INTRODUCTION
Chemotherapy for Chagas disease remains unsatisfactory in terms of both toxicities and lack of effectiveness. There is a growing consensus that the persistence of the parasite, together with the imbalance of host immune response, leads to a sustained inflammatory response that underlies the characteristic lesions of the infection. Machado et al. (Reference Machado, Souto, Rossi, Esper, Tanowitz, Aliberti and Silva2008) demonstrated that cardiomyocytes are a potential source of cytokines, chemokines and nitric oxide (NO) in vivo. In mice infection, these factors may contribute to the recruitment, placement and migration of inflammatory cells in heart tissue, leading to the persistence of myocarditis. Chemokines such as CCL2 have emerged as critical in infections and autoimmune myocarditis (Paiva et al. Reference Paiva, Figueiredo, Kroll-Palhares, Silva, Silvério, Gibaldi, Pyrrho Ados, Benjamim, Lannes-Vieira and Bozza2009). Specially in Trypanosoma cruzi infection, this chemokine is intensely produced in the heart of infected mice and its involvement in parasitic uptake and destruction by macrophage has been demonstrated (Aliberti et al. Reference Aliberti, Machado, Souto, Campanelli, Teixeira, Gazzinelli and Silva1999; Machado et al. Reference Machado, Martins, Aliberti, Mestriner, Cunha and Silva2000; Paiva et al. Reference Paiva, Figueiredo, Kroll-Palhares, Silva, Silvério, Gibaldi, Pyrrho Ados, Benjamim, Lannes-Vieira and Bozza2009). Another chemokine observed in macrophages and cardiomyocytes infected with T. cruzi is CCL5, and in several studies the role of this chemokine in attracting leucocytes and its probable involvement in controlling the growth of T. cruzi and NO production have been described (Sallusto et al. Reference Sallusto, LanzaVecchia and Mackay1998; Aliberti et al. Reference Aliberti, Machado, Souto, Campanelli, Teixeira, Gazzinelli and Silva1999; Cook et al. Reference Cook, Smithies, Strieter, Frelinger and Serody1999; Machado et al. Reference Machado, Martins, Aliberti, Mestriner, Cunha and Silva2000; Talvani et al. Reference Talvani, Ribeiro and Aliberti2000). These findings suggest that eradication of the parasite may be a prerequisite to contain the development of Chagas disease, preventing its irreversible long-term consequences. Thus, the identification of new drugs and assessing the impact of antiparasitic treatment on the prevention of morbidity for infected individuals remain as major challenges in health promotion and disease control (Urbina, Reference Urbina2010).
Current drugs for Chagas disease, such as nifurtimox and benznidazole, require long-term administration and often show efficacy problems, including insufficient activity in established, chronic forms of the disease (Cançado, Reference Cançado2000). However, nitroheterocyclics are a well-known class of pharmacologically active compounds that deserve special attention for the treatment of infectious diseases. The antimicrobial toxicity of nitroimidazoles is dependent on reduction by nitroreductases of the nitro group that generate cytotoxic species that cause damage to DNA, lipids and proteins (Do Campo and Moreno, Reference Docampo and Moreno1986; Hall and Wilkinson, Reference Hall and Wilkinson2011). Fexinidazole, a 2-substituted 5-nitroimidazole, was originally synthesized by Hoechst in the 1970s, and has recently been identified as a promising new drug candidate for treatment of African trypanosomiasis (Torreele et al. Reference Torreele, Trunz, Tweats, Kaiser, Brun, Mazué, Bray and Pécoul2010). Fexinidazole has previously been shown to act in vivo against T. cruzi (Raether and Seidenath, Reference Raether and Seidenath1983). These results were confirmed by Bahia et al. (Reference Bahia, de Andrade, Martins, Nascimento, Diniz, Caldas, Talvani, Trunz, Torreele and Ribeiro2012) where the authors demonstrated that treating mice with fexinidazole cured acute and chronic experimental T. cruzi infection and the efficacy of this drug was equivalent in benznidazole susceptible and resistant parasite stocks.
New treatment options also include inhibitors of the sterol biosynthesis pathway, in particular C14-α-demethylase inhibitors such as posaconazole and ravuconazole that represent promising target drugs (Molina et al. Reference Molina, Martins-Filho, Brener, Romanha, Loebenberg and Urbina2000; Diniz et al. Reference Diniz, Caldas, Guedes, Crepalde, de Lana, Carneiro, Talvani, Urbina and Bahia2010, Reference Diniz, Urbina, de Andrade, Mazzetti, Martins, Caldas, Talvani, Ribeiro and Bahia2013). It has been argued that the in vivo antiparasitic activities of these compounds are a result of a combination of their potent and selective intrinsic anti-T. cruzi activity and their pharmacokinetic properties, such as long terminal half-life and large volumes of distribution (Urbina, Reference Urbina2010).
Natural resistance of some T. cruzi strains to nitroderivative compounds might explain therapeutic failure in Chagas disease. However, drug-induced reduction of the parasite loads in infected tissues can exert positive effects in the clinical evolution of Chagas disease by reducing the associated inflammatory processes. In order to further characterize the role of parasite clearance time induced by different treatments in mice infected with a resistant T. cruzi VL-10 strain and the outcome of these treatments in the development of the acute and chronic phases of experimental Chagas disease, we used the blood and tissue qPCR to determine the dynamic relationship between the efficacy of the treatment with posaconazole, fexinidazole and benznidazole and the heart lesion/serum inflammatory mediators in experimental hosts.
