Introduction
Infections by gastrointestinal nematodes, mainly Haemonchus contortus, stand out as one of the most common constraints in small ruminant production, especially in tropical regions (Beiser et al. Reference Besier2016). The treatment of nematodiosis has employed synthetic anthelmintics. However, the nematode resistance to various available drugs (Singh et al. Reference Singh2016), the risk of residue in food animal origin and environmental contamination (Cooper et al. Reference Cooper2011) have led to the research for other control methods. The use of extracts and metabolites derived from plants can be a potential alternative for the control of helminth infections (Hoste and Torres-Acosta, Reference Hoste and Torres-Acosta2011).
Saponins and flavonoids are important secondary metabolites found in several plant species. They have a wide range of biological activities, such as the antiparasitic. Previous studies reported that fractions rich in steroidal and triterpenic saponins from Agave sisalana and Ziziphus joazeiro have in vitro ovicidal and larvicidal effect against nematodes of goats, respectively (Botura et al. Reference Botura2013; Gomes et al. Reference Gomes2016). The flavonoids naringenin, quercetin and luteolin induced the inhibition of exsheathment of H. contortus larvae (L3) (Klongsiriwet et al. Reference Klongsiriwet2015).
The lack of information on the anthelmintic activity of saponins and flavonoids isolated from plants has accounted for the development of this study, which aimed to evaluate the in vitro anthelmintic activity of saponins aescin (I) and digitonin (II), as well as their respective sapogenins (III and IV, respectively). The hecogenin acetate (V) and the flavonoids catechin (VI), hesperidin (VII), isocordoin (VIII) and a mixture of isocordoin and cordoin (VIII + IX) against gastrointestinal nematodes of goats. Also, toxicity of active compounds was analysed on Vero cell cultures.
Materials and methods
Reagents and equipment
Saponins digitonin (II) and hecogenin acetate (V) were obtained from Sigma-Aldrich® (St. Louis, MO, USA), while aescin (I) was acquired commercially at a local drugstore (Bahia, Brazil). The flavonoids catechin (VI) and hesperidin (VII) were purchased from Sigma-Aldrich® (St. Louis, MO, USA). Thin-layer chromatography (TLC) analyses were done using plates coated with silica gel F254 (0.025 mm, Merck, Darmstadt, Germany). 1H NMR (flavonoids and sapogenins) and 13C NMR (flavonoids) spectra were acquired on a Bruker spectrometer (models AC-300 and AC-400, Fällanden, Switzerland) using chloroform-d (flavonoids and digitogenin) and methanol (MeOH)-d 4 (aescin) as solvents. Tetramethylsilane was used as an internal standard and chemical shift (δ) was obtained in parts per million (ppm). High-resolution mass spectra (Bruker microTof), infrared (Shimadzu mod. IRAffinity, Kyoto, Japan), distortionless enhancement by polarization transfer, heteronuclear multiple bond correlation and heteronuclear multiple-quantum correlation spectral analyses of flavonoids were also acquired. All reagents were of analytical grade.
Obtaining of the sapogenins
The saponins aescin (I) and digitonin (II) were submitted to acid hydrolysis procedure to yield their sapogenins (aglycones). Three hundred milligrams of I was hydrolysed as described by Braz-Filho et al. (Reference Braz-Filho1986). One hundred forty milligrams of II was processed according to Ahmad et al. (Reference Ahmad, Baqai and Ahmad1995) with some modifications. This saponin was treated with hydrochloric acid (20%) in MeOH–chloroform solution (170:24 mL) under reflux (100 °C) for 5 h. After this time, the solvents were evaporated under reduced pressure. The acidic solution was diluted with water and extracted five times with chloroform. The chloroformic phase was collected and evaporated to obtain the digitogenin (IV). All sapogenins were analysed by TLC using a mobile phase ethyl acetate:ethanol (7:3) (aescin sapogenins, III) and ethyl acetate (100%) (digitogenin, IV).
