I. INTRODUCTION
Bismith(III) oxide exhibits an extraordinary rich phase polymorphism appearing in seven modifications: monoclinic α-, tetragonal β-, body-centered cubic γ-, face-centered cubic δ-, orthorhombic ɛ-, triclinic ω-, and a high-pressure hexagonal phase (Sillén, Reference Sillén1937; Gattow and Schröder, Reference Gattow and Schröder1962; Levin and Roth, Reference Levin and Roth1964a; Harwig, Reference Harwig1978; Harwig and Weenk, Reference Harwig and Weenk1978; Harwig and Gerards, Reference Harwig and Gerards1979; Gualtieri et al., Reference Gualtieri, Imovilli and Prudenziati1997; Sammes et al., Reference Sammes, Tompsett, Näfe and Aldinger1999; Cornei et al., Reference Cornei, Tancret, Abraham and Mentre2006; Ghedia, et al., Reference Ghedia, Locherer, Dinnebier, Prasad, Wedig, Jansen and Senyshyn2010). Among these seven polymorphisms, metastable γ- and high-temperature δ-Bi2O3 are found to be the most investigated because of their interesting structural and other properties. The high-temperature δ-Bi2O3 modification can be stabilized at room temperature by using many isovalent and aliovalent cations to substitute Bi3+ (Shuk et al., Reference Shuk, Wiemhofer, Guth, Gijpel and Greenblatt1996; Sammes et al., Reference Sammes, Tompsett, Näfe and Aldinger1999), but this often causes the formation of modulated structures based on a fluorite-related substructure (Watanabe, Reference Watanabe1997; Pang et al., Reference Pang, Feng, Tang and Xu1998; Darriet et al., Reference Darriet, Launaya and Zuniga2005). The metastable γ-Bi2O3 phase can also be stabilized down to room temperature by doping with many different cations, and in that case the structural features depend on the size, oxidation state, and concentration of dopant (Levin and Roth, Reference Levin and Roth1964b).
The title compound is one of the doped γ-Bi2O3 phases belonging to the Bi12SiO20 (sillenite) structure type. There are two cation sites in this structure: general 24f and special 2a. The cation, labeled as Bi1, is positioned at the 24f site, while the dopant cation, M, is positioned at the 2a site, i.e. in the origin and center of the bcc unit cell (space group I23). As long as the charge of dopant is 4 + , the crystal structure is composed of Bi1O7 polyhedra and ideal MO4 tetrahedra. Five oxygen atoms at shorter distances form a distorted square pyramid, and with two other oxygen atoms at very long distances create a 5 + 2 coordination polyhedron around Bi1. According to bond valence (BV) calculations (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007), the contribution of two distant oxygen atoms to the bond valence sum of Bi13+ ion is negligible (usually <3%), i.e. the coordination number of Bi1 can be described as five. Two square pyramids share a common edge and two pairs of adjacent pyramids are connected by the MO4 tetrahedra along [100]. Thus, two pairs of pyramids with two MO4 tetrahedra form a cavity that contains the 6s 2 lone electron pairs of Bi3+ ions.
If the charge of the M cation is less than 4+, the stoichiometry is more complicated, and the formulae of corresponding doped sillenite phases can be calculated from the general formula Bi112(Bi23+(4−n)/(5−n)M n+1/(5−n))O19+1/(5−n) assuming full occupancy of both cationic sites (Valant and Suvorov, Reference Valant and Suvorov2002). For such phases, two basic structural models have been proposed by Craig and Stephenson (CS-model; Craig and Stephenson, Reference Craig and Stephenson1975) and by Radaev (R-model; Radaev et al., Reference Radaev, Muradyan and Simonov1991; Radaev and Simonov, Reference Radaev and Simonov1992). According to the CS-model, Bi25+ and M n+ ions share the 2a site. Previously, by analysis of the geometry parameters and results of BV calculations for 30 structurally characterized sillenites, it was shown that the R-model is more acceptable (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007). This means that M cations and Bi23+ ions are slightly moved from the origin along the [111]-direction to the 8c site. Also, the vacancies of O atoms should be introduced for charge balance. The deficiency of positive charge is compensated for by a partial occupancy of O3 atoms forming a trigonal pyramid instead of tetrahedral coordination of the 8c site. If the dopant is a Pb2+ ion, the formula of the corresponding γ-Bi2O3 phase is Bi12(Bi2/3Pb1/3)O19.33 or Bi38PbO58.