MATERIALS AND METHODS
Animal model and parasite strain
Female Swiss mice from the Animal Facility at Federal University of Ouro Preto (UFOP) were used in this study. Animals were fed with commercial food, and water was available ad libitum. Swiss mice (18–22 g) were inoculated intraperitoneally with 5·0×103 bloodstream trypomastigotes of the VL-10 T. cruzi strain, DTU II, which is resistant to the benznidazole-treatment (Filardi and Brener, Reference Filardi and Brener1987).
Study drugs
Benznidazole: N-Benzyl-2-(2-nitro-1H-imidazol-1-yl) acetamide (produced by LAFEPE, Brazil), was administered orally in a water suspension with 0·5% w/v methyl cellulose; fexinidazole: 1-Methyl-2-{[4-(methylsulfanyl)phenoxy]methyl}-5-nitro-1H-imidazole, was administrated orally in suspension containing methyl cellulose 0·5% w/v, with 5% v/v of polysorbate 80 (Tween 80) and posaconazole: 4-{4-[4-(4-{[(5R)-5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-ylmethyl)oxolan-3-methyl]methoxy}phenyl) piperazin-1-yl]phenyl}-1-[(2S,3S)-2-hydroxypentan-3-yl]-4,5-dihydro-1H-1,2,4-triazol-5-one; Noxafil®, Schering-Plough) was diluted in ultrapure water and administered orally.
Determination of treatment efficacy by parasite quantification
The first set of experiments was designed to determine the kinetics of the parasite clearance induced by benznidazole, fexinidazole and posaconazole in mice infected with VL-10 strain. Groups of 30 infected mice were treated with the different compounds. The drugs were administered at the time of parasitaemia detection, which occurred at day 10 post-inoculation, for 20 consecutive days at the doses determined in previous studies. Benznidazole was administered at a dose of 100 mg kg−1 day−1 (Filardi and Brener, Reference Filardi and Brener1987), fexinidazole at a dose of 300 mg kg−1 day−1 (Bahia et al. Reference Bahia, de Andrade, Martins, Nascimento, Diniz, Caldas, Talvani, Trunz, Torreele and Ribeiro2012) and posaconazole at a dose of 20 mg kg−1 day−1 in two daily doses (Molina et al. Reference Molina, Martins-Filho, Brener, Romanha, Loebenberg and Urbina2000). A group of 30 animals infected with the parasite but receiving no treatment and 30 healthy mice treated with the different compounds were used as controls.
The kinetics of parasite clearance was investigated in peripheral blood and in cardiac muscle tissue samples. For blood evaluation, animals were bled at 10, 12, 14, 16, 20, 30, 40, 50, 60 and 120 days of infection (di) and parasite quantification was performed by microscopic fresh blood examination (FBE) and qPCR assays. For parasite tissue quantification, groups of 6 animals were euthanized at 10, 16, 30, 60 and 120 di and parasite quantification was performed in 30 mg of heart muscle tissue by qPCR. For FBE, the animals were bled from the tail vein, 5 μL of collected blood were used to estimate the number of parasites as described by Brener (Reference Brener1962). For the qPCR assay, animals were bled from the orbital venous sinus and 200 μL of collected blood was mixed with 35 μL of sodium citrate solution at 129 mm (DOLES, BR) and used for DNA extraction. DNA extraction was performed using the Wizard® Genomic DNA Purification Kit (Promega) with some modifications (Caldas et al. Reference Caldas, Caldas, Diniz, Lima, Oliveira, Cecílio, Ribeito, Talvani and Bahia2012). Assays of qPCR were performed using the SYBR Green system (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions and using the primers TCZ-F 5′-GCTCTTGCCCACAMGGGTGC-3′, where M = A or C and TCZ R 5′-CCAAGCAGCGGATAGTTCAGG-3′ (Invitrogen™) for T. cruzi DNA amplification (Cummings and Tarleton, Reference Cummings and Tarleton2003). The internal control (corresponding to a segment of the murine TNF-α gene) was amplified using the primers TNF-5241 5′-TCCCTCTCATCAGTTCTATGGCCCA-3′ and TNF-5411 5′-CAGCAAGCATCTATGCACTTAGACCCC-3′ (Invitrogen™) (Cummings and Tarleton, Reference Cummings and Tarleton2003). Cycles of amplification were carried out in StepOnePlus real-time PCR System, Applied Biosystems. The cycles consisted of an initial denaturation hold of 10 min at 95 °C followed by 40 cycles of 15 s at 94 °C and 1 min at 64·3 °C with fluorescence acquisition. Amplification was immediately followed by a melt programme with an initial denaturation for 15 s at 95 °C, cooling to 60 °C for 1 min and then a stepwise temperature increase of 0·3 °C s−1 from 60 to 95 °C. All samples were analysed in duplicate and the mean quantification values for T. cruzi DNA were normalized by the data obtained with the murine-specific (TNF-α) primers. Standard curves were performed using 5-fold serial dilutions of an initial suspension of 5×106 parasites/0·1 mL of mice blood (for qPCR in blood) or 106 parasites/30 mg of heart muscle tissue (for qPCR in tissue). Negative samples and reagent controls were processed in parallel in each assay, and all experiments were conducted under controlled conditions.
Myocardial tissue assessment
For morphometric analysis, mice were euthanized at 10, 30 and 120 di (6 animals/group/day) and heart tissues fixed with 10% formalin and embedded in paraffin. Blocks were cut into 4 μm sections and stained by hematoxylin-eosin (H&E) for inflammation assessment (10, 30 and 120 di) or Masson's trichromic for fibrosis quantitative evaluation (120 di). Twenty fields from H&E or Masson's trichromic slides were randomly chosen at 40× magnification for a total of 74931 μm2 analysed myocardium area. Images were captured using a Leica DM 5000 B microcamera (Leica Application Suite, model 2·4·0R1) and processed by the software Leica Qwin V3 image analyser.
