the summer (before Ethiopian mustard season) by planting cotton or peanut or leaving the plot fallow according to the main plot factors. The summer fallow treatments were regularly treated with glyphosate (Roundup PowerMax®, Bayer CropSciences, USA) at 1,156 g ai ha−1 throughout the summer to control the natural weed community characterized predominantly by S. obtusifolia, A. hybridus, Florida beggarweed [Desmodium tortuosum (Sw.) DC.], tropical spiderwort (Commelina benghalensis L.), goosegrass (Eleusine indica [L.] Gaertn.), and barnyardgrass [Echinochloa crus-galli (L.) P. Beauv]. Agronomic and weed management practices for cotton and peanut were followed according to the local recommendations (Ferrell et al. Reference Ferrell, MacDonald and Devkota2020a, 2020b; Wright et al. Reference Wright, Tilman, Small, Ferrell and DuFault2016, Reference Wright, Small and DuFault2017). There was no residual herbicide used during the cotton- or peanut-growing season to avoid residual effect during Ethiopian mustard–growing season. After cotton and peanut were harvested, fields were disked twice, rototilled, summer weed seeds were spread in the respective 1-m2 quadrats and lightly incorporated with a rototiller before planting Ethiopian mustard. In 2018 to 2019, field preparation, weed seed spreading, and planting of Ethiopian mustard was delayed until February 4, 2019, due to heavy and frequent rainfall (Table 1). Ethiopian mustard was planted on November 18, 2019, for the 2019 to 2020 season. Ethiopian mustard was seeded using a grain drill at 6 kg ha−1 with 36-cm row spacings (Great Plains 1206 NT, Salina, KS, USA). Planting was done only in the subplot treatments with Ethiopian mustard plus S-metolachlor and Ethiopian mustard without S-metolachlor. For the Ethiopian mustard with S-metolachlor treatment, herbicide was applied immediately after planting with a tractor-mounted sprayer calibrated to deliver 187 L ha−1 using XR11002 (TeeJet® Technologies, Wheaton, IL, USA) nozzles. Liming and fertilization were done according to soil tests results and based on recommendations for canola (Brassica napus L.), except for nitrogen, which was applied at 22 kg N ha−1 as urea immediately after planting, and additional 68 kg N ha−1 was top-dressed at the early bolting stage.
Table 1. Soil moisture and temperature for 2018–2019 and 2019–2020 cropping seasons at Jay, FL. a

a Data were obtained from HOBO-U 30 data loggers’ moisture and temperature sensors installed at 7-cm depth at the experimental site at the University of Florida/IFAS-West Florida Research and Education Center.
b Average soil moisture and temperature for 2018–2019 and 2019–2020 cropping seasons.
Weed Seedling Emergence and Data Collection
After planting of Ethiopian mustard, soil moisture and temperature data were recorded every 30 min at 7-cm depth in whole plots during both seasons using 12-bit temperature and ECH20 EC-5 soil moisture sensors, and HOBO U30 data loggers (Onset Computer Corporation, Bourne, MA). Seedlings of S. media, O. laciniata, and L. amplexicaule were counted in the designated 1-m2 quadrats and hand removed at a weekly interval throughout the Ethiopian mustard growing season from February 4 to April 8, 2019, for the 2018 to 2019 season, and November 18, 2019, to April 10, 2020, for the 2019 to 2020 season. Seedling emergence of summer annual weed species (S. obtusifolia and A. hybridus) was detected during the winter from November to February. Summer weed emergence was recorded from the respective 1-m2 quadrats and hand removed every week throughout the Ethiopian mustard growing season. After Ethiopian mustard harvest, the emergence of the two summer weed species continued and was recorded throughout the season until emergence ceased.
