Introduction
Within the framework of the project “Mortar technology and construction history at Müstair Monastery”Footnote 1, a selection from about 5000 mortar samples ranging from the 8th to the 20th century, collected in over 30 years of archaeological excavations on site, has been studied in detail. Such quality and number of samples is very rare and provides a unique opportunity for further analytical studies. Absolute dating of mortars is crucial to date the construction phases of archaeological sites and to confirm or challenge existing chronologies. The method of selective dissolution showed promising results in dating Roman and medieval mortars (Hajdas et al. Reference Hajdas, Trumm, Bonani, Biechele, Maurer and Wacker2012). Furthermore, an attempt to systematically test the possibility of dating mortars with various compositions was carried out by comparing methods and laboratories within the mortar dating intercomparison study (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017). But the wider variety of mineralogical phases in a dolomitic mortar, due to the more complicated model of the dolomitic lime cycle, were identified as a potential source of error for the dating process (Michalska et al. Reference Michalska, Czernik and Gosar2017). The alpine region around the monastery is characterized by dolomitic rock formations and the mortars used at Müstair are of a dolomitic nature (Cavallo et al. Reference Cavallo, Caroselli, Jornet and Cassitti2019), giving the possibility to test their potential to be dated with 14C.
Dolomite CaMg(CO3)2 is a calcium and magnesium carbonate that is formed through the substitution by Mg atoms in the structure of calcium carbonate (CaCO3). This process does not always occur completely and, as a result, dolomite limestones can contain variable amounts of dolomite (Warren Reference Warren2000). During the burning process of dolomite limestone, in addition to calcium carbonate, magnesium carbonate (MgCO3) decomposes, with the formation of magnesium oxide (MgO). The calcination of dolomite limestone happens between 510°C and 750°C and it is necessary not to exceed these temperatures to avoid the formation of sintered magnesium oxides, which have a very slow reactivity. Furthermore, due to the very low solubility in water of magnesium hydroxide (Mg(OH)2) its carbonation is very slow and often incomplete. The formation of magnesite is therefore often slower than calcite, while that of hydroxy-carbonates of magnesium (such as artinite, hydromagnesite, etc.) is favored (Diekamp et al. Reference Diekamp, Konzett and Mirwald2009). The slow carbonation of these mortars can cause a delay in the absorption of CO2 and result in an incorrect outcome of the age with radiocarbon (14C).
In particular, the objectives of this paper are the following:
-
1. To verify if the 14C dating can be applied to mortar made by dolomitic raw material, a verification which is possible because the age of the main church was clearly established by dendrochronology (Wacker et al. Reference Wacker, Güttler, Goll, Hurni, Synal and Walti2014);
-
2. To understand which type of sample preparation is suitable for dating dolomitic mortar, as in other studies lime lumps and bulk mortars showed different 14C contamination and they are highly complementary for a reliable mortar dating (Lindroos et al. Reference Lindroos, Ringbom, Heinemeier, Hodgins, Sonck-Koota, Sjöberg, Lancaster, Kaisti, Brock, Ranta, Caroselli and Lugli2018);
-
3. To investigate if the construction phases of the monastery can be chronologically distinguished with mortar dating.
The first results obtained by 14C dating of mortars belonging to the first Carolingian phase are presented and discussed in relation to their petrographic and mineralogical characterization. Once the potential feasibility of the dolomitic mortar dating is demonstrated, the method would be applied to investigate controversial building phases of the monastery, for which no well-established dating options are available.
