Introduction
Lymphatic filariasis is still a major public health problem in tropical and subtropical countries. Approximately 856 million people in 52 countries worldwide remain at risk (WHO, 2016). The disease is caused by mosquito-transmitted filarial worms, especially Wuchereria bancrofti (>90% of cases). Brugia malayi and B. timori cause about 10% of cases (WHO, 2016). Various genera of mosquitoes – that is, Anopheles, Culex, Aedes, Mansonia and Ochlerotatus act as vectors, depending on the geographical location and filarial species/strain (WHO, 2013). Wuchereria bancrofti can be physiologically classified into three strains according to the periodicity of microfilariae in peripheral blood: nocturnally periodic (urban and rural types), nocturnally sub-periodic and diurnal sub-periodic (the Pacific type) (Sucharit & Harinasuta, Reference Sucharit and Harinasuta1981; Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). In Thailand and Myanmar, only two types (nocturnally periodic and nocturnally sub-periodic) have been recognized (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). Data relating to morphometry, genetic diversity and periodicity of microfilariae are lacking for Lao PDR. Therefore, it is important to study bancroftian filarial epidemiology in this country (Sudomo et al., Reference Sudomo, Chayabejara, Duong, Hernandez, Wu and Bergquist2010). Apart from the species of mosquito vector used and the periodicity of microfilariae in peripheral blood, nocturnally periodic and nocturnally sub-periodic strains can also be clearly differentiated using morphological parameters (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). The number of nuclei between the cephalic space and the nerve ring and most body dimensions, are significantly lower in microfilariae and infective larvae of the nocturnally periodic type relative to the nocturnally sub-periodic type (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999).
Genetic variation of nematodes is an important factor that may influence the efficacy of available drugs as well as the development and spread of drug resistance among parasite populations (Anderson & Jaenike, Reference Anderson and Jaenike1997). Previous studies based on sequences of the mitochondrial cytochrome c oxidase subunit 1 (cox-1) gene in Papua New Guinea (Small et al., Reference Small, Ramesh and Bun2013) and Ghana (de Souza et al., Reference de Souza, Osei-Poku, Blum, Brown, Lawson, Wilson, Bockarie and Boakye2014) indicated that W. bancrofti exhibits high levels of genetic diversity. This was very evident also from a comparison of complete mitochondrial genomes from isolates of W. bancrofti from India, West Africa and Papua New Guinea (Ramesh et al., Reference Ramesh, Small, Kloos, Kazura, Nutman, Serre and Zimmerman2012). Thus, an understanding of genetic variation may provide useful tools for discerning epidemiological patterns and for designing strategies for prevention and control. Here, we compared morphometric measurements and genetic variation of W. bancrofti microfilariae recovered from three adjacent Asian countries – Lao PDR, Myanmar and Thailand. Morphometric data were obtained from microfilariae in Giemsa-stained thick blood films from W. bancrofti-infected carriers. We found that reported morphometric differences between localities were less clear-cut than previously thought. These blood films were also a source of DNA for amplification and sequencing of the mitochondrial cox-1 gene.
Materials and methods
Patients and specimens
Giemsa-stained thick blood films from nine human carriers of bancroftian filariasis were obtained in 1999 or 2007 and stored at room temperature until used in 2016 (table 1). Three samples were obtained from patients in Attapeu Province southern Lao PDR; three samples were obtained from infected Myanmar immigrants (nocturnally periodic strain); two from Mawlamyine, south-eastern Myanmar and one from Yangon in lower Myanmar. The remaining three samples (nocturnally sub-periodic strain) were obtained from Karen ethnic people, Mae Sot District, Tak Province in western Thailand, near the Myanmar border. Details of carriers and source of samples are summarized in table 1.
Table 1. Details of filariasis carriers and geographical sources of samples.

Strain identity, where known from previous work, is indicated. NP, nocturnal periodic strain; NSP, nocturnal sub periodic strain.
Morphometric measurements and statistical analyses
A total of 254 microfilariae were examined at a final magnification of 100× using a compound microscope. One hundred microfilariae obtained from three Lao PDR carriers, 97 microfilariae obtained from three Myanmar and 57 microfilariae obtained from three Thai carriers were measured using camera-lucida drawings by two experienced laboratory personnel. Six morphological parameters were measured for each microfilaria: body length, cephalic space length and width, length of head to nerve ring, body width at nerve ring and Innenkȍrper length. In addition, the number of column nuclei between the cephalic space and the nerve ring were counted. Statistical analyses were carried out in IBM SPSS Statistics for Windows, version 16.0 (SPSS Inc., Chicago, IL). One-way analysis of variance with Tukey's multiple comparison tests was used to identify significant (P < 0.05) differences between morphometric parameters among the three countries.