Obtaining of the flavonoids
The flavonoids isocordoin (VIII) and the mixture of isocordoin and cordoin (VIII + IX) were obtained from the roots of Bowdichia virgilioides (Fabaceae). The roots were collected in June 2012 in the campus of the Federal University of Bahia (UFBA, Salvador/Bahia). The plant was identified by Professor Luciano P. Queiroz at the herbarium of the Department of Biology, Feira de Santana State University, Bahia, Brazil. A voucher specimen was deposited under the number 12 672.
Dried and powdered roots (1.6 Kg) underwent extraction employing MeOH furnishing 116.5 g of MeOH crude extract. Sequentially, this extract was partitioned between dichloromethane (DCM) and MeOH:H2O (7:3). The DCM soluble fraction was then sequentially partitioned between hexane and MeOH:H2O (9:1) furnishing 10.7 g of MeOH soluble fraction. This extract underwent a silica gel 60 chromatographic column (Acros Organics) employing the mixtures of CHCl3:MeOH as eluent. The fractions were eluted with 10% of MeOH and underwent Sephadex LH-20 (Pharmacia) chromatography yielding pure isocordoin and a mixture of cordoin and isocordoin. The flavonoids VIII and a mixture of VIII and IX were submitted to the extract and their yields were 90 mg (1%) and 7 mg (0.06%), respectively.
In vitro anthelmintic evaluation
Fecal samples from donor goats naturally infected with gastrointestinal nematodes were used to obtain eggs and larvae (L3). The goats were kept in the Hospital of the School of Veterinary Medicine and Zootechny of the Federal University of Bahia (HOSPMEVZ – UFBA).
Eggs were recovered as described by Hubert and Kerbeoeuf (Reference Hubert and Kerboeuf1992) and infective larvae (L3) were obtained from coprocultures (Ueno and Gonçalves, Reference Ueno and Gonçalves1998). The fecal cultures indicated the presence of infection by Haemonchus spp. (80%), Oesophagostomum spp. (15%) and Trichostrongylus spp. (5%). Previous studies have showed the resistance of these nematodes to ivermectin, thiabendazole and levamisole (Borges et al. Reference Borges2015).
The anthelmintic activity of flavonoids, saponins and sapogenins was evaluated using the egg hatch (EHA) and larval motility assays (LMA). Initially, all compounds were tested at the concentration 1 mg mL−1. The substances that showed efficacy ⩾95% were tested in different concentrations for the assessment of EC50 and EC90: aescin (I) (0.5, 0.6, 0.7, 0.84, 1.0 mg mL−1 – EHA) and digitonin (II) (0.003, 0.015, 0.06, 0.25, 1.0 mg mL−1 – LMA). Two negative controls were prepared: distilled water and Tween 80 (1%). As a positive control, thiabendazole (0.025 mg mL−1) was used for the EHA test, and levamisole (0.25 mg mL−1) for the LMA test.
Egg hatch assay
The EHA was performed according to the methodology described by Coles et al. (Reference Coles1992), with modifications. The egg suspension (100 eggs/100 µL/well) was placed in 96-well microplates. Subsequently, the test substances (100 µL) were added in different concentrations. The cultures were incubated in a B.O.D. incubator at 27 °C and relative humidity at 80% for 48 h. After this time, Lugos’ iodine solution was added and then hatched larvae (L1) and eggs in each well were counted using a microscope. The percentage of inhibition of the egg hatching was determined by the ratio: [number of eggs/(number of eggs + number of larvae)] × 100.
Larval motility assay
For the LMA (Ferreira et al. Reference Ferreira2013), an infective larvae (L3) suspension (50 larvae/100 µL) and the compounds tested (100 µL) in different concentrations were distributed in 24-well microplates. The plates were incubated at 27 °C in a B.O.D. incubator for 24 h and the motile and non-motile larvae were counted. The movement of the larvae was stimulated through the agitation of the plates and exposition of larvae to a light source. These results were expressed as a percentage of motile larvae (% ML), which was calculated according to the following formula: % ML = number of motile larvae/(number of motile larvae + number of non-motile larvae) × 100.