CCL2, CCL5 and NO levels
For immunoassays, animals were bled at 10, 30 and 120 di. Plasma levels of CCL2 and CCL5 were measured by ELISA using commercially available antibodies and according to the procedures supplied by the manufacturer (DuoSet® ELISA Development System, RandD Systems®, Minneapolis, MN systems). ELISA measurements for a given experiment were conducted in duplicate in the same plate. Results were expressed as pg mL−1 and the detection limits of the ELISA assays were in the range of 250–3·9 pg mL−1 for CCL2 and 2000–31·25 pg mL−1 for CCL5.
Nitric oxide production was evaluated by nitrite measurement, and its stable degradation product by the Griess reaction. Total nitrite/nitrate levels were determined by the conversion of nitrate to nitrite using nitrate reductase in the presence of reduced nicotinamide adenine dinucleotide phosphate and flavinadenine dinucleotide. After centrifugation each plasma sample (50 μL) was added to 50 μL of the Griess solution (1% sulfanilamide, 0·1% N-(1-Naphthyl) ethylenediamine dihydrochloride, 2·5% H3PO4). Absorbance was measured at 550 nm in the microplate ELISA reader (model 680, BioRad). Nitrite concentrations were determined by using a standard sodium nitrite curve from 125 to 1 μ m.
Statistical analysis
Data were expressed as mean±s.d. Statistical difference for parasitological data among groups of mice at different days, as well as cytokine levels and intensity of cardiac inflammation and fibrosis, were determined by the non-parametric Kruskal–Wallis test. Values of P<0·05 were considered significant.
RESULTS
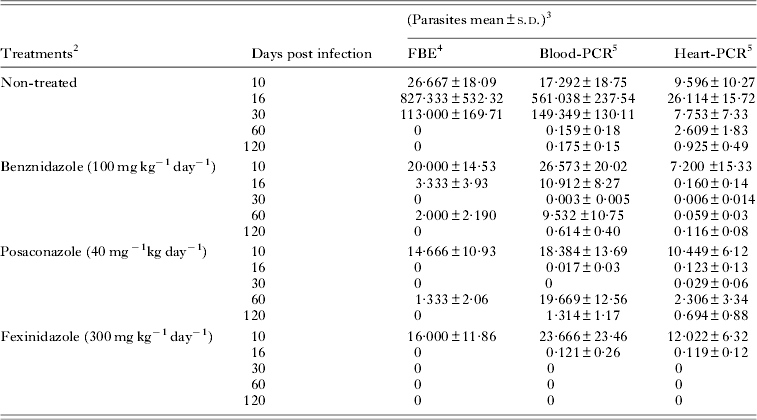
In the first set of experiments, we evaluated the clearance time of parasites induced by fexinidazole, posaconazole and benznidazole treatments in Swiss mice infected with T. cruzi stock that was resistant to benznidazole. Infection of Swiss mice with the VL-10 strain resulted in an acute parasitaemic phase easily detectable by FBE from 10 to 30 di, peaking at 16 di with average levels of 8·2×105 parasites/0·1 mL of blood. Similar results were observed in the blood and cardiac tissue using qPCR assay, since a higher parasite load was detected in blood (5·6×105 parasites/0·1 mL) and heart tissue (2·6×104 parasites/30 mg of tissue) samples at 16 di. However, in the chronic phase, the parasitism was only detected by qPCR assay, being higher in the cardiac tissue than in the blood tissue (Table 1).
Table 1. Parasite load in benznidazole-, posaconazole- and fexinidazole-treated mice infected with VL-10 Trypanosoma cruzi strain1, 2