Polymerase chain reaction (PCR) amplification, DNA sequencing and sequence analysis
DNA was extracted directly from individual slides positive for W. bancrofti using a commercial DNA extraction kit (NucleoSpin® Tissue, Macherey-Nagel, Germany) according to the manufacturer's instructions. Initial attempts to amplify relatively long portions of the cox-1 gene were unsuccessful. Assuming that the DNA had been degraded by Giemsa staining or long storage, we then amplified short DNA fragments using two primer pairs (approximate product sizes 151 and 139 bp), which were designed using the Primer Express Software Version 3.0 (Life Technologies, CA, USA) based on the cox-1 sequence of W. bancrofti (GenBank accession number HQ184469). The two regions that were amplified overlapped to give a contig of 244 bp (208 bp after trimming the primer sequences). Details of primers used are given in table 2. Each PCR was performed according to the previously published method (Jongthawin et al., Reference Jongthawin, Intapan and Sanpool2015). The thermocycling conditions for both sets of primers were as follows: 94°C for 5 min; ten cycles of 95°C for 30 s, 45°C for 30 s and 72°C for 30 s; 25 cycles of 95°C for 30 s, 50° C for 30 s and 72°C for 30 s; with a final step at 72°C for 10 min. Amplicons were separated by 1.5% agarose gel-electrophoresis and 151 and 139 bp fragments were excised and sequenced using the Applied Biosystems 3730 × I DNA Analyzer and ABI Big Dye sequencing kit, version 3.1 (Foster City, CA, USA). DNA sequencing was conducted at First BASE Laboratories Sdn Bhd (Selangor, Malaysia) in both directions, using the PCR primers as sequencing primers.
Table 2. Details of primers used in this study.

The identity of the partial cox-1 gene sequence from each sample was checked using the BLAST-N search tool (National Center for Biotechnology Information, Bethesda, MD, USA). The new cox-1 sequences from the three countries were aligned with sequences of filarial nematodes from the GenBank database (alignment length was 208 bp) using the BioEdit sequence alignment editor (Hall, Reference Hall1999). Phylogenetic relationships were analysed using the maximum likelihood (ML) method implemented in MEGA v6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). The best substitution model for cox-1 was Hasegawa–Kishino–Yano (TN93) + G. Support for clusters in each tree was calculated using 1000 bootstrap replications. Pairwise genetic distance values, which describe the number of differences (corrected according to the model used) between two DNA sequences, were calculated using the Tamura–Nei model (Tamura & Nei, Reference Tamura and Nei1993).
Results
Comparison of morphometric data of W. bancrofti microfilariae from Lao PDR, Myanmar and Thailand
Representative images of W. bancrofti microfilariae from Thailand, Myanmar and Lao PDR are shown in fig. 1. Microfilariae from the three countries were sheathed, lying in graceful coils without secondary kinking. Somatic nuclei were discrete, overlapping where crowded but with distinct borders, countable and no nuclei were present at the tip of the tail. Comparisons of measurement and count parameters of microfilariae from the three countries are shown in table 3. The body length differed significantly by country among specimens from Lao PDR, Myanmar and Thailand, with those of the Thailand type being the longest. Similarly, Innenkȍrper length and the number of nuclei between the cephalic space and nerve ring also differed significantly among countries (table 3). Microfilariae from Lao PDR had a significantly longer and wider cephalic space and a greater body width at the nerve ring than the two other types. Microfilariae from Myanmar had significantly shorter head to nerve ring lengths than did those from the other countries (table 3).