Emergence Pattern Modeling
Calculation of relative cumulative emergence for S. media, O. laciniata, and L. amplexicaule was done per quadrat as a percent of total emergence during the Ethiopian mustard growing season. Similarly, the relative cumulative emergence for S. obtusifolia and A. hybridus was calculated up to Ethiopian mustard harvest time and continued throughout the summer until weed emergence ceased. Thermal time was used to describe emergence patterns using cumulative growing degree days (GDD):
where
![]() ${T_{{\rm{mean}}}}$
represents the daily mean soil temperature in C, and
${T_{{\rm{mean}}}}$
represents the daily mean soil temperature in C, and
![]() ${T_{{\rm{base}}}}$
is the minimum temperature at which S. media, O. laciniata, L. amplexicaule, S. obtusifolia, and A. hybridus seeds germinate. The base temperature was 10 C for S. media, 0 C for O. laciniata and L. amplexicaule, and 15 C for S. obtusifolia and A. hybridus (Creel et al. Reference Creel, Hoveland and Buchanan1968; Grundy et al. Reference Grundy, Phelps, Reader and Burston2000; Guo and Al-Khatib Reference Guo and Al-Khatib2003; Hill et al. Reference Hill, Renner and Sprague2014; Patterson Reference Patterson1993; Teem et al. Reference Teem, Hoveland and Buchanan1980; Wright et al. Reference Wright, Coble, Raper and Rufty1999). Cumulative thermal time (GDD) was calculated beginning at soil preparation for winter annual weeds and beginning January 1 for summer annual weeds.
${T_{{\rm{base}}}}$
is the minimum temperature at which S. media, O. laciniata, L. amplexicaule, S. obtusifolia, and A. hybridus seeds germinate. The base temperature was 10 C for S. media, 0 C for O. laciniata and L. amplexicaule, and 15 C for S. obtusifolia and A. hybridus (Creel et al. Reference Creel, Hoveland and Buchanan1968; Grundy et al. Reference Grundy, Phelps, Reader and Burston2000; Guo and Al-Khatib Reference Guo and Al-Khatib2003; Hill et al. Reference Hill, Renner and Sprague2014; Patterson Reference Patterson1993; Teem et al. Reference Teem, Hoveland and Buchanan1980; Wright et al. Reference Wright, Coble, Raper and Rufty1999). Cumulative thermal time (GDD) was calculated beginning at soil preparation for winter annual weeds and beginning January 1 for summer annual weeds.
Thermal time models were developed to describe the observed cumulative weed emergence for each crop history—cotton, peanut, and summer fallow—by fitting the data to the Gompertz equation (Equation 2; Forcella et al. Reference Forcella, Arnold, Sanchez and Ghersa2000) using SigmaPlot v. 11 (Systat Software, San Jose, CA 95131):
where y is the relative cumulative emergence for time x, a is the asymptote (theoretical maximum for y normalized to 100%), x 0 is the lag period before emergence begins, and b is the rate of emergence.
Each experimental unit of the study design generated an independent emergence set for the weed species tracked over the growing season. A total of 135 emergence sets were recorded for each species between the 2 yr. Each set with cumulative emergence of at least 20 plants m−2 was included in the analysis.
Preliminary analyses indicated that the interaction of crop history and weed management treatment was not significant (0.20 < P < 0.94) for all the weed species evaluated (data not shown), so only the main effects were modeled for the emergence patterns. Models were developed for each crop history by pooling weed management treatments, because although the weed management treatments affected total weed density (Tiwari et al. Reference Tiwari, Reinhardt Piskáčková, Devkota, Mulvaney, Ferrell and Leon2021), they did not modify emergence patterns (data not shown). After removing sets with fewer than 20 plants m−2 cumulative emergence, each winter weed species had 15 sets per crop history, and each summer weed species had 45 sets per crop history. For both years, models were fit to nine randomly selected sets of each crop history for S. media, L. amplexicaule, and O. laciniata and 30 randomly selected sets for S. obtusifolia and A. hybridus using PROC NLMIXED and PROC REG in SAS (SAS Institute, Cary, NC, USA 27513). Akaike information criterion (AIC) and root mean-square error (RMSE) were used to compare the fitness of different models to the data set. The remaining sets for each crop history that were not used for modeling were used to validate the models: 6 and 15 sets for each winter and summer weed species, respectively. Regression (PROC REG) was done with the predicted values from fitted Gompertz models with the observed values of the validation data sets. This procedure is a robust method for describing weed emergence of the main plot treatments with nonlinear models (Hill et al. Reference Hill, Renner and Sprague2014). Additionally, to test whether a single model adequately described all treatments, the model for summer fallow (control) was regressed with the validation data sets for the other two crop history treatments. The summer fallow treatment did not receive fertilization or residual herbicides; therefore, it was considered as the crop history treatment that could provide the least biased assessment of weed seedling emergence. The model generated for summer fallow was used for validation against the independent data sets from the other crop history data sets.