In vitro cytotoxicity assay
Vero cell cultures
The Vero cell line (African green monkey kidney normal cell line – ATCC CCL81) was grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% equine fetal serum and antibiotics (100 UI mL−1 penicillin G, 100 mg mL−1 streptomycin) at 37 °C in an incubator at 5% CO2. The cells were cultivated in culture flasks (25 cm2) and the medium was replaced three times a week. This cell line was chosen once that it is easily available and widely used as a triage model for cytotoxic assays of compounds isolated from plants, including substances with effects against ruminant parasites (Nchu et al. Reference Nchu2011; Adamu et al. Reference Adamu, Naidoo and Eloff2013).
Cell viability assay
The cell viability was evaluated through the 3-4,5-dimethylthiazol-2-yl,2,5diphenyltetrazolium bromide (MTT) test, according to Hansen et al. (Reference Hansen, Nielsen and Berg1989). It is a colorimetric method based on the principle of the conversion of the MTT (yellow colour) to violet-coloured formazan crystals through the activity of a mitochondrial enzyme (succinate dehydrogenase) in living cells.
The cells were distributed in 96-well microplates (3.5 × 104 cells/well) and the plates were incubated for 24 h at 37 °C in a 5% CO2 incubator. The culture medium was then removed, and the cells were treated with the compounds (100 µL) in the following concentrations: 0.011, 0.016, 0.023, 0.032, 0.045 mg mL−1 for aescin (I) and 0.001, 0.003, 0.0092, 0.027, 0.083 mg mL−1 for digitonin (II). After 24 h, the culture medium was removed and MTT solution in RPMI medium (1 mg mL−1, 100 µL) was added to the wells. After 2 h of incubation (37 °C and 5% CO2), a 100 µL of lysis buffer (20% sodium dodecyl sulphate and 50% dimethylformamide/pH = 4.7) was added to each well to dissolve MTT formazan crystals. The plates were kept at 37 °C for 12 h and the absorbance was measured using a microplate reader (405–600 nm). The results were presented as a percentage of cell viability (average and standard deviation) in relation to the negative control (RPMI medium), which was considered as 100%.
Cell integrity assay
The membrane integrity was evaluated through the propidium iodide (PI) test, according to Chen et al. (Reference Chen2015), with adaptations. This is a fluorimetric method based on the principle of the impermeability of the cell membrane to the PI. The presence of any rupture in the membrane, dead cells or cells in late apoptosis allows a passage of this substance. PI is a red-fluorescent agent that binds to DNA by intercalating between base pairs (Vermes et al. Reference Vermes, Haanen and Reutelingsperger2000; Rieger et al. Reference Rieger2011).
For this assay, the cells were seeded in 24-well microplates at a density of 3.5 × 104 cells/well. After 24 h of incubation (37 °C and 5% CO2), the cells were submitted to treatment with the saponins aescin (I) and digitonin (II) for 24 h. These saponins were tested at three concentrations that were defined from the IC50 value of the MTT test: 0.013, 0.02 and 0.03 mg mL−1 (I) and 0.003, 0.010 and 0.03 mg mL−1 (II). The negative and positive controls were a culture medium and hydrogen peroxide (3%), respectively.
After exposure of cells to the saponins, the culture medium was removed and 500 µL of PI (5 µg mL−1) was added to each well. After an hour of incubation, the PI solution was discarded and the wells were washed three times with PBS-glucose (0.6%). The plate was sealed with a plastic film and put under an inverted fluorescence microscope, with an excitation wavelength of 536 nm and emission wavelength of 617 nm. Nine random fields on each well were selected for images of both brightfield and PI staining. The total number of cells and the number of PI-positive cells were counted in each field. The data were presented as percentages of PI-negative cells (viable cells).
Statistical analysis
The biological assays were independently repeated three times with five replicates, except for the PI test, which was performed with three replicates. Data are expressed as means and standard deviation (s.d.) values. The difference between groups was evaluated by analysis of variance, followed by a Tukey test. Significance level to reject the null hypothesis was P < 0.05. The determination of the EC50, EC90 (parasitologic assays) and IC50 (cell viability test) was done through non-linear regression analysis. The statistical analysis was performed through the GraphPadPrism® software (version 5.0 for Windows).