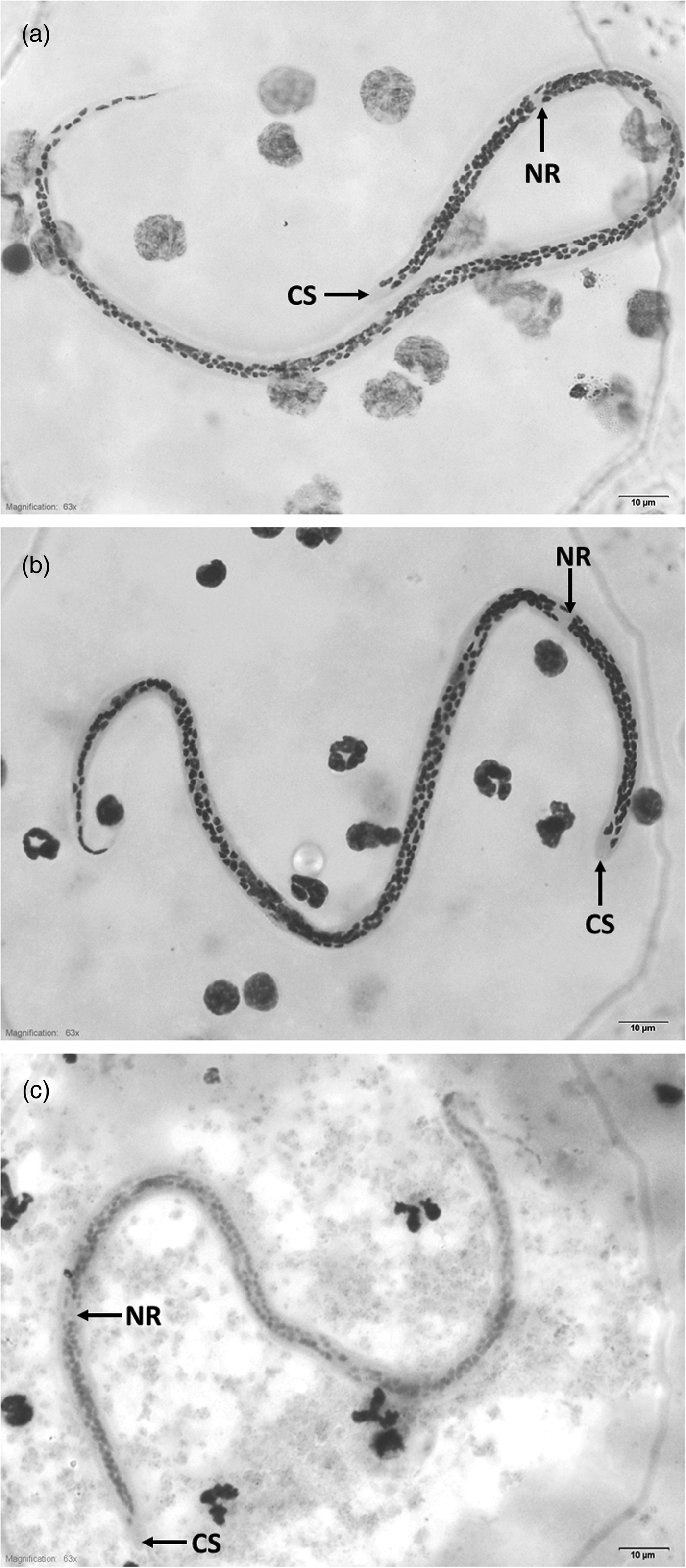
Fig. 1. A typical microfilaria from Thailand (a), Myanmar (b) and Lao PDR (c), observed at a final magnification of 100×. Scale bars: 10 μm. Abbreviations: CS, cephalic space; NR, nerve ring.
Table 3. Morphometric measurements and counts of column nuclei between the cephalic space and nerve ring of microfilariae of W. bancrofti from Lao PDR, Myanmar and Thailand.

Measurements in μm ± SD. Range in parentheses.
a Significant difference among Lao PDR, Myanmar and Thailand.
b Significant difference between Lao PDR and each of the other two countries.
c Significant difference between Myanmar and each of the other two countries.
Molecular phylogenetic analysis of W. bancrofti microfilariae
A contig, 244 bp in length, of cox-1 sequence was obtained from nine samples of microfilariae. After trimming the primer sequences, the final length was 208 bp. Eight variable sites were found among these sequences from three countries, and genetic distances ranged from 0.00 to 0.027 (see supplementary fig. S1). To verify the species, phylogenetic relationships of roundworms in the family Onchocercidae were reconstructed using the ML method (fig. 2). All partial cox-1 sequences of microfilariae obtained in this study were located in a clade containing W. bancrofti sequences from various geographical localities including Mali (GenBank accession number JN367461), Papua New Guinea (JF775522), India (JQ316200) and Italy (AM749235), with 98–100% similarity (BLAST-N search), confirming that the microfilariae specimens obtained from Lao PDR, Myanmar and Thailand were W. bancrofti. These new partial cox-1 sequences have been deposited in the GenBank database under the accession numbers MK138550–MK138558.