Ethiopian Mustard Phenological Stages
Dates of select Ethiopian mustard growth stages were recorded. GDD were calculated for Ethiopian mustard using 5 C as the base temperature based on winter B. napus (Vigil et al. Reference Vigil, Anderson and Beard1997).
Results and Discussion
Winter Weed Emergence Model Fitness
In general, the winter weeds studied in this trial exhibited defined emergence patterns across years and treatments. All models had a good fit as demonstrated by low AIC values (Table 2). Models for O. laciniata had AIC values less than −100, and L. amplexicaule and S. media values were less than −200. Another metric to evaluate fitness is the RMSE, which can be interpreted as a ratio of the data variation not described by the model. Thus, all models described more than 80% of the variation of the data. Based on these results, the reported models adequately described the emergence pattern of the three winter weed species following each crop history treatment.
Table 2. Relationship between thermal time (cumulative growing degree days) and Lamium amplexicaule, Oenothera laciniata, or Stellaria media cumulative seedling emergence described with Gompertz models, y = a*exp{−exp[−(x − x0)/b]}, during the Ethiopian mustard growing season.a

a Here, y is the relative cumulative emergence for time x, a is the asymptote (theoretical maximum for y normalized to 100%), x0 is the lag period before the emergence begins, and b is the rate of emergence. A summer fallow predictive model was validated with independent data from three crop histories.
b Seedling emergence data were collected from November to April at Jay, FL.
c AIC is the Akaike information criterion used for comparing models. The more negative values are the better fit.
d Root mean-square error (RMSE) and R2 reflect the fit of Gompertz equation used to create the model.
e Validation was done by comparing the predictive equation for fallow with validation data sets of each of the crop history treatments.
While the emergence of winter weed species was described well with the model for each crop history treatment, the model parameters were very similar across crop histories (Table 2). Therefore, we evaluated whether a single model (i.e., summer fallow model) could be used to describe seedling emergence regardless of the crop history. The summer fallow model was validated with independent weed emergence data from the cotton and peanut crop history treatments, and more than 80% of the emergence variation was properly described (Table 2). This provides evidence that seedling emergence patterns of the studied winter annual weed species may not need to be adapted based on every crop history and weed management strategy; rather, they can be modeled primarily by accounting for soil temperature (Figures 1–3). However, we acknowledge that this might only be the case for areas where moisture is not a frequent limiting factor (Davis et al. Reference Davis, Clay, Cardina, Dille, Forcella, Lindquist and Sprague2013).

Figure 1. Relationship between a thermal time (cumulative growing degree days [GDD]) model of Stellaria media seedling emergence and independent sets of emergence data during winter Ethiopian mustard production with the prior-summer crop history: cotton, peanut, and non-crop fallow. Days in the x axis represent the time required to attain corresponding cumulative GDD after Ethiopian mustard planting.
Emergence Timing of Winter Weed Species during Ethiopian Mustard Season
Ethiopian mustard is usually competitive after canopy closure, but weed control is essential at early growth stages (Leon et al. Reference Leon, Ferrell and Mulvaney2017). Therefore, it is necessary to know the critical time frame for winter weed species emergence within the Ethiopian mustard season.
Stellaria media
Cotton, peanut, and summer fallow crop history showed similar emergence patterns for S. media (Figure 1). The summer fallow history model was an adequate fit for all treatments, accounting for at least 90% of the variation (Table 2). According to the summer fallow history model, S. media reached 50% emergence by 250 GDD (Figure 1), that is, before Ethiopian mustard reached the 4-leaf stage (Figure 4). Likewise, a majority of the Ethiopian mustard plants were at the 12-leaf stage at 500 GDD when 90% of S. media emergence had occurred (Figures 1 and 4). Bullied et al. (Reference Bullied and Van Acker2003) reported B. napus emergence in a conventional system at 25% (E25), 50% (E50), and 80% (E80) is 446, 511, and 594 CDD, respectively, and 355, 420, and 504 GDD were reported for E25, E50, and E80, respectively, for wild mustard (Sinapis arvensis L.) in a similar system.
Oenothera laciniata and Lamium amplexicaule
The emergence of O. laciniata and L. amplexicaule surpassed 50% between 500 and 700 GDD (Figures 2 and 3). The required number of GDD to attain 50% emergence for these weed species was almost twice long than for S. media (Figures 2 and 3). For these weed species, 90% of emergence occurred between 1,000 and 1,200 GDD, which was equivalent to approximately 85 to 90 d after planting (Figures 2–4) and corresponded to after 50% flowering to before pod initiation stage for Ethiopian mustard.