Results
Obtaining of the natural compounds
Sapogenins of aescin (I) and digitonin (II) were obtained after acidic hydrolysis (III and IV, respectively). The flavonoids isocordoin (VIII) and a mixture of isocordoin and cordoin (VIII + IX), obtained from B. virgilioides, showed a molecular mass on negative ESI HRMS spectra of m/z 307.1343, besides the M + 1 and M + 2 isotopic contributions.
In vitro anthelmintic evaluation
Ovicidal activity
The saponins aescin (I) and digitonin (II) significantly inhibited (P < 0.05) the egg hatching of gastrointestinal nematodes of goats when compared with the negative control. However, only saponin I showed a 99% hatching inhibition efficacy, with an EC50 and EC90 of 0.67 and 0.79 mg mL−1, respectively. The treatment with the highest concentrations of this saponin (1.0 and 0.84 mg mL−1) did not differ (P > 0.05) from the positive control thiabendazole (0.025 mg mL−1) (Fig. 1A). In contrast, the sapogenins from these saponins and hecogenin acetate did not interfere in this stage of the parasite (Fig. 1B).
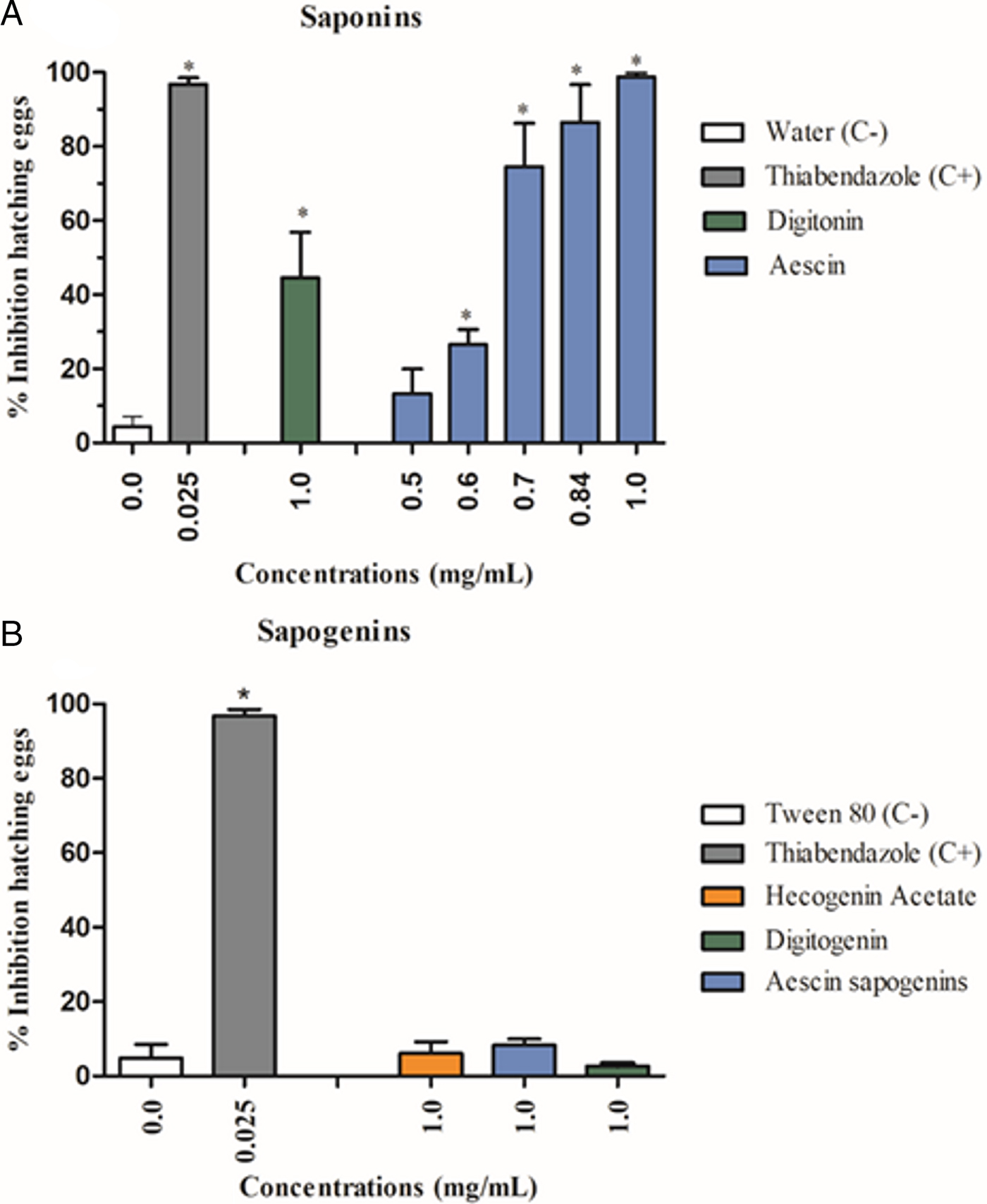
Fig. 1. Percentage of inhibition of the egg hatching (mean ± standard deviation) from the gastrointestinal nematodes treated with saponins (A) and sapogenins (B). (C−) Negative control. *Significant difference from negative control values (P < 0.05).