1 Swiss female (n = 6) weight 18 to 22 g inoculated with 5×103 trypomastigotes.
2 Treatment was initiated at 10 day after inoculation followed by 20 days and it was administered per oral route.
3 Data refer to the number of parasites in 0·1 mL of blood or 30 mg of heart tissues ×103.
4 FBE – fresh blood examination.
5 Real-time PCR in blood (Blood-PCR) and in heart tissues (Heart-PCR).
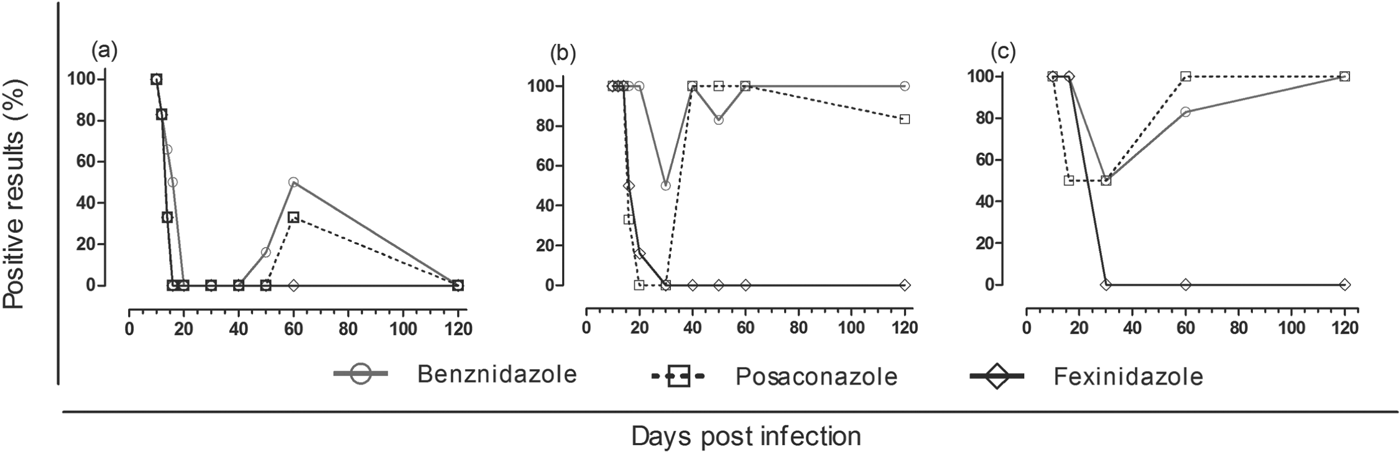
Our results showed that fexinidazole and posaconazole induced a faster parasitaemia clearance than benznidazole (parasites were not detected at the 6th day of treatment, 16 di, by FBE) (Fig. 1a and Table 1). This result was confirmed by qPCR assay, since the parasitaemia detected was several times lower in blood samples of animals treated with fexinidazole (121 parasites/0·1 mL) and posaconazole (17 parasites/0·1 mL) than detected in samples collected from benznidazole-treated animals (1·09×104 parasites/0·1 mL). At this time, all mice treated with benznidazole had positive parasitaemia in the qPCR assay, while the same results were detected in 33% of samples collected in posaconazole and in 50% of fexinidazole-treated animals (Fig. 1b). Additionally, at the last day of treatment (30 di), the qPCR assay had negative results in all blood samples of fexinidazole- and posaconazole-treated mice (Fig. 1b). The same result was found in only 50% of animals treated with benznidazole, and the positive results had lower levels (3 parasites/0·1 mL of blood) than the infected and non-treated group (1·49×105 parasites/0·1 mL blood) (Table 1).

Fig. 1. Trypanosoma cruzi clearance curves. Parasite clearance time in mice infected with VL-10 benznidazole-resistant T. cruzi strain during (10 to 30 di) and after (40 to 120 di) benznidazole, posaconazole and fexinidazole treatment. (a) Results determined by fresh blood examination; (b) real-time PCR in blood and (c) in heart tissues of treated mice. n = 6; di, days of infection.
As the clearance of parasites in the peripheral circulation may not always be correlated with the clearance in the tissue, we also evaluated the cardiac parasitism of treated mice. The treatments induced a reduction of cardiac parasite load (119–160 parasites/30 mg of heart tissue) at the 6th day of treatment, while in the samples of infected and non-treated mice, there were 2·6×104 parasites/30 mg of heart tissue (Table 1). At this time, parasites were detected in 50% of the tissue samples collected from posaconazole-treated animals and in all of the samples of fexinidazole- and benznidazole-treated mice, suggesting a quick response of posaconazole in the management of tissue parasitism in low or ‘controlled’ levels during a therapeutic regimen (Fig. 1c), since during the treatment, multiplication of the parasite was low in cardiac tissue, as compared with that observed before treatment, or between non-treated animals (Table 1). However, at the last day of treatment, the qPCR had negative results in all fexinidazole-treated animals, while negative results were detected in only 50% of those treated with benznidazole and posaconazole (Fig. 1c). Interestingly, our results with posaconazole showed that the parasite clearance was first detected in peripheral blood (Table 1 and Fig. 1a–c). For these mice, at the last day of the treatment, the qPCR had negative results in all blood samples and in 50% of the tissue samples. However, after the end of the treatment, an increase of parasitism was detected in both peripheral blood and heart tissue samples of animals treated with posaconazole and benznidazole, reaching similar or higher levels than those detected in samples obtained in infected and non-treated animals (Table 1). The parasitaemia reactivation was detected faster in benznidazole-treated animals than in mice treated with posaconazole (Fig. 1a and b). Different data were detected in fexinidazole-treated animals, since after the 6th day of treatment until the end of evaluation (at 120 di), the qPCR had negative results (Table 1 and Fig. 1c). Interestingly, the qPCR assay carried out after the end of the treatment with samples of mice treated with benznidazole or posaconazole showed a higher parasitism level in blood than in the cardiac tissue, this result being opposite to that found in the infected and untreated animals (Table 1).
In all experiments, the evaluated compounds were well tolerated by animals and mortality was not detected among treated mice. Additionally, no differences in weight gain were found among treated and non-infected animals during all of the evaluated period (data not shown).
