Introduction
Arbuscular mycorrhizal fungi (AMF, phylum Glomeromycota) are a key functional component of soil biota in agroecosystems and form symbiotic associations with the majority of economically important crop plants (Smith and Smith, Reference Smith and Smith2012) including vegetables (Baum et al., Reference Baum, El-Tohamy and Gruda2015). Arbuscular mycorrhiza (AM) have been reported to improve plant growth and health by increasing the uptake of mineral nutrients and water and providing tolerance to biotic and abiotic stresses (Brundrett, Reference Brundrett2009; Verbruggen et al., Reference Verbruggen, van der Hijden, Weedon, Kowalchuk and Röling2012). A plant's demand for mineral nutrients, particularly phosphorus (P), is determined by its genes (Krishna et al., Reference Krishna, Shetty, Dart and Andrews1985) and the fertility status of the soil. Some soil factors such as available nutrients, pH and organic carbon (OC) regulate the proliferation of AMF in the rhizosphere and secretion of the enzyme phosphatase by the fungal hyphae that help in hydrolysing phosphate from insoluble organic P compounds (Oehl et al., Reference Oehl, Laczko, Bogenrieder, Stahr, Bösch, van der Heijden and Sieverding2010). Many AMF species are widespread in different terrestrial ecosystems (Öpik et al., Reference Öpik, Moora, Liira and Zobel2006), whereas others appear to be restricted to specific types of land use, vegetation or climates (Oehl et al., Reference Oehl, Laczko, Bogenrieder, Stahr, Bösch, van der Heijden and Sieverding2010). Field crops depend obligately on AMF symbiosis and inoculation with these fungi has been found to be highly beneficial to crop yields in a range of soils (Rouphael et al., Reference Rouphael, Franken, Schneider, Schwarz, Giovannetti, Agnolucci, De Pascale, Bonini and Colla2015). Therefore, knowledge about the diversity of indigenous AMF prevalent in a specific region and associated with cultivated crops is an essential step for any application. The colonization patterns of AMF within the host roots tend to vary with the plant and fungal species involved (Dickson et al., Reference Dickson, Smith and Smith2007). Furthermore, the ability of AMF to colonize roots and to provide nutrients to the host system may vary among different morphological types i.e. Arum-, Paris- or Intermediate (Dickson, Reference Dickson2004). Though the association of AMF has been reported in the majority of cultivated crops, their root colonization patterns are yet to be determined (Muthukumar and Tamilselvi, Reference Muthukumar and Tamilselvi2010).
Recent reviews have reported the significance of AMF inoculation on growth and yield improvements of vegetable crops through alteration in plant growth physiology and morphology (Baum et al., Reference Baum, El-Tohamy and Gruda2015; Rouphael et al., Reference Rouphael, Franken, Schneider, Schwarz, Giovannetti, Agnolucci, De Pascale, Bonini and Colla2015). However, the characterized effect of AMF on plant growth and development in horticultural and agricultural crops is not always consistent, because of the complexity of interactions involved between the host plant genotypes and AMF, types of inoculation methods and differing environmental conditions (Baum et al., Reference Baum, El-Tohamy and Gruda2015). Application of microbial inoculants such as AMF and rhizobacterial communities, also termed biofertilizers and biostimulants, has attracted universal attention due to the negative impacts of excessive use of chemical fertilizers and pesticides, and to an increased awareness about sustainable organic agriculture (Gosling et al., Reference Gosling, Hodge, Goodlass and Bending2006; Srivastava et al., Reference Srivastava, Singh, Tripathi and Raghubanshi2016) and the beneficial association between rhizosphere microbiota and plants (Alori et al., Reference Alori, Glick and Babalola2017). Among these, phosphate solubilizing bacteria (PSB), plant growth promoting rhizobacteria (PGPR) and AMF consortia have received notable interest due to their synergistic beneficial effects as growth promoters to improve plant health in agriculture and horticulture (Antoun, Reference Antoun2012). In this context, Pseudomonads and the nitrogen-fixing Azotobacter are popular bioinoculants used widely to promote the growth of a wide variety of crop species (Soleimanzadeh and Ghooshchi, Reference Soleimanzadeh and Ghooshchi2013; Dey et al., Reference Dey, Sarkar, Dutta, Murmu and Mandal2017; David et al., Reference David, Chandrasehar, Selvam, Prasad, Gill and Tuteja2018). These bacteria promote plant growth through making plant nutrients available, synthesis of phytohormones, solubilization of phosphate, production of siderophores, antibiotics and lytic enzymes that degrade the fungal cell wall, and degradation of the pathogenic toxins (David et al., Reference David, Chandrasehar, Selvam, Prasad, Gill and Tuteja2018). They may also act as mycorrhizal helper bacteria in promoting mycorrhizal formation and function. Previous studies have shown that co-inoculation of Pseudomonads and Azotobacter along with AMF can improve plant growth and yield in several plant species (Sabannavar and Lakshman, Reference Sabannavar and Lakshman2008, Reference Sabannavar and Lakshman2011; Pérez-de-Luque et al., Reference Pérez-de-Luque, Tille, Johnson, Pascual-Pardo, Ton and Cameron2017).