All the flavonoids (1 mg mL−1) showed low inhibition percentages: 4, 6, 9 and 12% for hesperidin (VII), catechin (VI), isocordoin (VIII) and a mixture of isocordoin and cordoin (VIII and IX), respectively.
Larvicidal activity
The digitonin (II) and hecogenin acetate (V), at the 1 mg mL−1 concentration, showed significant inhibitory activity on the L3 larvae motility (P < 0.05), with mobile larvae percentages of 5 and 25%, respectively. The digitonin (II) was the most active and showed larvicidal effect in a concentration-dependent manner, with EC50 and EC90 values of 0.03 and 0.49 mg mL−1, respectively. Moreover, no statistically significant difference was observed between digitonin (1 mg mL−1) and the positive control levamisole (0.25 mg mL−1). The treatment with aescin saponin (Fig. 2A), aescin sapogenins complex and digitogenin (Fig. 2B) did not induce alteration in the motility of infective larvae (mobile larvae percentages: 86–98%).
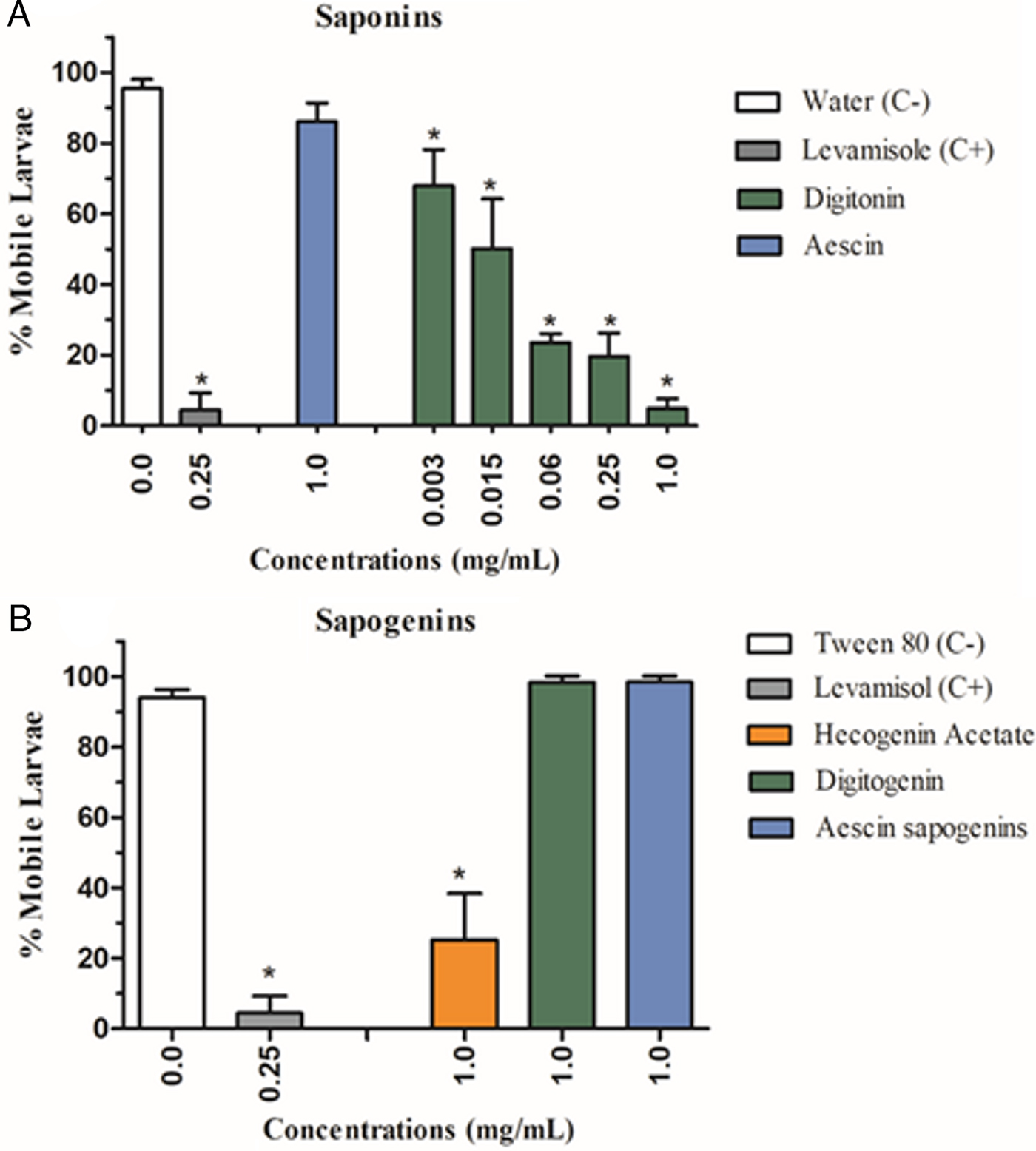
Fig. 2. Percentage of inhibition of mobile larvae (mean ± standard deviation) from the gastrointestinal nematodes treated with saponins (A) and sapogenins (B). (C−) Negative control. *Significant difference from negative control values (P < 0.05).
All flavonoids evaluated had a low larvicidal activity. The percentages of mobile larvae were: 81% (catechin, VI), 85% (hesperidin and isocordoin, VII and VIII, respectively) and 90% (mixture of isocordoin and cordoin, VIII and IX, respectively). Only the last treatment did not differ from the negative control (P > 0.05).
Cytotoxic evaluation
Cell viability
The saponins aescin and digitonin reduced the cell viability (MTT test) in a concentration-dependent manner (P < 0.05). The IC50 of saponin I (0.02 mg mL−1 or 18 µ m) was approximately three times higher than that of II (0.0074 mg mL−1 or 5.7 µ m). The cell viability percentages, between the highest and lowest concentrations tested, ranged from 8 to 97% for compound I and 6 to 97.5% for II (Fig. 3).

Fig. 3. Percentage of Vero cell viability, through the MTT test, after being treated with aescin and digitonin. (C−) Negative control. *Significant difference from negative control values (P < 0.05).
Cell integrity
In the PI test, the treatments with both the saponins [aescin (I) and digitonin (II)] did not cause alteration in the percentage of viable Vero cells. The percentages of cell viability varied between 96–99% (digitonin) and 94–97% (aescin) and did not differ from the negative control group (Fig. 4). However, these saponins induced morphology alterations, characterized by the cell spherical shape and loss of confluence in the cell monolayer.

Fig. 4. Percentage of Vero cell viability, through the PI test, after being treated with aescin and digitonin. (C−) Negative control; (C+) positive control. *Significant difference from negative control values (P < 0.05).