Description of the Case Study
The monastery complex of St. John in Müstair is an outstanding example of Carolingian art and architecture. It is located in the Eastern Alps, in the Vinschgau/Val Venosta region which is known for its many early medieval and Romanesque churches. The region was of strategic importance at least since Roman times because of its mountain passes, which allowed a safe crossing of the main alpine divide (Figure 1). The via Claudia Augusta, built by the Romans after the conquest of Raetia in the late 1st century BC, remained one of the most important north-south routes through the Alps well into the early modern period (Grabherr Reference Grabherr, Walde and Grabherr2006). From the 6th century onward the area came under the influence of the Merovingian and Frankish rulers, who had to contend with the neighboring Lombards to the south and Bavarians to the east. In 774 Charlemagne conquered the Lombard Kingdom. This bestowed even greater strategic importance to the alpine passes in the Val Müstair and Vinschgau/Val Venosta region.
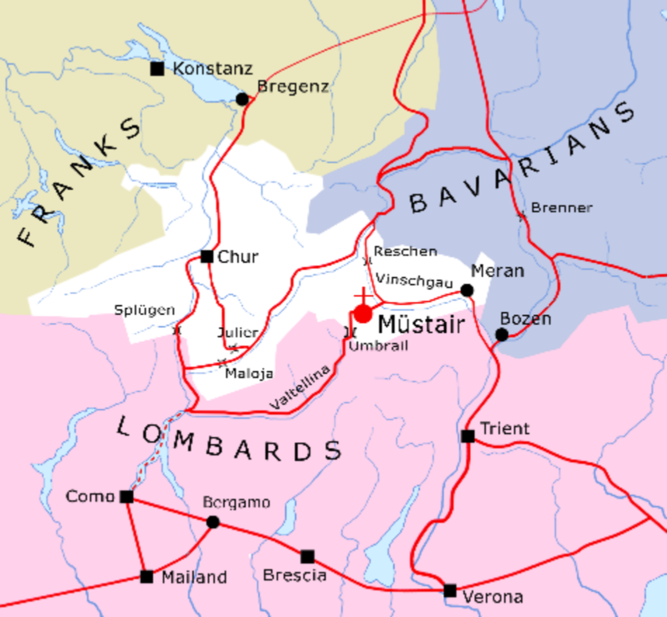
Figure 1 The territory of Raetia Curiensis in the second half of the 8th century with the main roads and mountain passes; see Goll et al. (Reference Goll, Exner and Hirsch2007, with modifications).
It is within this historical and political context that the monastery of St. John was built. The size of the first Carolingian monastery, as known from archaeological excavations (Sennhauser 1996), is monumental, and comparable to other large monasteries of the time, such as the monastery of Reichenau, on Lake Constance, SW-Germany (Zettler Reference Zettler1988), or San Vincenzo al Volturno, in the Molise region of central Italy (Hodges Reference Hodges1993). It possessed two sacred buildings, the monastery church, which is a three apse aisleless church with two annexes to the north and south, both with their own apse and connected to the nave by large arches, and the Holy Cross chapel, with a trefoil-shaped layout, an upper floor and a lower floor used as crypt (Figure 2). The monastery church and the Holy Cross chapel were decorated with mural paintings and sculpted marble choir screens. The Holy Cross chapel was also provided with rich stucco decoration.
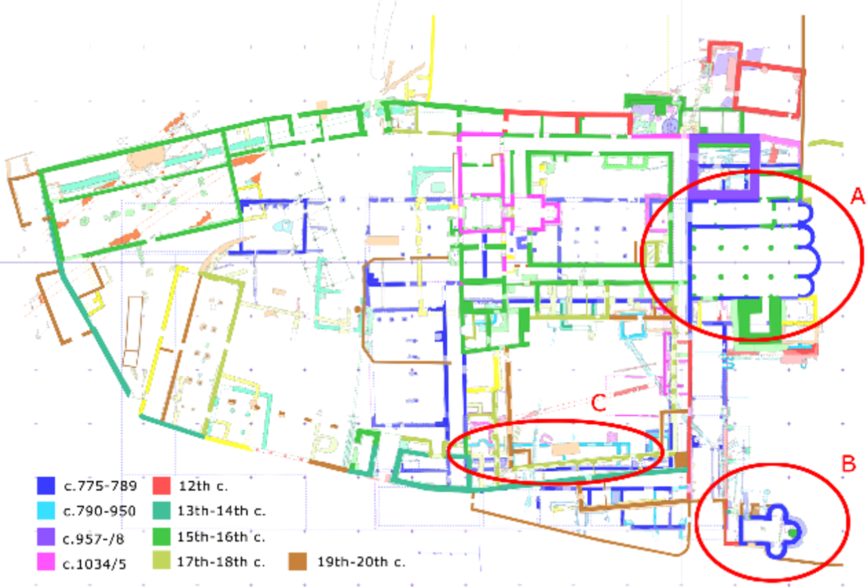
Figure 2 Archaeological phase plan of the monastery of St. John: (A) Monastery church; (B) Holy Cross chapel; (C) Loggia building (cyan). Sennhauser and Courvoisier (Reference Sennhauser, Courvoisier and Sennhauser1996) with modifications.