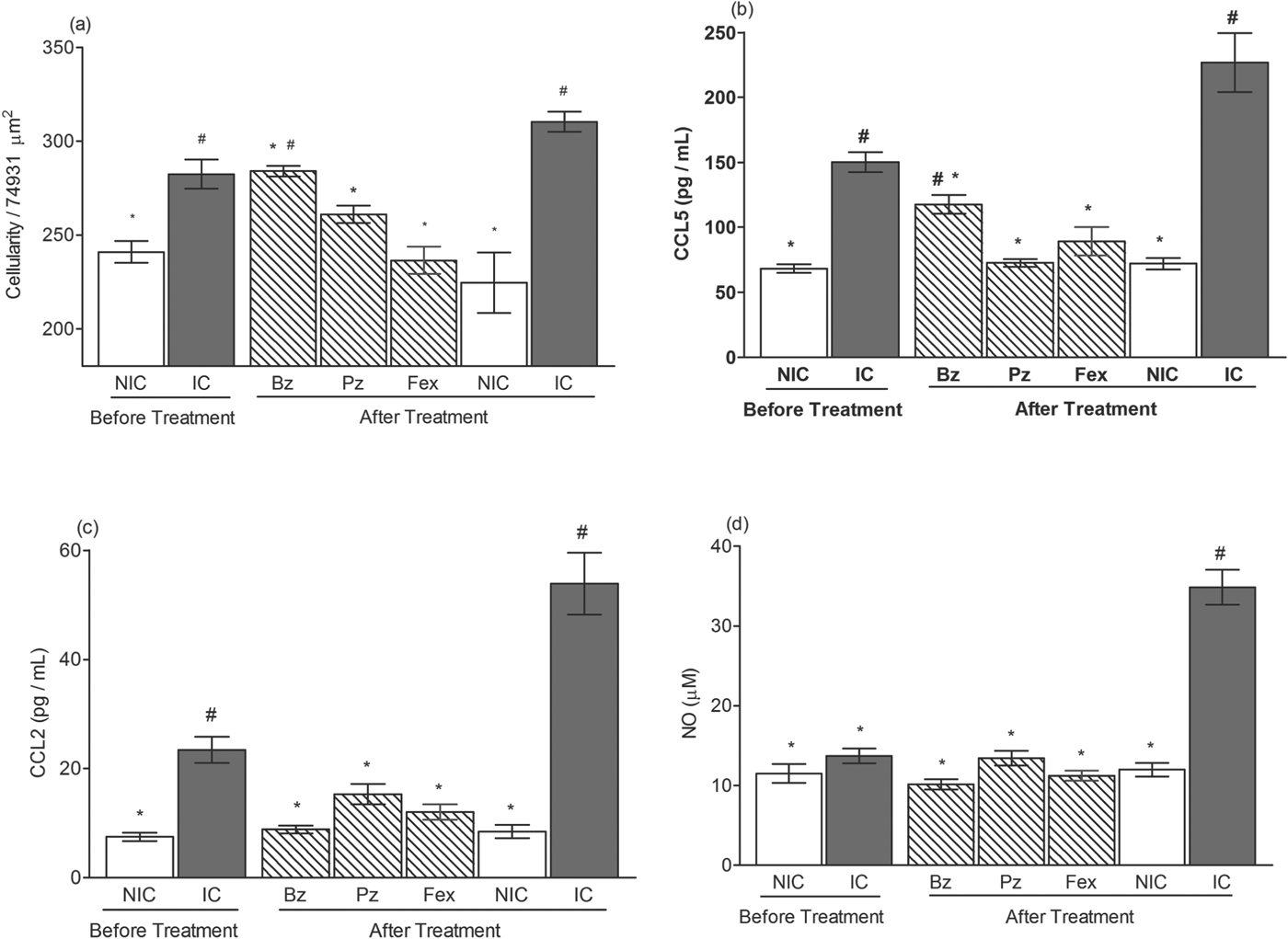
In order to assess the effectiveness of these different treatments in reducing or preventing the development of myocardial lesions in infected mice, a quantitative analysis of inflammation in the heart tissue was performed before treatment (10 di), at the acute (30 di) and chronic (120 di) phases of the infection. Treatments were able to reduce the number of inflammatory cells at the acute phase of experimental T. cruzi infection, as observed by a significant increase of inflammatory infiltrates in the hearts of the infected non-treated animals. However, mice treated with benznidazole presented a higher number of inflammatory cells than the healthy mice (Fig. 2a). In contrast, fexinidazole and posaconazole treatments prevent heart inflammation, as illustrated by similar levels of inflammatory intensity between treated and healthy animals (Fig. 2a).

Fig. 2. Inflammatory outcome from treated mice during acute phase of Trypanosoma cruzi infection. Mice were infected with 5000 trypomastigotes of VL-10 strain and treated with benznidazole (Bz), posaconazole (Pz) or fexinidazole (Fex). Animals were euthanized before treatment (10 days of infection) and after treatment (30 days of infection). For controls, infected and non-treated (IC) and non-infected (NIC) groups were evaluated. (a) Myocardial inflammatory cell count in heart muscle of mice. (b) CCL5, (c) CCL2 and (d) NO levels in plasma sample compared with control groups before and after treatment. # denotes significant differences in relation to non-infected mice (P<0·05). * denotes significant differences in relation to infected and non-treated (P<0·05).