Discussion
In the present work, the acidic hydrolysis method was efficient to obtain the sapogenins from the saponins aescin (I) and digitonin (II). The 1H-RMN data of hydrolysed products were in agreement with the information already described for the saponins I and II aglycones (Muhr et al. Reference Muhr, Likussar and Schubert-Zsilavecz1996; Glensk et al. Reference Glensk2011; Farag et al. Reference Farag, Porzel and Wessjohann2015). Glycosylated molecules present strong signs in the middle-lower region of the 1H NMR spectrum (Vliegenthart et al. Reference Vliegenthart, Dorland and Halbeek1983; Press et al. Reference Press2000). These signs were not observed in the sapogenins of saponins I and II, what accounts for the acidic hydrolysis. The main sapogenins after acidic hydrolysis of aescin complex are protoescigenin and barringtogenol C (III) (Wulff and Tschesche, Reference Wulff and Tschesche1969; Gruza et al. Reference Gruza2013).
The chemical analyses of flavonoids obtained from B. virgilioides led to the characterization of isocordoin (VIII) and cordoin (IX). The analysis of HRMS indicated that both compounds were constitutional isomers with a C20H20O3 molecular formulae. The NMR spectra of IX presented all the peaks of the flavonoid structure. The flavonoid VIII could be identified in the mixture comparing the other NMR signals with the literature (Parmar et al. Reference Parmar1992).
Among the compounds evaluated, the saponins I, II and hecogenin acetate (V) showed in vitro anthelminthic activity against gastrointestinal nematodes of goats. According to Vercruysse et al. (Reference Vercruysse2001), the efficacy of an anthelmintic product is assured when it has per cent effectiveness of at least 90%. Considering this parameter, only I and II can be considered as effective against nematodes eggs and larvae L3, respectively. Antiparasitic effects of these saponins have been described against protozoans (Baldissera et al. Reference Baldissera2014; Krstin et al. Reference Krstin, Peixoto and Wink2015). However, no reports were found on their activity on helminths of goats.
In our study, the triterpenic saponin aescin (I) was more active against eggs than the L3 larvae stage, while the steroidal saponin digitonin (II) and sapogenin hecogenin acetate (V) showed the highest activity on L3 larvae. These findings are in agreement with the data reported for the fraction of triterpenic saponins from the Z. joazeiro (Gomes et al. Reference Gomes2016), which inhibited egg hatching of goat nematodes and did not interfere in L3 larvae motility. Botura et al. (Reference Botura2013) described that the steroidal saponin fraction of the A. sisalana, containing hecogenin aglycone, has only in vitro larvicidal effect.
The comparison of anthelmintic effects of saponins and sapogenins revealed that saponins were more effective, and this difference was more prominent between the saponins I and II and their respective sapogenins. Then, the removal of sugar chains promoted a high reduction of nematocidal activity.
Differences in biological activities, such as anti-inflammatory (Ye et al. Reference Ye, Xing and Chen2013), antimicrobial (Avato et al. Reference Avato2006), antifungal (Yang et al. Reference Yang2006) and haemolytic, were reported among the saponins and the sapogenins. Yang et al. (Reference Yang2006) evaluated antifungal activity of 22 saponins and six sapogenins, and observed that the presence of the sugar chains increased the efficacy of the saponins. These authors attribute the efficacy of the saponins to their aglycone portion and the quantity and the structure of the monosaccharide units in their sugar chains. Biological activities of saponins have been associated with their amphipathic characteristics, which facilitate the formation of complexes with cellular membrane components (steroids, proteins and phospholipids) leading to a pore formation and consequent increase in membrane permeability (Fuchs et al. Reference Fuchs2009; Doligalska et al. Reference Doligalska2011; Bottger et al. Reference Böttger, Hofmann and Melzig2012). The anthelmintic mode of action of saponins may be associated with their ability to act on these cellular components present in different stages of nematodes (Doligalska et al. Reference Doligalska2011).
The cytotoxicity of active saponins (aescin and digitonin) was evaluated using Vero cells. In the PI test, these saponins showed low toxicity, although morphology cell alterations were observed in both treatments. The apparent reduction of the cell, volume increase and the spherical shape might be an indicator of apoptotic cell death (Paris et al. Reference Paris2011). These alterations can be related with an increase of in-cell membrane permeability, yet without the presence of membrane rupture (Platonova et al. Reference Platonova2015). Gilabert-Oriol et al. (Reference Gilabert-Oriol2013) related membrane permeabilising effects of digitonin on human urinary bladder carcinoma cell line (ECV-304). This saponin promoted moderated effect at a concentration of 24 µ m and complete degradation at 48 µ m in PI assay. The PI acts as a DNA intercalator, staining the DNA of dead cell with membrane rupture (Rieger et al. Reference Rieger2011). Then, this test is not able to evaluate enzymatic alterations that may also indicate the mechanisms of cell death (Puttonen et al. Reference Puttonen2008).