Both sacred buildings are still standing and in use today. Their construction date can be precisely determined by dendrochronology. A wooden beam preserved in the eastern gable of the monastery church was felled in the winter of 775/6 AD, while the wooden beams and planks used for the construction of the ceiling of the Holy Cross chapel, which are to a large part still in place and in use today, were felled between 784/5 and 788/9 (Hurni et al. Reference Hurni, Orcel, Tercier and Sennhauser2007). In the medieval period, timber for the construction of buildings was usually felled in winter and processed in the following months (Descoeudres Reference Descoeudres2007). This means that the construction of the church was probably completed in 776, and that work on the Holy Cross chapel probably ended in 789 or shortly after. The remaining convent buildings, which are not preserved above ground and therefore did not yield any dendrochronological date, were most likely built in between these dates.
Sometimes after the completion of the first Carolingian monastery, the so-called “loggia building” was added to the southern tract (Figure 2C). The monastery remained in use throughout the centuries up to the present time. It was never completely rebuilt, but buildings were continually replaced or added to the site, so that today it features architectural and archaeological remains from 12 centuries of European history. This makes it an ideal site for studying and testing mortar dating methods.
Material and methods
The choice of the first two samples (Table 1) to be used as a test for mortar dating was made considering the results of the petrographic characterization of 52 Carolingian samples. Within this group of samples two different groups of bedding mortar have been identified with different petrographic characteristics: one group comes from the main church and one from the large adjacent building complex to the west, which consists of the convent and the Holy Cross chapel (Caroselli et al. Reference Caroselli, Bläuer, Cassitti, Cavallo, Hajdas, Hueglin, Neukom and Jornet2019). Furthermore, high presence of lime lumps has been considered as an important element for the selection of a sample. An embedded charcoal fragment in one sample has been extracted and dated to obtain independent data necessary to support or contrast the results of the dating of the mortar binder.
Table 1 Description of samples used for dating. ff = binder fine fraction, LL = lime lumps.

Polished thin sections were prepared by a specialized laboratory. Polarized light microscopy (PLM) on the thin sections was carried out for mineralogical and textural analysis; a Zeiss Axioskop 4.0 Polarizing Light Microscope (PLM) was used and micrographs were acquired with a digital camera, and processed with the software Axiovision (Zeiss, release 4.5.1). The following interesting features for mortar dating were observed: binder (structure, color, birefringence, homogeneity), lime lumps (types, internal structures, quantity and size), aggregate grains (grain sizes, mineral and rock types present, estimation of the grain size distribution), additions (brick grains or organic material), macro-porosity and especially secondary calcite or hydromagnesite fillings of voids.
The binder enriched fraction of the two samples was extracted by dry sieving. The samples were broken avoiding fine splinters of aggregates. The crushed material was vibrated in a sieve series. The fine grain-size fraction (ff) 45–63 μm and the fraction 63–75 μm were homogenized and divided in two parts: one part was analyzed by X-ray powder diffraction (XRPD) and the other was used for mortar dating. Lime lumps (LL) were mechanically separated from the mortar bulk under the stereomicroscope.