If the charge of the M cation is 5+, then the occupancy of the 2a site should be limited to 0.80. However, there is at least one sillenite, Bi12.03V0.89O20.27, where the occupancy of the 2a site exceeds 0.80 (Radaev and Simonov, Reference Radaev and Simonov1992). In this case, an excess of positive charge is compensated for by a partial occupancy of the 6b site (the middle of the unit-cell edges) with an additional O4 atom.
As was shown in our previous work on sillenites (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007), considering the geometry parameters and oxidation numbers of M cations, new structural characterizations are needed for at least three systems: Co-, Mn-, and Pb-doped γ-Bi2O3. To explore their structural complexity, we began to investigate Pb2+-incorporating sillenites. Our earlier (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007) and some new results (to be published) have revealed that single-phase Pb-doped γ-Bi2O3 specimens with Bi:Pb ratios of 11:1, 12:1, 24:1, 36:1, and 38:1 can easily be prepared by high-temperature solid state reaction. Also, the application of some other treatments, such as mechanochemical synthesis, allows the preparation of Pb-doped γ-Bi2O3 even from starting mixtures with 1:1 and 2:1 Bi:Pb ratios (Zyryanov, Reference Zyryanov2004). Independently of the type of synthesis, the Pb-doped γ-Bi2O3 unit-cell parameters are very close to those of undoped γ-Bi2O3, which is a logical consequence of the great similarity of Bi3+ and Pb2+ ions: they are isoelectronic, with similar ionic radii, and both have the lone electron pair.
Previously, Murray et al. (Reference Murray, Catlow, Beech and Drennan1986) described the structure of Bi12PbO19. The analysis was performed using high-resolution neutron powder diffraction data. Two structural models, both of the CS type with some oxygen vacancies, were presented: the first with Pb2+ at the 2a site and Bi3+ at the 24f site, and the second with a random distribution of cations over the 2a and 24f sites. None of the models can be regarded as satisfactory, since the isotropic atomic displacement parameters (ADPs) of the cations at the 2a site were very high (B iso = 4.2–5.3 Å2) and the observed Pb–O distances were too short (1.95 Å). Besides, there are two papers describing the crystal structures of Bi24Pb2O40 (Rangavittal et al., Reference Rangavittal, Row and Rao1994) and Bi12Pb0.89O19.78 (Mazumdar, Reference Mazumdar1993) refined using X-ray powder diffraction data. In both cases, the presence of Pb4+ ions was assumed. However, these results are of very low reliability and will not be considered further. For example, the published unit-cell parameters are 10.292(8) Å for Bi24Pb2O40 and 10.21 Å for Bi12Pb0.89O19.78. Both values are well out of the range of known unit-cell parameters for Pb-doped γ-Bi2O3 phases, which are grouped at about 10.26 Å (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007).
In addition to the very interesting structural properties, Pb-doped Bi2O3 phases also have a great potential application. For example, the mixed oxides from the Bi2O3–PbO system are good high-temperature ionic conductors, but also exhibit mixed ionic/electronic conductivity that could be useful in oxygen selective membranes (Honnart et al., Reference Honnart, Boivin, Thomas and De Vries1983; Fee and Long, Reference Fee and Long1996; Fee et al., Reference Fee, Sammes, Tomsett, Soto and Cartner1997). Next, mixed heavy metal oxide glasses based on Bi2O3 and PbO have interesting physical properties such as high density, high linear or non-linear refractive index, and long infrared cut-off (Pan and Ghosh, Reference Pan and Ghosh2000; Knoblochova et al., Reference Knoblochova, Ticha, Schwarz and Tichy2009; Reshak et al., Reference Reshak, Lakshminarayana, Proskurina, Yushanin, Calus, Chmiel, Miedzinski and Brik2010; Shi and Qian, Reference Shi and Qian2010; Salem and Mohamed, Reference Salem and Mohamed2011). Moreover, the mixed Bi–Pb oxides with sillenite-type structure possess other extraordinary characteristics: they are wide-band-gap high-resistivity semi-insulating, photoconductive, photoluminescent, electronic, optoelectronic, acoustic, and piezoelectric materials (Mitsuyu et al., Reference Mitsuyu, Wasa and Hayakawa1976; Manier et al., Reference Manier, Champarnaud-Mesjard, Mercurio, Bernache and Frit1988; Sammes et al., Reference Sammes, Tompsett and Cartner1995; Borowiec et al., Reference Borowiec, Kozankiewicz, Szymczak, Zmija, Majchrowski, Zaleski and Zayarnyuk1999; Valant and Suvorov, Reference Valant and Suvorov2001, Reference Valant and Suvorov2002).