Figure 2. Relationship between a thermal time (cumulative growing degree days [GDD]) model of Oenothera laciniata seedling emergence and independent sets of emergence data during winter Ethiopian mustard production with the prior-summer crop history: cotton, peanut, and non-crop fallow. Days in the x axis represent the time required to attain corresponding cumulative GDD after Ethiopian mustard planting.

Figure 3. Relationship between a thermal time (cumulative growing degree days [GDD]) model of Lamium amplexicaule seedling emergence and independent sets of emergence data during winter Ethiopian mustard production with the prior-summer crop history: cotton, peanut, and non-crop fallow. Days in the x axis represent the time required to attain corresponding cumulative GDD after Ethiopian mustard planting.

Figure 4. Relationship between a thermal time (cumulative growing degree days [GDD]) model developed from the predicted sets from previous summer non-crop fallow for Stellaria media, Lamium amplexicaule, and Oenothera laciniata seedling emergence during the Ethiopian mustard growing season. Phenological stages of fall-planted Ethiopian mustard are mentioned on the top right with the corresponding cumulative GDD.
Summer Annual Weed Emergence Model Fitness
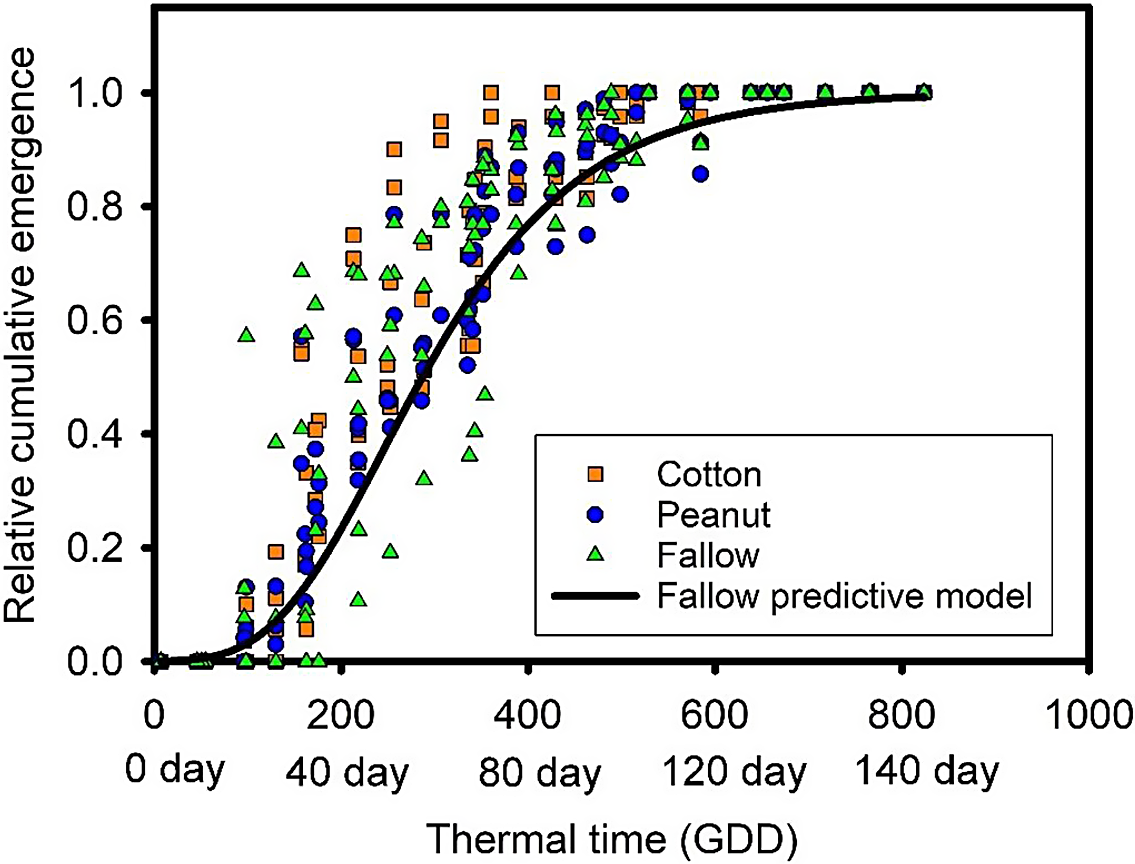
Models of S. obtusifolia emergence following each crop history exhibited excellent fitness, with AIC values all less than −600 and accounting for more than 90% of the variation in the data (Table 3). Still, the emergence pattern seen in each crop history treatment was explained well by the model developed for the emergence following the summer fallow with RMSE validation values of 0.09 to 0.11 (Table 3). Based on these results, S. obtusifolia followed a consistent emergence pattern regardless of crop history or management that can be described well by a single model (Figure 5).