The mineralogical analyses of the mortar binders were carried out using XRPD. After grinding in an agate mortar, randomly oriented samples were prepared and deposited in the hollow of a Si monocrystal zero background plate, supplied by Assing spa, Monterotondo, Italy. A Rigaku Miniflex system, operating in θ:2θ mode was used; generator setting 30 kV, 10 mA, Cu anode (Cu Kα -1.5418 Å), Ni filter, 2 θ range 5–55°, step size 0.02°, scan speed 0.3° min–1. Qualitative phase determination was carried out using the software QualX2.0 (Altomare et al. Reference Altomare, Corriero, Cuocci, Falcicchio, Moliterni and Rizzi2015) and the correlated COD database (Gražulis et al. Reference Gražulis, Chateigner, Downs, Yokochi, Qiurós, Lutterotti, Manakova, Butkus, Moeck and Le Bail2009).
Sequential Dissolution Method for 14C Dating of Mortar
The method of sequential dissolution targets the fast-dissolving component of the binder. The procedure follows the method described in detail by Hajdas et al. (Reference Hajdas, Maurer and Röttig2020 in this issue). The sieved mortar fraction of grain size 45–63 μm and lime lumps were used. Prior to the sequential procedure the whole samples (bulk) was dissolved in acid and graphitized for the AMS analysis. This step was to estimate carbon content of each sample as well as to measure the range of the ages of the carbonates. Measurement of bulk is considered exploratory although, dependent on the type of mortar, this fraction has a potential of providing the accurate ages.
For sequential dissolution, subsamples containing ca. 50 mg of the mortar powder fraction were placed in one of the chambers of the special dual chamber glass vessel. The second chamber has been filled with 10 mL of concentrated phosphoric acid (85% H3PO4). The vessel was then closed and evacuated at room temperature, prior to pouring of acid to the chamber, which contained mortar. This process was timed and freezing of purified (passing through a water trap) CO2 in liquid nitrogen (LN) was performed in sequence: 4 consecutive fractions were collected after each 3 s. Carbon content of each collected fraction was measured and 10–100 μg of C was trapped in a 4 mm tube to be flame sealed for analysis using Gas Ion Source (GIS) AMS facility at ETHZ (Ruff et al. Reference Ruff, Szidat, Gaggeler, Suter, Synal and Wacker2010). Graphite samples were also measured using the MICADAS at ETH Zurich (Synal et al. Reference Synal, Stocker and Suter2007). Solid and gas formed samples were analyzed together with corresponding size of standard (OXA II) and background samples (C-1, IAEA).
Results
Petrographic Analyses
The binder of sample 6577, which was taken from the western wall of the monastery church (Figure 2), shows heterogeneous clear and dark beige color with radial dark spots, under the microscope. In this mortar, lime lumps sensu strictu, underburned and overburned fragments (Elsen Reference Elsen2006) can be distinguished, the last two being potentially source of error for mortar dating (Lubritto et al. Reference Lubritto, Caroselli, Lugli, Marzaioli, Nonni, Marchetti Dori and Terrasi2015). The aggregate grain size is prevalently medium and coarse, not well selected. The composition is: rock fragments of gneiss, rich in quartz and feldspar, schists and micas (muscovite and chlorite). The absence of carbonate minerals (neither dolomite nor calcite) avoids the potential harmfulness of dead carbon contamination from the aggregate (Figure 3A), but the contribution of the binder related particles (BRP) left in the binder which during the production process did not completely lose former CO2, and therefore still containing dead carbon, should be targeted. Indeed, in this particular mortar a relevant presence of overburned BRP is observed, showing the typical glassy structure and texture, as well as the neoformation of tiny calcite crystals (Figure 3B).