In this paper, we report the synthesis and detailed structural properties of Pb-doped γ-Bi2O3 with a Bi:Pb ratio of 24:1 investigated by X-ray powder diffraction, Rietveld analysis and BV calculations.
II. EXPERIMENTAL
A. Synthesis conditions
The yellow-orange polycrystalline sample with nominal composition Bi24PbO37 (12Bi2O3·PbO) was prepared by high-temperature solid state reaction. Stoichiometric amounts of bismite, α-Bi2O3 (99.975%, Alfa Aesar), and massicot, PbO (99.9%, Alfa Aesar), were dry homogenized for about 30 min in an agate mortar, heated at 690 °C for 1.5 h (heating rate 4 °C min−1) in an open Pt crucible, and then annealed to room temperature.
B. Rietveld refinement
X-ray powder diffraction data of the re-ground polycrystalline product were collected using a SEIFERT XRD3000TT X-ray powder diffractometer with monochromated CuKα radiation (λ = 1.5418 Å) in a range 4–140° 2θ with a step-width of 0.02° and a constant counting time of 10 s per step. The FULLPROF software was used for the Rietveld refinement (Rodriguez-Carvajal, Reference Rodriguez-Carvajal1993) in WINPLOTR environment (Roisnel and Rodriguez-Carvajal, Reference Roisnel and Rodriguez-Carvajal2001).
The Rietveld refinement was performed adhering to the previously published recommendations (Karanović et al., Reference Karanović, Petrović-Prelević and Poleti1999; McCusker et al., Reference McCusker, Von Dreele, Cox, Louër and Scardi1999). The profiles were described by the pseudo-Voigt function as the most frequently used function in this type of analysis (Young and Wiles, Reference Young and Wiles1982; Hill, Reference Hill1992; Hill and Cranswick, Reference Hill and Cranswick1994), with n in n × FWHM equal to 30 and the limit for the peak asymmetry set to 90° 2θ. In the first stage of the refinement, the background was modeled using a linear interpolation between 80 selected points and in the last cycles of refinement the Fourier filtering method with the window size set to 2000 was used.
After an initial full-profile fitting, the structural refinement was performed by testing five structural models described later in detail. During the refinement of isotropic ADPs of O2 and O3 atoms, either non-positive ADP values were observed or the refinement became unstable. This can be explained by the significant difference between the X-ray scattering powers of Bi/Pb and O atoms. To avoid this problem, ADPs for all O atoms were restrained to be equal, i.e. O atoms were refined with a common isotropic ADP. This approach resulted in a rather acceptable common ADP value [1.7(3) Å2]. In the final cycles of the refinement, a total of 21 parameters were refined using 6501 data points and 210 reflections. The site occupation factors were not refined.
C. Bond valence calculations
The bond valence calculations were performed using the equation ∑ν ij = ∑exp[(R 0− R ij)/B] (Brown, Reference Brown2009), where ν ij is the bond valence for an interatomic bond, ∑ν ij is the overall bond valence summed over all interatomic bonds of one ion, R 0 is the bond valence parameter that represents the length of a bond of unit valence and was taken to be 2.094 and 2.112 Å for Bi3+–O and Pb2+–O, respectively (Brese and O'Keeffe, Reference Brese and O'Keeffe1991), R ij is the experimentally obtained bond length and B is a 'universal' constant equal to 0.37 Å (Brown and Altermatt, Reference Brown and Altermatt1985).
III. RESULTS AND DISCUSSION
A. Rietveld refinement and valence bond calculations
As mentioned in the Introduction, the formula Bi38PbO58 should represent the stoichiometrically ideal Bi:Pb molar ratio according to the R-model (Radaev et al., Reference Radaev, Muradyan and Simonov1991; Radaev and Simonov, Reference Radaev and Simonov1992). However, we found that a single Pb-doped γ-Bi2O3 phase with the composition BixPbO3x/2+1 can be obtained in the relatively wide x range, 11 ≤ x ≤ 38, indicating the possibility of isomorphous replacement of Pb2+ for Bi3+ at both crystallographic sites. The sample with composition Bi24PbO37 was chosen for this study because it was in the middle of the above interval.