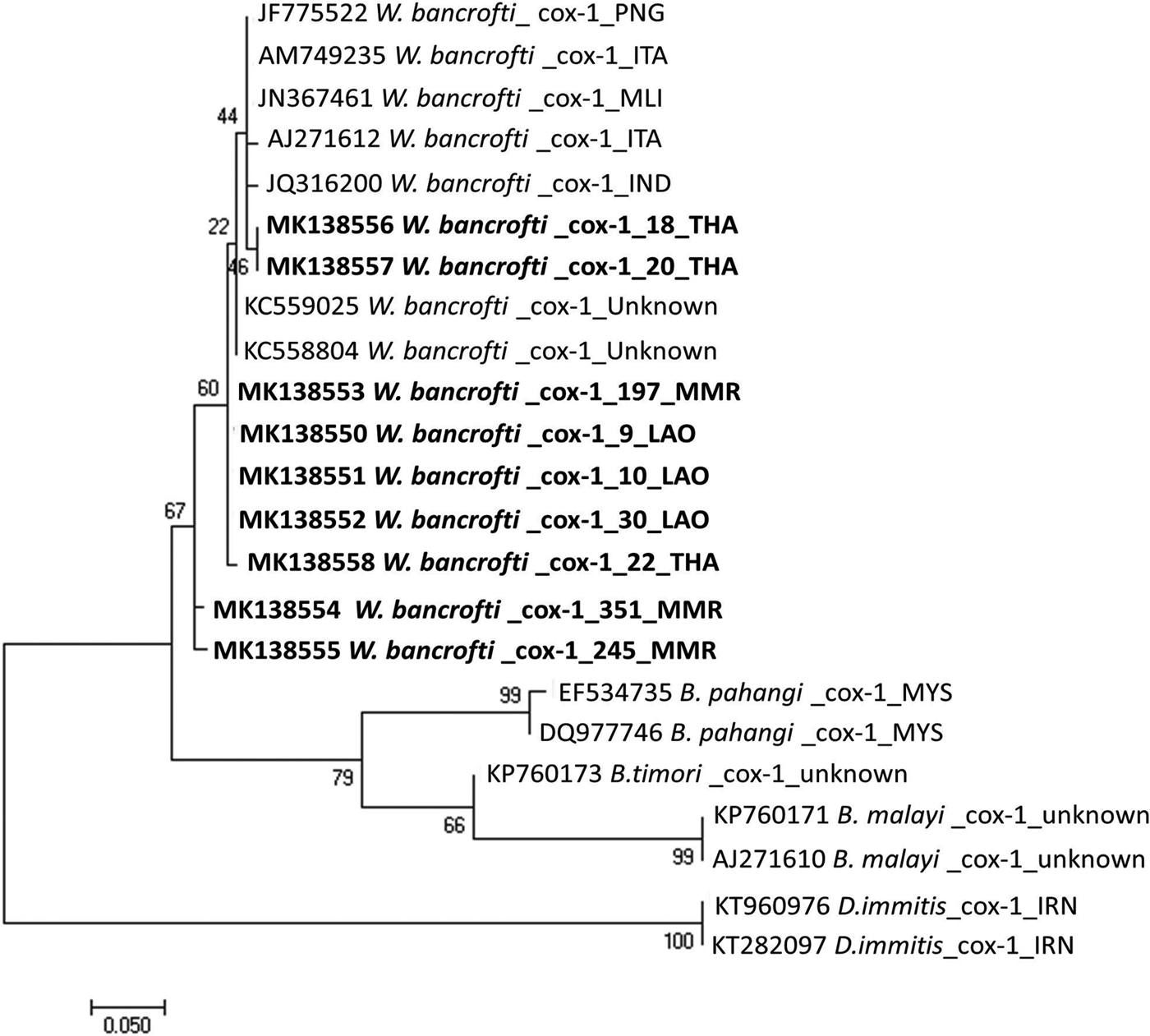
Fig. 2. The ML tree reconstructed from cox-1 gene sequences of W. bancrofti and other related species. Bootstrap scores (percentages of 1000 replications) are presented for each node. Sequences obtained in this study are indicated in bold font with accession numbers (MK138550–MK138558), species name, sample codes and country codes (ISO 3166-1 alpha-3 code). Other sequences from the DNA database are shown with accession numbers, species names and country codes. Scale bar indicated nucleotide substiutions per site.
Discussion
Morphology of our samples from Lao PDR, Thailand and Myanmar indicate that the microfilariae belong to W. bancrofti. Microfilariae of this species are generally 244–296 µm in length and 7.5–10 µm in width. Other conspicuous features distinguish them from microfilariae of B. malayi: nuclei do not reach the tail end, there are no terminal nuclei and nuclei are regularly spaced and well dispersed. In addition, the body is bigger and wider than in B. malayi, exhibits graceful sweeping curves, a short head space and is bluntly rounded anteriorly and pointed caudally (Beaver et al., Reference Beaver, Jung, Cupp, Beaver, Jung and Cupp1984).