Figure 3 Thin section images 5×, XPOL: (a) Sample 6577 bedding mortar of the church; the aggregate of this mortar is mainly of metamorphic siliceous composition; (b) same sample of A, here a big overburned BRP is shown with a glassy structure and the neoformation of tiny calcite crystals; (c) sample 957 bedding mortar of the courtyard with big dolomite crystals as aggregate.
The sample 957 is a wall mortar coming from the buildings on the western side of the Carolingian courtyard (Figure 2). The binder is homogeneous, with a clear beige color. The lime lumps are frequent and dark phases can be observed within them. The grain size of the aggregate varies from very fine to medium-coarse, not sorted. The aggregate is composed by fragments of gneiss, schists, quartz, feldspar, dolomite, limestone, calcite and micas (Figure 3C). In this mortar the potential source of dead carbon is the relevant presence of diverse carbonate sand (Caroselli et al. Reference Caroselli, Bläuer, Cassitti, Cavallo, Hajdas, Hueglin, Neukom and Jornet2019).
XRPD Analysis Results
Qualitative mineralogical analysis of the sieved fraction 43–63 μm of the samples is reported in Table 2. Results indicate that the binder of the samples 6577 is dolomitic due to the presence of hydromagnesite. The presence of phyllosilicate minerals, quartz and dolomite in both samples is due to the fraction of aggregates that has passed the 63 µm sieve.
Table 2 XRPD results. Cal = calcite, Qz = quartz, Dol = dolomite, Ilt/Ms = illite/muscovite, HMgs = Hydromagnesite.

Mortar Dating Results
Results of 14C dating are summarized in Table 3. The measured 14C content of mortar (F14C) and corresponding 14C ages of different fractions which were calculated following Stuiver and Polach (Reference Stuiver and Polach1977) are listed along with the weight of the carbon that was released in each fraction. AMS allows for measurement of ∂13C however the fractionation in the process of the sequential dissolution is possibly changing the original isotopic signal of the specific mortar fraction (Folk and Valastro Reference Folk and Valastro1976).
Table 3 Results of AMS 14C analysis. Sample description gives information about the grain size used. Fraction defines the collection time and the technique of AMS measurement. GIS stands for gas ion source, graphite for solid samples. F14C is the measured 14C content and the 14C ages are calculated based on this value. The amount of carbon released and measured in the samples is given as mg of C. ff= binder fine fraction, LL= lime lumps.

Figures 4–7 allow the assessment of the method of removing the old (geological) component that is assumed to dissolve at lower speed (Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Braskén and Sveinbjörnsdóttir2007). The presence of such a component, which is an indicator of contamination with geological material, can be quickly assessed by comparison of the ages of the fractions.
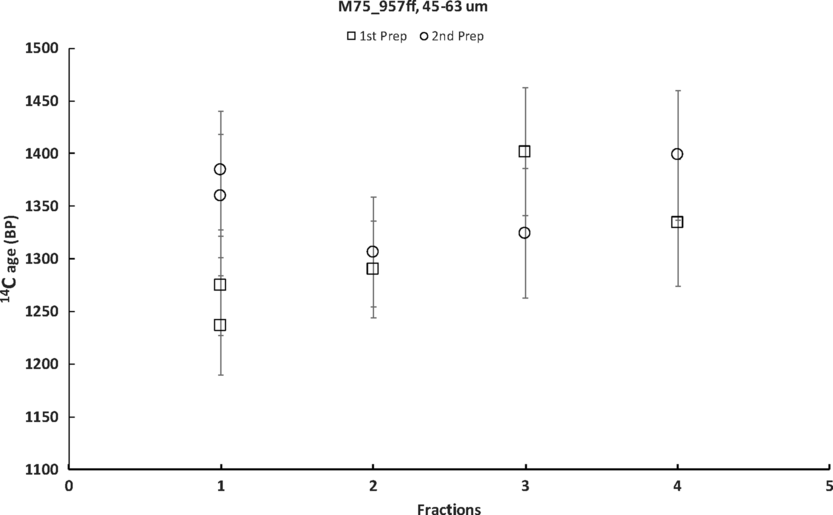
Figure 4 Results of sequential dissolution from 2 independent preparations. In both preparations the fast fractions#1 (1–3 s) resulted in sufficient amount of CO2 for duplicate GIS measurements. Result of measurement on the bulk is not shown as it is out of scale: 2585 ± 24 BP. ff= binder fine fraction.