At the beginning, the structural model published by Murray et al. (Reference Murray, Catlow, Beech and Drennan1986) with Pb2+ and Bi3+ at the 2a site was tested for comparison. Irrespective of whether the coordination around the 2a site is tetrahedral or trigonal, very similar results, i.e. very high ADPs for cations at the 2a site and O3 atom (about 4.0 and 3.0 Å2, respectively), were obtained. These results clearly confirmed that CS-model (Craig and Stephenson, Reference Craig and Stephenson1975) is inappropriate for the investigated sample.
Therefore, the R-model should be applied, which means that 2a cations are slightly displaced along the [111]-direction to the 8c site (with an occupancy of 0.25). This also caused an oxygen vacancy generation at the O3 position, resulting in the formation of a trigonal pyramidal geometry with a cation at the apex. Such coordination is asymmetrical and more favorable than a tetrahedral one for large Pb2+ and Bi3+ ions with their inert electron pairs. Actually, the presence of O3 vacancies was first suggested by Murray et al. (Reference Murray, Catlow, Beech and Drennan1986), but in their model Bi2 or Bi2/Pb2 cations were kept at the 2a site.
The main objective of this study was whether Pb2+ was preferentially situated at the 24f or 8c site. To test this, five structural models with the possible cation distributions were generated and refined by the Rietveld method. In all cases, it was assumed that no extra vacancies are present at both cationic sites, and a small amount of an additional O4 atom was placed at the 6b site to achieve charge balance.
The considered cation distributions over two sites indicated by formulae representing the unit-cell content are listed in Table I. In model A all Pb was placed at the 8c site, and in model E all Pb was placed at the 24f site. Model C was in accordance with the R-model and the content of the 8c site was Bi1.33Pb0.67, i.e. characteristic for M n+ cations with n = 2 (Valant and Suvorov, Reference Valant and Suvorov2002). The cation distributions for models B and D were chosen to be exactly at the midpoints between A and C, and C and E, respectively. Therefore, the general formula for all structural models can be represented as (Bi13+24−yPb12+y)(Bi23+2−xPb22+x)O38.48, where x + y = 1.04.
Table I. Cation distribution over two sites and comparison of BVS and OS values for the 8c site.

aR ij in equation ∑ν ij = ∑exp[(R 0− R ij)/B] was 2.15 Å for all models.
All parameters were refined as described in the Experimental section, but the results will not be described in detail because the refined coordinates of corresponding atoms, as well as most of their ADPs, were practically identical. For example, the common ADPs for cations distributed over the 8c site were 2.5(8) for model A, 2.6(8) for models B, C, and D, and 2.7(9) Å2 for model E. Also, R wp factors were equal for all five models (10.40%), whereas R B factors were found to be in a very narrow range (3.05–3.07%). Apparently, these results were not useful to determine the most probable structural model. Since Pb2+ and Bi3+ as isoelectronic ions have a negligible difference in scattering power, the resolution between them is not possible by X-ray diffraction.
To estimate cation distribution, bond valence analysis was performed and the results were compared with the calculated oxidation state (OS) of cations positioned at the 8c site (Table I). OS was calculated as a mean value considering the proposed Bi/Pb ratio and the charge of cations, i.e. 3+ and 2+, respectively. The BV sum (BVS) was calculated assuming that the 8c site is occupied by Bi or by Pb, and then averaged according to the Bi/Pb ratio. In an ideal case, BVS should be equal to OS, i.e. the BVS/OS ratio must be 1, and therefore model C seems to be very close to the most probable structural model (Table I).
The substitution of Pb2+ for Bi3+ at the 8c site causes an approximately linear increase of the BVS/OS ratio (Figure 1). The linear fit using the least-squares method yielded the equation
where x represents the Pb2+ content at the 8c site. According to Eq. (1), if BVS/OS = 1, then x = 36%. Thus, the formula for the most probable structural model is (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48, and this composition was further refined by the Rietveld method.

Figure 1. BVS/OS ratio vs. Pb2+ content at the 8c site.
B. Crystal structure of Pb-doped γ-Bi2O3 phase
The results of Rietveld refinement for Bi24PbO37 or (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48 solid solution are given in Table II, and its X-ray powder diffraction profiles are given in Figure 2. The interatomic distances are listed in Table III. About 1.3% of Bi is substituted by Pb at the 24f site, and 36% of Bi is substituted by Pb at the 8c site. This allows one to conclude that Pb2+ is preferentially situated at the 8c site. Otherwise the main structural characteristics follow the well-known pattern of doped γ-Bi2O3 phases interpreted according to the R-model (Poleti et al., Reference Poleti, Karanović and Hadži-Tonić2007). The polyhedral presentation of the crystal structure of the title compound is shown in Figure 3.