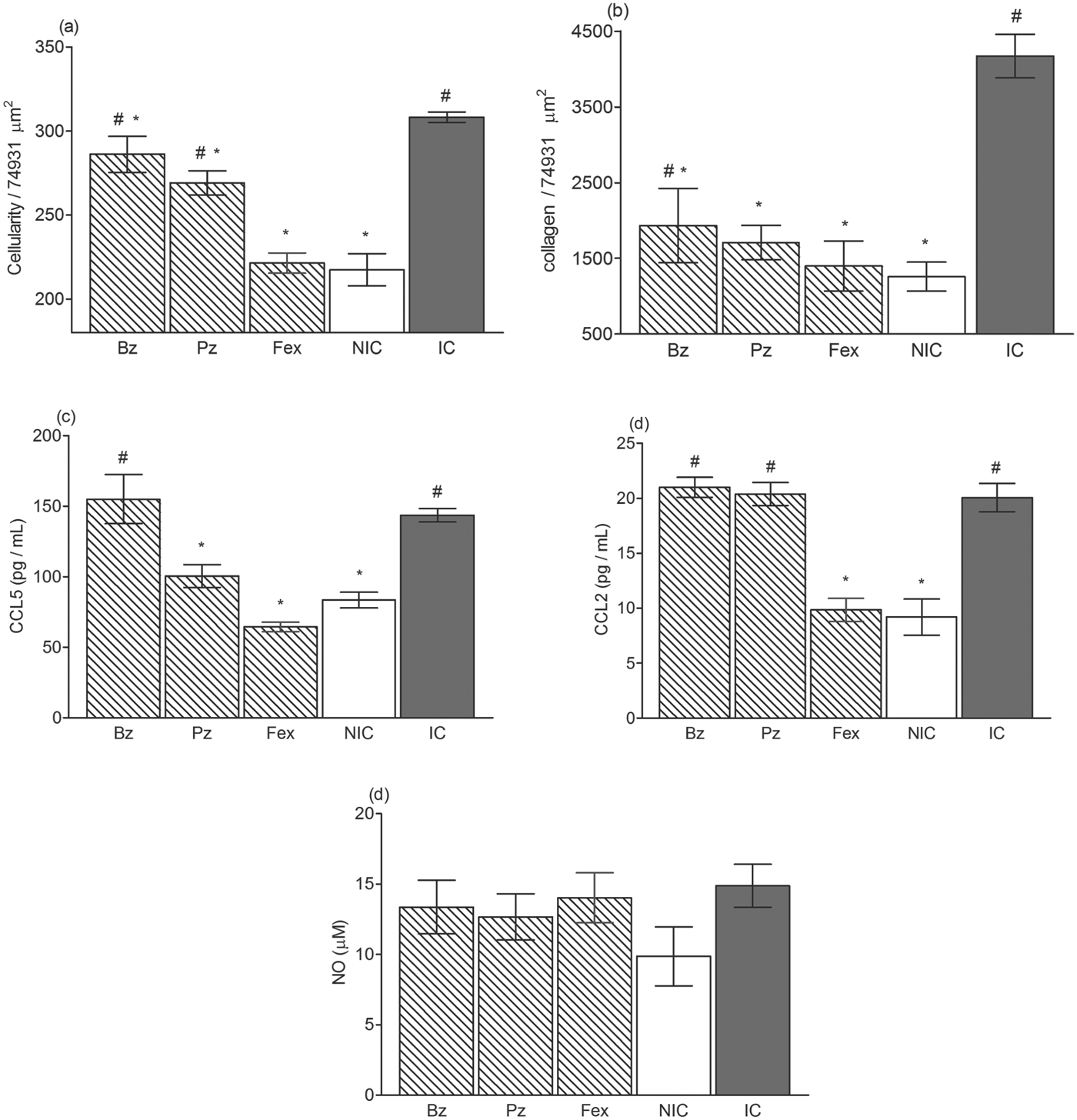
The increased parasite loads during the chronic phase of the infection are in line with the increased amount of inflammatory cells in the cardiac tissue of benznidazole- and posaconazole-treated mice. At this time, mice treated with benznidazole and posaconazole had a higher number of inflammatory cells than did the healthy mice (Figs 3a and 4). Interestingly, the comparison of the number of inflammatory cells in the heart tissue of animals treated with different compounds allowed ordering of the effects of the drugs as follows: (benznidazole <posaconazole<fexinidazole). The means and standard deviations, including the values of controls, were: benznidazole = 286·1±10·8 cells/74931 μ m2; posaconazole = 269·1±17·7 cells/74931 μ m2; fexinidazole = 221·4±13·3 cells/74931 μ m2 and healthy animals = 217·5±23·4 cells/74931 μ m2. Moreover, analysis of the fibrotic area in the hearts of the treated mice showed that all treatments were efficient in reducing fibrosis in the chronic phase of experimental infection. Interestingly, among animals treated with posaconazole and fexinidazole, a similar heart fibrosis intensity with healthy mice was detected, but significant differences were observed when the heart fibrotic area from healthy and benznidazole-treated mice were compared (Figs 3b and 4).

Fig. 3. The outcome of treatment on the development of the chronic phase of experimental Chagas disease. Mice were inoculated with 5000 trypomastigotes of VL-10 strain and treated with benznidazole (Bz), posaconazole (Pz) or fexinidazole (Fex) and euthanized at 90 days after treatment (120 days after inoculation). For controls, infected and non-treated (IC) and non-infected (NIC) groups were evaluated. (a) Myocardial inflammatory cell count in heart muscle of mice. (b) Fibrosis area in heart muscle of mice. (c) CCL5, (d) CCL2 and (e) NO levels in plasma sample of mice compared with control groups, 90 days after treatment. # denotes significant differences in relation to non-infected mice (P<0·05). * denotes significant differences in relation to infected and non-treated (P<0·05).

Fig. 4. Treatment reduces chronic Chagas disease-associated inflammatory and fibrotic pathology. Analysis of histological sections of hearts from mice infected with VL-10 strain of Trypanosoma cruzi, 40× magnification. (A–E) Hematoxylin-eosin staining for inflammation assessment and (F–J) Masson's trichromic for fibrosis assessment. (A and F) Myocardial sections from healthy mice, (B and G) from infected control non-treated mice, (C and H) from benznidazole-treated mice, (D and I) from posaconazole-treated and (E and J) from fexinidazole-treated mice at 120 days of infection. # denotes significant differences in relation to non-infected mice (P<0·05). * denotes significant differences in relation to infected and non-treated mice (P<0·05).
We also evaluated the CCL2, CCL5 and NO levels in the plasma of the treated and control animals in order to compare the efficacy of the treatments on the leucocyte chemotractant mediators and NO profiles. The profile of chemokines CCL5 (Fig. 2b) and CCL2 (Fig. 2c) detected in the plasma of infected and non-treated animals was correlated with the parasite load, showing an increase in the early phase of the infection (10 di) peaking at 30 di with plasma levels ∼3-fold (CCL5) and ∼6-fold (CCL2); higher compared with healthy mice. The level of these chemokines was reduced during the chronic phase of infection, but remained significantly higher than those detected in the plasma of healthy mice (Fig. 3c and d). A different pattern was observed in animals treated with the evaluated compounds, and correlated with their anti-T. cruzi activity. At the end of the treatments, the chemokine levels among the treated animals were similar to those detected in healthy animals (Fig. 2b and c). However, at the chronic phase, the chemokine pattern presented particularities according to the compounds used: (i) similar between infected untreated and benznidazole-treated mice; (ii) posaconazole-treated mice had similar CCL2 and smaller CCL5 plasma levels (P<0·05) than the infected mice; and (iii) fexinidazole-treated mice had plasma chemokine levels indistinguishable from those of healthy animals (Fig. 3c and d).