Table 3. Relationship between thermal time (cumulative growing degree days) and Amaranthus hybridus and Senna obtusifolia cumulative seedling emergence described with Gompertz models, y = a*exp{−exp[−(x − x0)/b]}, during the Ethiopian mustard growing season up to late summer until weed emergence ceased. a

a Here, y is the relative cumulative emergence for time x, a is the asymptote (theoretical maximum for y normalized to 100%), x0 is the lag period before the emergence begins, and b is the rate of emergence. A summer fallow predictive model was validated with independent data from three crop histories.
b Seedling emergence data were collected from February to early September at Jay, FL.
c AIC is the Akaike information criterion used for comparing models. The more negative values are the better fit.
d Root mean-square error (RMSE) and R2 reflects the fit of Gompertz equation used to create the model.
e Validation was done by comparing the predictive equation for fallow with validation data sets of each of the crop history treatments.

Figure 5. Relationship between a thermal time (cumulative growing degree days [GDD]) model of Senna obtusifolia seedling emergence and independent sets of emergence data during and after the Ethiopian mustard growing season with the prior-summer crop history: cotton, peanut, and non-crop fallow. Cumulative GDD was calculated beginning January 1 up to the subsequent summer until emergence ceased for S. obtusifolia. Days in the x axis represent the period after January 1 required to attain corresponding cumulative GDD.
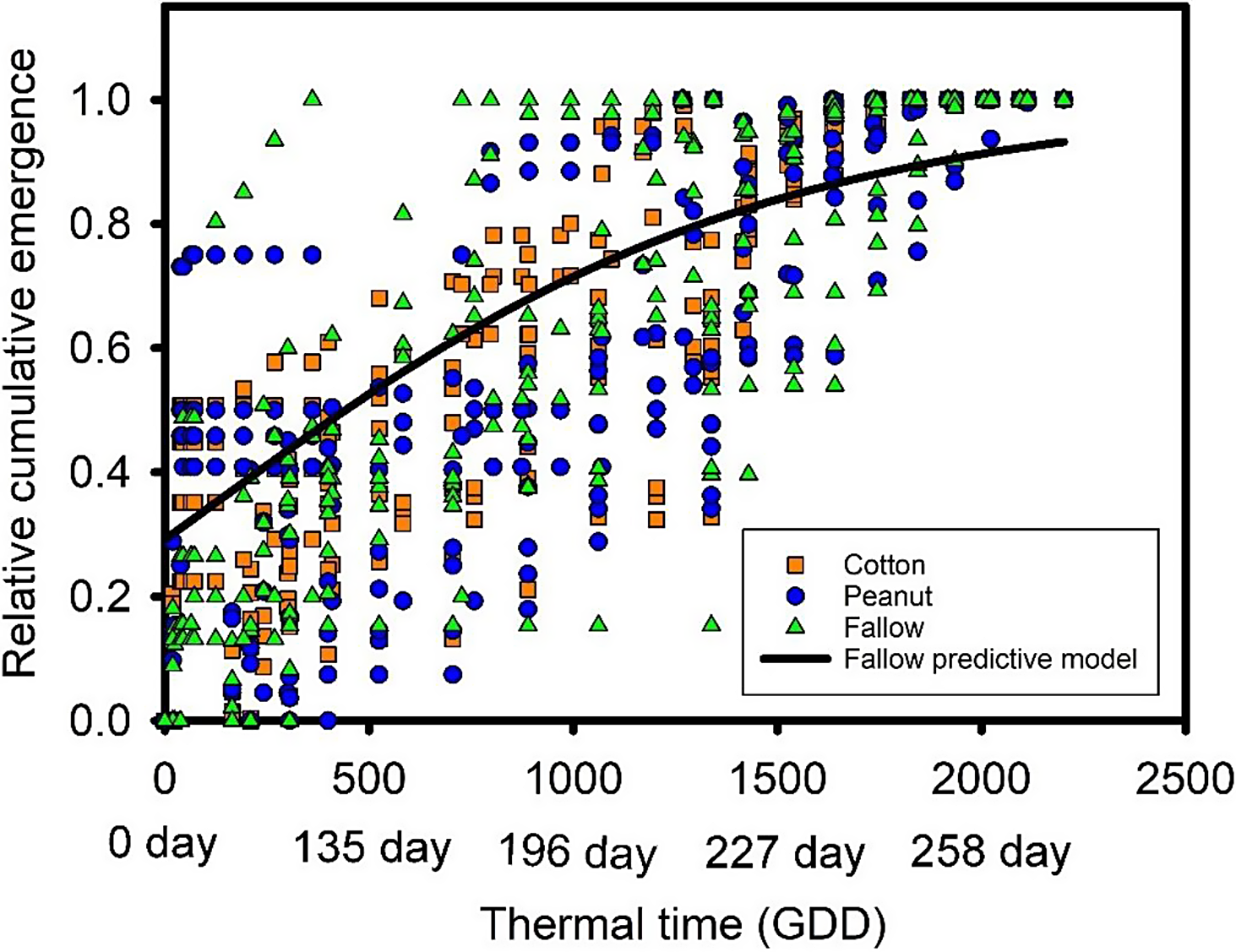
Emergence patterns of A. hybridus following each crop history adequately fit the models, and these models accounted for greater than 80% of the variation in emergence (Table 3). Greater than 80% of the variation was also explained with the fallow model (Table 3). However, there was significant variation between sampling points (Figure 6). For example, in some quadrats, emergence reached 100% in 250 GDD, while others took almost 1,500 to 1,700 GDD before no emergence was observed or until the end of the summer season (Figure 6).