For a better characterization of the cytotoxic effect, the MTT test was performed. In this assay, treatments with both saponins promoted cytotoxicity, and digitonin (II) was more toxic (IC50 = 5.7 µ m) in relation to aescin (I) (IC50 = 18 µ m). Gulden et al. (Reference Gülden, Schreiner and Seibert2015) also observed cytotoxicity of digitonin on the embryonic mouse fibroblast cells (Balb/c 3T3) using MTT assay. The Vero cells were more sensible to I (IC50 = 18 µ m) when compared with the human renal cancer cell lines (IC50 = 35.0–40.6 µ m) investigated by Yuan et al. (Reference Yuan2017). These authors reported that the aescin caused apoptosis though G2/M arrest and reactive oxygen species leading to mitochondrial membrane potential dysfunction in the cancer cells. In our study, saponins presented greater toxicity in MTT test in comparison to PI test, which suggests a possible mitochondrial mechanism of action.
Variations in toxicity between different groups of saponins have been reported in the literature. These differences may be associated with the structural chemical difference of saponins, mechanisms of action, selectivity and specificity properties of these components according to their target molecules or cells (Wang et al. Reference Wang2007; Thakur et al. Reference Thakur2011).
Even though the cytotoxic concentration (IC50) of the aescin (I) and digitonin (II) was lower than the effective concentration (EC50) for the anthelmintic activity, it must be highlighted that only the in vitro assays are not enough to fully evaluate their therapeutical and toxicological potential. Adverse effects might be observed in every medication, whether it is natural or synthetic, depending on its concentration, dosage and target organism (Klaassen and Watkins, Reference Klaassen and Watkins2010). The Aesculus hippocastanum extract, containing an active compound I, is a traditional herbal remedy used for chronic venous insufficiency and haemorrhoids in humans and it is generally well tolerated (Sirtori, Reference Sirtori2001). Then, in vivo studies on the toxic effects of saponins I and II in goats are needed (Thakur et al. Reference Thakur2011).
The flavonoids showed low anthelmintic activity on the eggs and larvae of nematodes. Similar results were reported by Desrues et al. (Reference Desrues2016), which demonstrated that the catechin was not active against L1 larvae of the cattle parasites (Ostertagia ostertagi and Cooperia oncophora). However, high in vitro anthelmintic activity of the flavonoid fraction (containing homoisoflavonoids) of A. sisalana and ethyl acetate of Digitaria insularis (containing flavonols tricin and diosmetin) were described against the eggs of goats nematodes (Botura et al. Reference Botura2013; Santos et al. Reference Santos2017). Despite the variety of investigations about the anthelmintic activity of plant extracts containing flavonoids, the data on the action of these isolated compounds are still scarce (Kerboeuf et al. Reference Kerboeuf, Riou and Guégnard2008). Moreover, structure–activity relationships can also be considered for nematicidal effect of flavonoids (Kerboeuf et al. Reference Kerboeuf, Riou and Guégnard2008; Kumar and Pandey, Reference Kumar and Pandey2013).
Conclusion
Aescin and digitonin have a high in vitro anthelmintic activity against gastrointestinal nematodes of goats. The presence of the sugar chains in the structure of these saponins has increased this biological effect, demonstrating the importance of the sugar chain in this activity. However, the flavonoids analysed had a low anthelmintic effect. The low in vitro toxicity of the saponins with anthelmintic activity was observed in the PI assay; this toxicity, however, had been high in the MTT test, which suggests the necessity of complementary studies in other cell types and in vivo testing.
Acknowledgements
The authors would like to thank the Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
Declaration of interest
None.
Ethical standards
The experimental procedures were approved by the Ethics Committee for the Use of Animals of the UFBA (Protocol number 37/2016).