Levels of NO were also measured in both phases of infection, as shown in Figs 2d and 3e. At 30 di, plasma concentrations of NO reached ∼3·3-fold higher than those observed for healthy mice (Fig. 2d). Interestingly, when the parasitism was controlled, there was a decrease in NO, reaching levels similar to those of healthy animals at 120 di, and the treated ones, who always had NO levels similar to healthy mice (Fig. 3e).
To confirm the influence of evaluated compounds on CCL2, CCL5 and NO levels, healthy animals were treated using the same therapeutic regime, and chemokines and NO dosages were performed after treatment. Our results showed no significant difference between the chemokines and NO levels detected in the plasma sample of healthy animals, treated or not, showing that drugs used, by themselves, do not interfere in these plasma inflammatory mediators.
DISCUSSION
Some studies consider that despite their inability to eradicate the parasite, the drug-induced reduction of the parasite loads in infected tissues has positive effects on the clinical evolution of the T. cruzi infection by reducing the severity of the associated inflammatory processes (Andrade et al. Reference Andrade, Stocker-Guerret, Pimentel and Grimaud1991; Tarleton et al. Reference Tarleton, Sun, Zhang and Postan1994; Hardison et al. Reference Hardison, Wrightsman, Carpenter, Kuziel, Lane and Manning2006; Caldas et al. Reference Caldas, Talvani, Caldas, Carneiro, de Lana, Guedes and Bahia2008).
To characterize the role of parasite clearance time induced by different treatments and their outcome on Chagas disease, we used qPCR and defined the dynamic relationship between the efficacy of the treatments and the heart tissue damage during the acute and chronic stages of the infection in mice. In the acute infection, the treatment efficacy to induce parasitic clearance and disease severity showed absolute correlation. Furthermore, drug clearance of parasites induced by the fexinidazole treatment resulted in the disappearance of inflammatory lesions and the resolution of the disease. Previous studies failed to show a correlation between parasitic burden, disease progression and severity (Ãnez et al. 1999; Tarleton and Zhang, Reference Tarleton and Zhang1999; Caldas et al. Reference Caldas, Talvani, Caldas, Carneiro, de Lana, Guedes and Bahia2008). Here, the comparisons of blood and tissue parasite loads along the course of infection indicate that all treatments initiated in the acute phase were able to induce a global decrease of parasite loads in both peripheral blood and cardiac tissue, but with different efficacies. Posaconazole and fexinidazole treatments induced a faster parasite clearance in relation to benznidazole. Among the posaconazole-treated mice, the blood parasite clearance was detected upon both FBE and qPCR. However, the parasites were still detected in 50% of tissue samples from the heart. These results indicate that the determination of parasite DNA in blood does not reflect the actual parasitic load in cardiac tissue when performed immediately after the end of treatment and it is not sufficient at this time to assess the effect of treatment. As an overall analysis, our results indicated that evaluated compounds presented potent activity against the VL-10 T. cruzi strain, but with individual particularities in their potency. The potent anti-T. cruzi activity of fexinidazole could be related to its pharmacokinetic properties. Fexinidazole is rapidly oxidized in vivo in two more therapeutically relevant species, sulphoxide and sulphone metabolites. A high and prolonged systemic bioavailability of these metabolites is achieved a few hours after drug administration (Sokolova et al. Reference Sokolova, Wyllie, Patterson, Oza, Read and Fairlamb2010). Although both metabolites present anti-T. cruzi activity (Bahia et al. Reference Bahia, Nascimento, Mazzeti, Marques, Gonçalves, Mota, Diniz, Caldas, Talvani, Shackleford, Koltun, Saunders, White, Scandale, Charman and Chatelain2014), fexinidazole sulphoxide is extensively converted to sulphone, the final metabolite to appear in the blood. Fexinidazole sulphone presents a better pharmacokinetic profile, as half-life and plasma concentrations, superior to sulphoxide metabolite and benznidazole (Workman et al. Reference Workman, White, Walton, Owen and Twentyman1979; Bahia et al. Reference Bahia, Nascimento, Mazzeti, Marques, Gonçalves, Mota, Diniz, Caldas, Talvani, Shackleford, Koltun, Saunders, White, Scandale, Charman and Chatelain2014) and is the species most likely associated with efficacy following administration of fexinidazole.
Our data demonstrate that early treatment prevents severe disease progression, due to the control of parasitic replication and its consequent immunopathological implications. Probably, these events induced a balance between the clearance of the parasites and the prevention of the immune-mediated pathology. The degree of regression relates to the successful treatment with cured mice showing the most pronounced reversal of the pathological signs.
In agreement with our data, several authors have shown that even in cases of treatment failure, there was a reduction in the parasitic load as well as in the cardiac lesions, events followed by a slow progression of chronic cardiac injury (Andrade et al. Reference Andrade, Stocker-Guerret, Pimentel and Grimaud1991; Garcia et al. Reference Garcia, Ramos, Senra, Vilas-Boas, Rodrigues, Campos-de-Carvalho, Ribeiro-Dos-Santos and Soares2005; Caldas et al. Reference Caldas, Talvani, Caldas, Carneiro, de Lana, Guedes and Bahia2008; Diniz et al. Reference Diniz, Caldas, Guedes, Crepalde, de Lana, Carneiro, Talvani, Urbina and Bahia2010; Santos et al. Reference Santos, de Lima, Gravel, Martins, Talvani, Torres and Bahia2012).