Figure 6. Relationship between a thermal time (cumulative growing degree days [GDD]) model of Amaranthus hybridus seedling emergence and independent sets of emergence data during and after the Ethiopian mustard growing season in fields that had cotton, peanut, and non-crop fallow during the previous summer. Cumulative GDD was calculated beginning January 1 up to the subsequent summer until emergence ceased for A. hybridus. Days in the x axis represent the period after January 1 required to attain corresponding cumulative GDD.
Emergence pattern is a function of seed dormancy release and the presence of adequate germination conditions, such as soil moisture, and temperature (Karseen and Bouwmeester Reference Karseen and Bouwmeester1992; Roberts Reference Roberts1964). In the present study, the winter weed species, which exhibit physiological dormancy, were able to release dormancy during the summer (Baskin and Baskin Reference Baskin and Baskin1976, Reference Baskin and Baskin1981; Steiner Reference Steiner1968; Taylorson and Hendricks Reference Taylorson and Hendricks1976), resulting in uniform and well-defined sigmoidal emergence patterns (Figures 1–3).
Early Spring Emergence of Summer Weed Species
Abundant seedling emergence of the two summer annual weeds (S. obtusifolia and A. hybridus) during the winter was unexpected and resulted in seedling mortality due to frost damage. However, from the end of January to April, there were multiple days with temperatures above the base temperature for these weed species (Table 1), which could trigger the germination of nondormant seeds. Senna obtusifolia nondormant seeds germinate over a wide range of temperatures (Teem et al. Reference Teem, Hoveland and Buchanan1980). This species possesses physical dormancy that was likely reduced by scarification caused by cultivation in the fall, microbial activity, and temperature fluctuations (Baskin and Baskin Reference Baskin and Baskin2004). Meanwhile, A. hybridus (a small-seeded weed) possesses physiological dormancy (Gallagher and Cardina Reference Gallagher and Cardina1998), which is quickly reduced under moist cold conditions such as those present from December to February. Therefore, in northern Florida, where temporary warm periods are not uncommon during the winter–spring transition, nondormant S. obtusifolia and A. hybridus seeds can germinate, but these emergence events are not consistent during the winter months, due to the return of cold temperatures, which results in a variable emergence pattern, as observed in the current study (Figure 6; Leon and Owen Reference Leon and Owen2006).