Figure 5 Results of sequential dissolution. The fast fractions#1 (1–3 s) resulted in sufficient amount of CO2 for duplicate GIS measurements. Result of measurement on the bulk is shown as the fraction #5. LL= lime lumps.
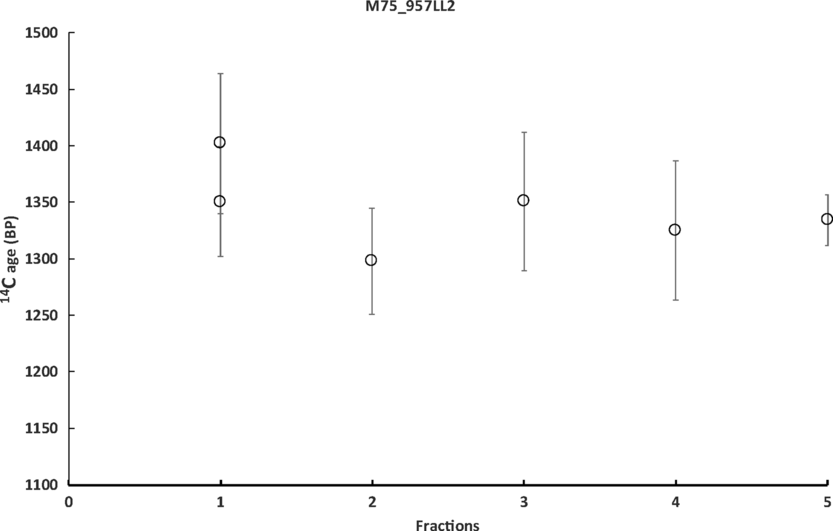
Figure 6 Results of sequential dissolution. The fast fractions#1 (1–3 s) resulted in sufficient amount of CO2 for duplicate GIS measurements. Result of measurement on the bulk is shown as the fraction #5. LL= lime lumps.

Figure 7 Results of sequential dissolution. The fast fractions#1 (1–3 s) resulted in an insufficient amount of CO2 even for GIS measurement. Result of measurement on the bulk is shown as the fraction #5. ff= binder fine fraction.
Calibration of the evaluated final ages was performed using OxCal 4.2 (Ramsey and Lee Reference Ramsey and Lee2013)with the INTCAL13 dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). Calibrated ages are summarized in Table 3 and shown in Figures 4–7.
The principle of 14C dating of mortar using sequential dissolution relies on the separation of carbon from the fast-dissolving component. In the ideal case of mortar samples free of contamination (old and young) the 14C ages of all the fractions are in agreement, or forming an age-plateau (Heinemeier et al. Reference Heinemeier, Ringbom, Lindroos and Sveinbjornsdottir2010). Although such distribution is rather unusual, an assessment of reliability of 14C ages is based on the distribution of 14C ages of fractions in sequence. The first fraction is considered to be the best estimated of the 14C and if followed by an agreement with the 2nd fraction, such 14C age can be considered the most accurate measure of the moment when the binding reaction occurred.
In this case, each of the samples tested presents a somewhat different picture. The 14C ages of bulk samples (fraction #5 in Figures 4–7) are very helpful for evaluating the sequence development and effectiveness of the method. In the case of sample 957ff, 45–63 μm, the age of bulk 2585 ± 24 BP is more than 1000 years older than the first fraction. A repeated sequential dissolution resulted in an agreement between the results of the 1st and 2nd preparations. This result means that bulk mortars were highly contaminated with dead carbon and that after sequential dissolution treatment a part of the contamination has been eliminated. The 14C age of the sample 957 is based on a mean value of ages of 1st fractions from 2 preparations (Table 4, Figure 4).