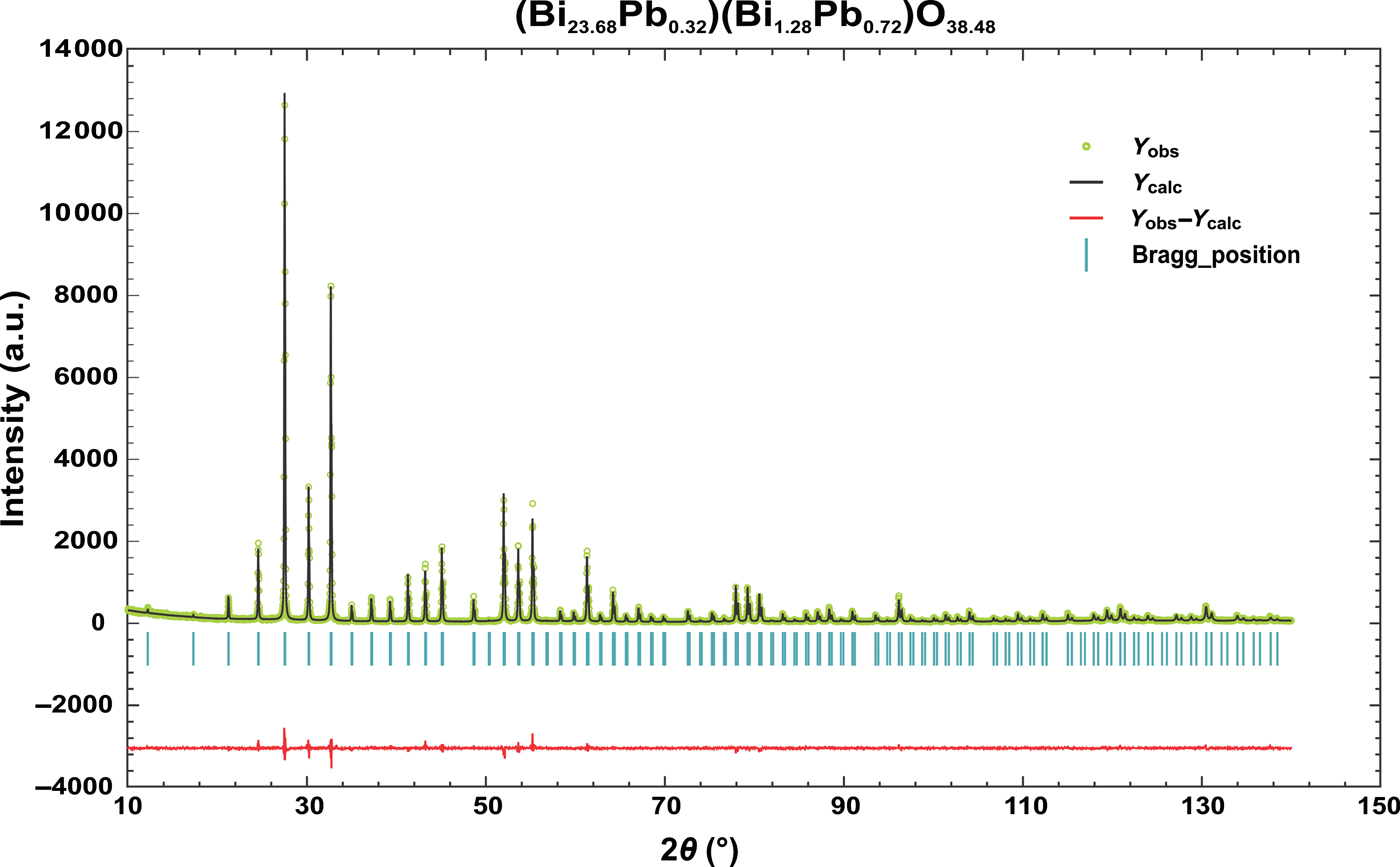
Figure 2. (Color online) Observed, calculated and difference profiles for the Rietveld refinement of (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48 compound.

Figure 3. (Color online) Polyhedral presentation of (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48 compound.
Table II. Structural parameters for (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48.

Table III. Comparison of interatomic distances for Bi24PbO37 (this study), Bi24Pb2O38 (Murray et al., Reference Murray, Catlow, Beech and Drennan1986) and Bi12.8O19.2 (Radaev et al., Reference Radaev, Simonov and Kargin1992) compounds.