According to Andrade et al. (Reference Andrade, Stocker-Guerret, Pimentel and Grimaud1991), the presence of the parasite and the inflammatory process underlie the pathological evolution of chronic Chagas disease. In the present work, chemokines and NO were measured in the plasma samples at the same time that parasitic load and cardiac tissue lesion evaluations were performed. The increased parasite load is in line with the increased amount of CCL2 and CCL5 observed in the plasma of these animals as compared with healthy or cured mice. Interestingly, at the chronic phase, only benznidazole-treated animals presented a significantly higher fibrotic area in the cardiac tissue in comparison with healthy animals, as well as higher levels of CCL2 and CCL5 chemokines. However, the intensity of heart fibrosis was higher in infected and untreated animals, demonstrating the beneficial effect of the reduction of the tissue parasitism induced by different treatments in the disease outcome, even in the absence of parasitological cure. In agreement, a study involving chemokine expression in the heart of chronically T. cruzi-infected beagle dogs with the cardiac form also showed increased mRNA expressions of CXCR3, CCL4 and CCL5 as compared with those in the indeterminate form (Guedes et al. Reference Guedes, Urbina, de Lana, Afonso, Veloso, Tafuri, Machado-Coelho, Chiari and Bahia2004).
In the present study, all evaluated compounds induced a strong reduction in the parasite load, displaying their potent anti-T. cruzi activity. However, a relapse of parasitaemia was detected in benznidazole- and posaconazole-treated mice showing that these compounds were not effective in inducing parasitological cure in mice infected with VL-10 strain. These results were in line with Filardi and Brener (Reference Filardi and Brener1987) who showed the full resistance of the VL-10 strain to benznidazole. Molina et al. (Reference Molina, Martins-Filho, Brener, Romanha, Loebenberg and Urbina2000) have reported the efficacy of posaconazole in curing mice infected with benznidazole-resistant T. cruzi strain in a long-term treatment (28 days, 7 days rest and another 15 days). However, a phase II clinical trial to investigate the efficacy and safety of posaconazole in Spain (http://clinicaltrials.gov/ct2/show/NCT01162967?term=posaconazole) resulted in good safety, but unfortunately, this compound had little to no sustained efficacy in treating patients in the chronic phase of Chagas disease as a single medication (Israel Molina, International Congress of Tropical Medicine http://ictmm2012.ioc.fiocruz.br/program_25_sept.html). These results highlight the need to investigate alternative dosing regimens and possible combination therapies to improve treatment efficacy. This idea is in line with others; Pinazo et al. (Reference Pinazo, Espinosa, Gállego, López-Chejade, Urbina and Gascón2010) described a successful resolution of a T. cruzi infection (a case of chronic Chagas disease and systemic lupus erythematosus) following treatment with posaconazole (400 mg for 12 h during 90 days). Previous treatment of the same patient with benznidazole induced a reduction, but not a complete elimination of the parasite. These results indicate that more prolonged therapeutic regimens may be needed to improve Chagas disease treatment efficacy with azoles derivatives. Additionally, the different mechanisms of action and pharmacological characteristics of benznidazole, posaconazole and fexinidazole (Wilkinson and Kelly, Reference Wilkinson and Kelly2009; Urbina, Reference Urbina2010; Bahia et al. Reference Bahia, de Andrade, Martins, Nascimento, Diniz, Caldas, Talvani, Trunz, Torreele and Ribeiro2012) might also contribute to explaining the diversified cure rate in VL-10 infected mice. This idea is corroborated by the observations of Guedes et al. (Reference Guedes, Urbina, de Lana, Afonso, Veloso, Tafuri, Machado-Coelho, Chiari and Bahia2004) showing that different therapeutic strategies using benznidazole (60-day treatment) and albaconazole (90-day treatment) induced a definitive cure in dogs infected with T. cruzi Y strain, while the administration of albaconazole for 60 days was able to cure only 25% (1 of 4) of the animals. In this scenario, the individualized therapy schemes, considering both the drug concentration and the time of treatment, might be important for therapeutic success.
Finally, the most important observations of this study were related to the severity of the cardiac lesions in both the acute and chronic phases of the infection and the drug-induced parasitaemia clearance time. These observations suggest that the prevention of T. cruzi infection progression in Chagas disease severity might be achieved through chemotherapeutic strategies that enhance parasite clearance and reduce parasite load in a permanent way, thereby contributing to a better balance in the host-parasite relationship.
FINANCIAL SUPPORT
This study was funded by DNDi, Fundação de Amparo a Pesquisa do Estado de Minas Gerais and Universidade Federal de Ouro Preto. SC is thankful to FAPEMIG for the BIP fellowship programme. MTB and AT are grateful to the CNPq research fellowship programme. LFD is thankful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for CAPES/PNPD 2012. DNDi would also like to thank the following donors for their support: Department for International Development (DFID), UK; Médecins sans Frontières/Doctors without Borders (MSF), International; Spanish Agency for International Development Cooperation (AECID), Spain; Swiss Agency for Development and Cooperation (SDC), Switzerland; and private foundations and individual donors.
COMPETING INTERESTS
None to declare.
ETHICAL APPROVAL
This study was approved by the Ethics Committee in Animal Research at UFOP [number 2009/17].