Approximately 11% of S. obtusifolia seeds initially placed in the quadrats emerged (average of the cumulative emergence) by Ethiopian mustard harvest, which was between 150 and 180 d after January, or mid-May to early June for reference (Figure 5; Table 4). Also, the results showed less than 10% of A. hybridus seed emerged from the added seeds during the Ethiopian mustard growing season (Table 4). More importantly, about 40% of the total emergence of S. obtusifolia occurred before planting of summer crops (i.e., between 500 to 1,000 GDD or between May to July; Figure 5). Therefore, almost half of the total season emergence was eliminated during the Ethiopian mustard growing season and before summer crops were established (Figure 5). Different factors such as germination, seed decay, microbial infection, predation, and fatal germination could account for the loss of weed seeds in the soil and low seedling emergence (Buhler et al. Reference Buhler, Hartzler and Forcella1997; Davis and Renner Reference Davis and Renner2007; Martinkova and Honěk Reference Martinkova and Honěk2013; Murdoch and Ellis Reference Murdoch, Ellis and Fenner2000; Schwinghamer and Van Acker Reference Schwinghamer and Van Acker2008). Germinable and extractable weed seedbank analysis, similar to that of Reinhardt and Leon (Reference Reinhardt and Leon2018), also showed few viable seeds for S. obtusifolia emergence at Ethiopian mustard harvest (data not shown), which indicated that there could be an important reduction in the viable seedbank during the winter–spring season. Therefore, winter–spring seedling emergence (during the Ethiopian mustard production season) might be an important source of pre-summer season population reduction for the two summer weeds currently studied.
Table 4. Percentages of seedling emergence of Amaranthus hybridus and Senna obtusifolia during and after the Ethiopian mustard growing season. a

a Seedling emergence percentage in relation to seeds initially placed in the 1-m2 quadrat. Data were taken from the UF/IFAS-WFREC for 2018–2019 and 2019–2020 cropping seasons at Jay, FL. Same-letter assignment within vertical column indicates no significant differences for the percentages of emerged weed among the crop history for both the seasons based on Fisher’s LSD.
Implications of Winter and Summer Weed Emergence
Short-Term Weed Management Implications in Ethiopian Mustard
Winter weed emergence patterns illustrate the critical timing when growers can implement short-term weed management options during the Ethiopian mustard production season. Stellaria media, O. laciniata, and L. amplexicaule are prevalent winter weed species in the southeastern United States. While predictive emergence models for these species can help in timing weed control, it is equally important to consider the relationship between weed emergence timing and crop growth to maximize both weed control efficacy and yield (Reinhardt Piskackova et al. Reference Reinhardt Piskackova, Reberg-Horton, Richardson, Jennings and Leon2020b). The best-fit model proposed here can be used to predict the timing of winter weed emergence and develop effective weed management strategies. Martin et al. (Reference Martin, Van Acker and Friesen2001) reported that the critical weed-free period for spring B. napus is the 4- to 6-leaf stage. Similar information on Ethiopian mustard about its critical weed-free period will be important for implementing weed control programs.
Long-Term Weed Management Implications
Emergence of summer annual weed species during the Ethiopian mustard growing period illustrates how weed management can be improved in a diversified crop rotation cycle on a long-term basis. When Ethiopian mustard was introduced as a winter crop into the existing cotton–peanut rotation, a major proportion of the emerged S. obtusifolia and A. hybridus seedlings died before summer crops were planted. It is worth mentioning that well-defined and consistent emergence patterns were not observed for summer annual weed species in the present study due to temperature variability during the winter. Therefore, the thermal time models developed here are not likely to be useful to predict S. obtusifolia and A. hybridus emergence timing for summer crops, particularly because field preparation for planting (e.g., chemical burndown or cultivation) will reset the thermal time accumulation count. Despite this caveat, our models can be used to better understand weed seedbank and population dynamics of S. obtusifolia and A. hybridus in subtropical conditions like those of the Florida Panhandle. In addition, our results illustrate the importance of considering off-season seedbank dynamics for weed management.
Acknowledgments
This research was supported by the U.S. Department of Agriculture–National Institute of Food and Agriculture grant 2017-6505-26807 and by Hatch Projects FLA-WFC-005843, FLA-WFC-005953, and NC02653. We want to thank Southeast Partnership for Advanced Renewables from Carinata (SPARC). The authors would particularly like to thank Mike Dozier, Moo Brown, and Chad Stewart for providing field support in this research. No conflicts of interest have been declared.