aOnly Bi2 in the case of Bi12.8O19.2.
bBi2/Pb2 or Bi2 is positioned at the 2a site with coordinates 000; O3 vacancies are also present.
At the 8c site (x, x, x), the cations are surrounded by three oxygen atoms (three symmetry equivalents of O3) forming a trigonal pyramidal geometry with x = 0.011(4) [Figure 4(a)]. Such geometry is typical for As3+, Sb3+, and Bi3+, although more common to As3+ and Sb3+ with a pronounced electron lone pair effect (Makovicky, Reference Makovicky and Merlino1997). The Bi2/Pb2–O bond distances (2.15 vs. 1.95 Å) and Bi2/Pb2 ADP value (2.6 vs. 4.4–5.3 Å2) are much more reasonable than in the model of Murray et al. (Reference Murray, Catlow, Beech and Drennan1986). It is generally overlooked, but it should be mentioned that around the atoms at the 8c site there are three additional O2 atoms at very long distances of 3.24(4) Å forming an irregular 3 + 3 polyhedron around Bi2/Pb2. These three atoms do not contribute significantly to the BVS of Bi2/Pb2 atoms (compare with Bi1/Pb1 atoms), but clearly show the direction of the Bi2/Pb2 lone electron pairs.

Figure 4. (Color online) Geometry of 8c (a) and 24f sites (b, c).
Bi1/Pb1 bond distances deviate significantly from the values obtained by Murray et al. (Reference Murray, Catlow, Beech and Drennan1986), but they are in rather good agreement [Table III and Figure 4(b)] with data published for the undoped γ-Bi2O3 phase (Radaev et al., Reference Radaev, Simonov and Kargin1992). This once again confirms that the Pb content at the 24f site is practically negligible. Since the excess positive charge in (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48 solid solution is compensated for by partial occupancy of the 6b site, if the two farthest O atoms are neglected, one small percentage of Bi1/Pb1 is enclosed by six oxygen ions forming a highly deformed octahedron [Figure 4(c)].
Recently, for a mechanochemically prepared γ-Bi2O3 phase with formula Bi2.09Pb1.05O4.17 and unit-cell parameter a = 10.262(6) Å, a small shift of the M cation from the tetrahedral 2a to the 8c site was also noticed (Zyryanov, Reference Zyryanov2004). According to this study, all Pb2+ is positioned at the 24f site and the 8c site is only partially occupied by Bi3+, with a Bi2–O3 distance of 2.20(2) Å. Therefore, the structural formula of the investigated sample is (Bi115.6Pb18.4)(Bi21.12)O33.36, and the distribution of cations is just the opposite of our conclusions. However, the credibility of these results is questionable. For example, the ADP of Bi2 is equal to 0 within 3σ, whereas BVS values for cationic sites are 3.37 v.u. for the 8c site and 2.26 v.u. for the 24f site.
IV. CONCLUSION
The yellow powder of single Pb-doped γ-Bi2O3 phase with nominal composition Bi24PbO37 was easily prepared starting from a mixture of bismite (α-Bi2O3) and massicot (PbO) after heat treatment at 690 °C for 1.5 h. The sample was characterized by the Rietveld method using X-ray powder diffraction data, but it was necessary to apply bond valence calculations to resolve ambiguity about cation distribution over the two mixed sites. This approach yielded (Bi23.68Pb0.32)(Bi1.28Pb0.72)O38.48 as the most probable structural formula. According to this formula, Pb2+ is preferentially situated at the 8c site, i.e. only 1.3% of Bi3+ is substituted by Pb2+ at the 24f site and 36% of occupied sites at the 8c site.
Apart from the typical properties of sillenite structure, the investigated structure of Pb-doped γ-Bi2O3 phase, Bi24PbO37, possesses some exceptional characteristics: novel chemical composition and the occurrence of two crystallographic independent mixed cationic sites, one of which is slightly [x = 0.011(4) Å] moved from the origin and center of the unit cell (2a site) along the [111]-direction. The key to the exceptional properties of Pb-doped γ-Bi2O3 phase lies in the fact that both Pb2+ and Bi3+ are large, isoelectronic ions of similar sizes, with the lone electron pair. Therefore, the relatively small tetrahedral 2a site is not suitable for them, and as a result the transformation of the fully occupied 2a site to the partly occupied 8c site appears. At the partly occupied 8c site, Bi2/Pb2 cations with three O2– ions form a trigonal pyramid with cations at the apex of the pyramid.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from the Ministry of Education and Science of the Republic of Serbia (Grant No. III 45007).