Table 4 Combined and calibrated ages (95.4% confidence level) obtained using OxCal v.4.2 calibration program (Ramsey and Lee Reference Ramsey and Lee2013) with IntCal data set (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). ff = binder fine fraction, LL = lime lumps. In the last column the dendrochronology results obtained from the church according to Hurni et al. (Reference Hurni, Orcel, Tercier and Sennhauser2007: 111, Table 4) were included for comparison.

The samples lime lumps LL1 and LL2 from the sample 957 were also treated with sequential dissolution. The results show slight difference between the lime lumps, but all 3 samples result in a coherent age interval between 650 and 880 CE (Figure 8).
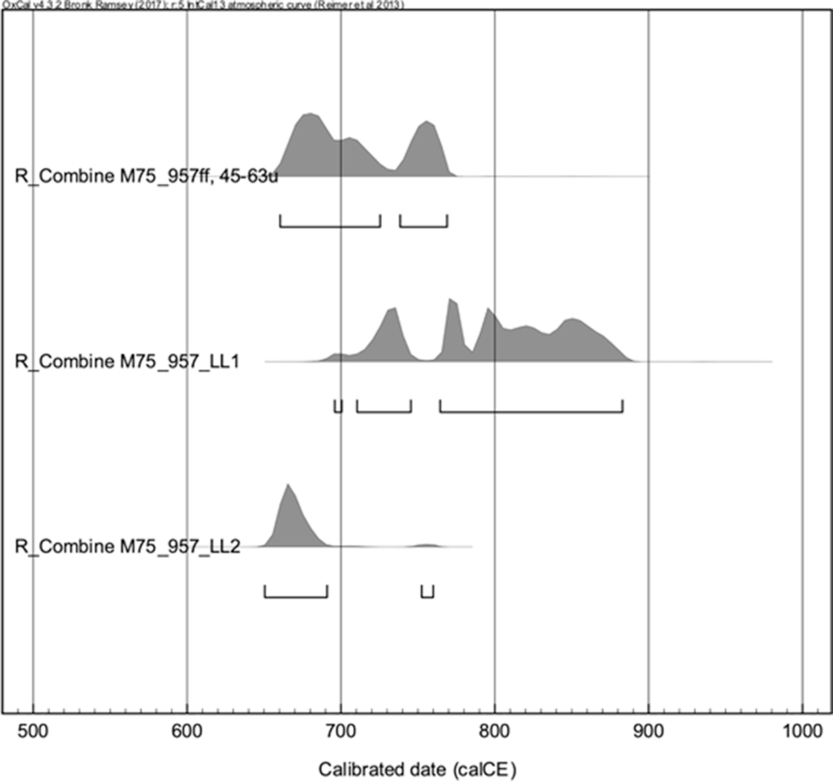
Figure 8 Calibrated 14C age of the sample 957 sieved fraction (ff) 45–63 and the lime lumps LL1 and LL2. The results show slight difference between the lime lumps but all 3 samples result in a coherent age interval between 650 and 880 CE (Table 4). ff = binder fine fraction, LL = lime lumps.
The Sample 6577ff, 45–63 μm was prepared and analyzed only once and the first (1–3 s) dissolution fraction was too small even for GIS analysis. However, the ages of all the 3 following fractions were so coherent that the ages were combined and calibrated resulting in 1180 ± 32 BP and calendar age of 729–961 CE. This is a little younger than the age of the whole bulk of this sample 1341 ± 29 BP and charcoal 1331 ± 21 BP, both calibrated fall into the interval 644–765 CE (Table 4 and Figure 9).

Figure 9 Calibrated age of the sample 6577ff, 45–63 μm (fractions 2-3-4) 729–961 CE, compared to the age of the whole bulk of this sample and the charcoal, both falling into the interval 644–765 CE. ff = binder fine fraction, LL = lime lumps.