Wuchereria bancrofti in the Karen ethnic group living near the Thai–Myanmar border is generally regarded as the nocturnally sub-periodic type (Thailand type), whereas the Myanmar strain is the nocturnally periodic type (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). The types are said to differ in morphology and use different mosquito vectors (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). The body length and cephalic space (length and width) of the Thai nocturnally sub-periodic strain of W. bancrofti were significantly larger than those of the Myanmar nocturnally periodic strain (Nuchprayoon et al., Reference Nuchprayoon, Junpee and Poovorawan2007).
Morphometric findings in the present study indicate that there is a wide geographical variation in length and width of microfilariae and in the number of nuclei between the cephalic space and nerve ring. This might result from either natural variability or reflect subspecies variations, which are currently undescribed in these countries. Analysis of many W. bancrofti populations from various geographical localities will be needed to clarify the situation. According to data in the previous study (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999), the microfilariae we obtained from Thailand and Myanmar morphologically conform to the nocturnally sub-periodic and nocturnally periodic types, respectively. However, there are some discrepancies between our observations and those of Jitpakdi et al. (Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999) and Nuchprayoon et al. (Reference Nuchprayoon, Junpee and Poovorawan2007). We found the body dimensions and the numbers of nuclei between the cephalic space and nerve ring of both types to be smaller than previously observed (Jitpakdi et al., Reference Jitpakdi, Choochote, Panart, Insun, Panart, Pitasawat and Prajakwong1999). In contrast, the Innenkȍrper length of microfilariae from Thailand was larger than reported by Nuchprayoon et al. (Reference Nuchprayoon, Junpee and Poovorawan2007).
Little is known about the periodicity of W. bancrofti microfilariae in Lao PDR. We observed that W. bancrofti microfilariae from Lao PDR have morphometric parameters overlapping those of Thailand and Myanmar strains. Several parameters relating to body dimension were very similar to those of the nocturnally sub-periodic type (Thailand strain), but the number of nuclei between cephalic space and nerve ring was closest to that in the nocturnally periodic type (Myanmar strain). In the absence of parasitological knowledge about microfilarial periodicity of W. bancrofti from Lao PDR, the differences in morphometric data need further investigation.
Random amplified polymorphic DNA (RAPD) markers can be used for differentiating Thailand and Myanmar strains of W. bancrofti (Nuchprayoon et al., Reference Nuchprayoon, Junpee and Poovorawan2007). Here, we successfully PCR-amplified DNA extracted from Giemsa-stained blood films using two primer pairs targeting short, overlapping fragments of the mitochondrial cox-1 gene. The samples had been stored at room temperature for nine or 17 years. Phylogenetic relationships among W. bancrofti and other related species based on their partial cox-1 sequences were constructed. All new cox-1 sequences (MK138550–MK138558) were located among known sequences of W. bancrofti cox-1 from the GenBank database, confirming them as belonging to W. bancrofti. However, the phylogeny we reconstructed differed from that based on RAPD markers by Nuchprayoon et al. (Reference Nuchprayoon, Junpee and Poovorawan2007). Our W. bancrofti cox-1 sequences from Lao PDR, Myanmar and Thailand were located in the same clade, while the relationship inferred using RAPD markers identified two very distinct clusters (Nuchprayoon et al., Reference Nuchprayoon, Junpee and Poovorawan2007). This could be due to the use of different molecular methods in the two studies or possibly the cox-1 sequences do not allow easy discrimination between nocturnally periodic and nocturnally sub-periodic strains.
In conclusion, this study highlights the morphological differences among microfilariae and also the genetic variability within W. bancrofti from Lao PDR, Myanmar and Thailand. The obtained data are a contribution towards the understanding of bancroftian filariasis epidemiology in these countries. Further studies are needed to validate the biological data and assess the microfilarial periodicity in Lao PDR.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X19000865.
Acknowledgement
We would like to thank David Blair for his valuable suggestions and assistance with the presentation of this paper through the Khon Kaen University Publication Clinic.
Financial support
This study was supported by the Distinguished Research Professor Grant, Thailand Research Fund (P.M.I. and W.M., grant number DPG6280002); Khon Kaen University Grant; and a Scholarship under the Doctoral Training Program from Graduate School Research Affairs and Khon Kaen University (L.S., grant number 60164).
Conflicts of interest
None.
Ethical standards
The protocol was approved and registered by the Khon Kaen University Ethics Committee for Human Research, Khon Kaen, Thailand (HE601114).