The 14C ages obtained for 6577_LL2 were not calibrated as the ages appear to be significantly younger than the charcoal and the bulk fraction discussed above.
Results of 14C dating bulk (no sequential solution) of the remaining samples are a primary assessment of the samples for future analysis. Samples 957ff, 63–75 μm and 6577ff, 63–75 μm were analyzed to see how the different grain sizes relate to the isotopic composition of the 45–63 μm fraction. It appears that the 6577ff, 63–75 μm has the same 14C age as the 45–63 μm fraction i.e. 1200 ± 30 BP.
Discussion
In the sample belonging to the main church 6577 characterized by mostly quartz and feldspar sand and without dolomite mineral, the dating has provided results consistent with the dendrochronological dating (Hurni 2007). The charcoal sample was a little older than the mortar and this is also consistent. The bulk mortar turned out to be older and this is consistent with a slight contamination that could be due to small fractions of carbonate sand, whose presence cannot be excluded, and/or fragments of BRP that did not completely react during the burning process. The 14C ages obtained for the lump of this mortar 6577_LL2 were significantly younger and thus they were not calibrated. This result can be explained by the presence of the overburned lumps, in which secondary calcite formed in more recent times can be observed (Figure 3B). The presence of delayed carbonation reaction of some lumps can also not be excluded.
On the contrary, in the sample of the courtyard 957, in which the presence of dolomitic sand is abundant, this component was not completely separated with the sequential dissolution, providing data in agreement with each other but discordant with the dendrochronology (with the binder dating giving a slightly older age due to the residual presence of dead carbon). From this, it can be assumed that the speed of acid hydrolysis of residual dolomite was not always significantly slower than the hydrolysis of the calcite binder. Indeed, the solubility of dolomite is depending on the Mg content and the order of the molecules (Railsback Reference Railsback2006). The dolomite rocks in the Val Müstair show a high variability both of the size of the crystals and of the texture (Cavallo et al. Reference Cavallo, Caroselli, Jornet and Cassitti2019) and therefore it cannot be excluded that some of them were already dissolved in the first fractions of the sequential dissolution. It can be said, however, that the treatment has rejuvenated the results by converging towards a correct value, but further refinement of the technique of sample preparation for eliminating dolomitic sand residue is necessary for achieving fully reliable results.
Finally, the presence of a plateau in the calibration curve in the range between 700 and 900 CE results in a wider uncertainty on the dating of the Carolingian phase than for other historical periods.
Conclusion
This preliminary study demonstrates that 14C dating with sequential dissolution has a potential for dating the mortars of Müstair, made from dolomitic raw material even if the dolomite presence complicates the situation. The results obtained for the main church samples are in agreement with its dendrochronological dating, but when a contamination from fine dolomitic aggregate is present further investigation is required to develop a more adequate sample preparation technique. This case has confirmed that for dating dolomitic mortar, lime lumps and 45–63 μm sieved fractions are complementary materials for a reliable mortar dating, while significant differences emerge in using the bulk. The dating of 63–75 μm sieved fractions or even the bulk of lime lumps can result in spurious ages.
ACKNOWLEDGMENTS
The authors thank the anonymous reviewers for suggestions and comments. The authors would like to acknowledge Christine Bläuer for the helpful suggestions that improved the text. Special thanks go to the Department of Chemical and Geological Sciences of the University of Modena and Reggio Emilia and in particular Dr. Simona Marchetti Dori under the scientific direction of Dr. Francesco Ronchetti for the preparation of thin sections and Prof. Alessandro Gualtieri for XRPD analyses. This project has been made possible thanks to the financial support provided by SNSF (Swiss National Science Foundation, Switzerland – Grant project number 105211_169411) and Biosfera Val Müstair (https://www.biosfera.ch/de).