Chilli (Capsicum spp., family Solanaceae) is among one of the most important horticultural and commercial crops in the Asian continent and its diverse cultivars are grown for vegetables, spices, condiments, etc., which are used mainly for colouring and pungency properties (Pereira et al., Reference Pereira, Vieira, Freitas, Prins, Martins and Rodrigues2016). India is the largest producer and consumer of chilli, contributing about 25% to the total global production, and remains in the prime position in terms of international trade by exporting 39% from its total production, earning about US$30 million per annum (Thilagar and Bagyaraj, Reference Thilagar and Bagyaraj2015; SBI, 2017). Global production of chilli and hot peppers has increased gradually during 2000–2010 from 20.8 to 27.6 million tonnes (FAO, 2010). The agroclimatic conditions of North Eastern (NE) India are suitable for cultivation of profitable quantities of various spices and the area is also perceived as a hot spot for various indigenous chilli species (Mathur et al., Reference Mathur, Dangi, Dass and Malhotra2000). Naga King chilli/Umorok (Capsicum chinense Jacq. cv.) has been cultivated widely in north-east India by traditional shifting cultivation since time prehistoric, with an extensive amount of genetic variability between the landraces (Hazarika and Neog, Reference Hazarika and Neog2014). It has received attention from the global scientific community due to its extremely high pungency [1 001 304 Scoville Heat Units (SHU)] and unique aroma (Meghvansi et al., Reference Meghvansi, Siddiqui, Khan, Gupta, Vairale, Gogoi and Singh2010) and has immense scope in domestic and international markets due to its remarkably high capsaicin content.
Recent studies in India have reported the positive role of AMF in the growth and yield of C. annum (Tanwar et al., Reference Tanwar, Aggarwal, Kadian and Gupta2013; Thilagar and Bagyaraj, Reference Thilagar and Bagyaraj2015) and C. frutescens (Dai et al., Reference Dai, Singh and Nimasow2011). Nevertheless, information on AMF diversity, root colonization level, the type of AM morphology produced therein and the impacts of AMF and/or bioinoculants on growth performances of C. chinense, in India, has not been explored to date. To our knowledge, only one previous study from Tabasco, Mexico has examined the response of C. chinense to inoculation with AMF and rhizobacteria (Constantino et al., Reference Constantino, Gómez-Álvarez, Álvarez-Solís, Geissen, Huerta and Barba2008). In view of the potential importance of AMF inoculation technology for sustainable agriculture, a detailed survey of indigenous AMF diversity in sub-tropical agroecosystems of the Indo-Burma biodiversity hotspot region would provide a basis for their multifunctional field applications in future. Therefore, an attempt has been made in the current investigation (1) to determine the spore density and species diversity of AMF in the C. chinense rhizosphere, (2) to record AM morphology as well as the root colonization patterns of AM during different stages of plant growth and (3) to evaluate the influence of the most abundant AM fungus on growth and yield response of Naga King chilli individually and along with rhizobacterial inoculants in sterilized and non-sterilized soils under greenhouse conditions of north-east India.
Materials and methods
Endomycorrhizal association in field cultivated C. chinense plants
Study site and crop growth conditions
The current work was conducted at a shifting cultivated agricultural field located on the gentle slope of a hillock along the Taphou Naga hill range of Senapati District (25°16′N, 94°01′E, 1117–1142 m asl) at a distance of 62 km north of Imphal, the capital city of Manipur, India. Geographically, about 90% of the total area of Manipur is covered by hill ranges that are offshoots of the eastern Himalayas, with ~1813 km2 of the central valley area (Sehgal et al., Reference Sehgal, Sen, Chamuah, Singh, Nayak, Saxena, Baruah and Maji1993). The sub-tropical humid climate of the area is characterized by three distinct seasons: warm, moist summer (April to June), wet, rainy months (July to September) and cool, dry winter (November to February). March and October comprise the transitional periods between winter/summer and rainy/winter seasons, respectively. The annual temperature of the study site ranged from 3.4 to 34.4 °C. The mean monthly averages of relative humidity (RH) varied from 76 to 92%, whereas the total annual rainfall was 1454 mm. According to United States Department of Agriculture classification (Soil Survey Staff, 1999), the soil is Ultisol developed from shale and sandstone on gently sloping narrow valleys to steep hill slopes and is heterogeneous in nature (Sehgal et al., Reference Sehgal, Sen, Chamuah, Singh, Nayak, Saxena, Baruah and Maji1993). The soil of the study site was sandy loam in texture and slightly acidic in nature.
Generally, C. chinense prefers deep, loose sandy loam or clay loam soil that is rich in organic matter with a pH ranging from 5.0 to 6.5 (Bhagowati and Changkija, Reference Bhagowati and Changkija2009). The crop is cultivated as a sporadic intercrop in slash and burn agriculture or Jhum (shifting cultivation) fields in areas with an annual rainfall range of 1200–4050 mm and a temperature range of 6–36 °C. In the hilly region, planting of Naga King chilli is carried out during February/March and harvesting is from May/June onwards, whereas in plain areas this crop is planted in August/September and harvested in November/December (Meghvansi et al., Reference Meghvansi, Siddiqui, Khan, Gupta, Vairale, Gogoi and Singh2010). The negative effects of Jhum cultivation, such as loss of soil fertility and biodiversity, warrant efforts to reduce the reliance on such cultivation practices.
Sample collection
Fine roots and rhizosphere soil samples belonging to Naga King chilli were collected between February and June 2015 during three different plant growth stages: (1) pre-flowering, at 40–45 days after sowing (D1), (2) flowering, 85–90 days after sowing (D2) and (3) fruit ripening, 130–135 days after sowing (D3). The roots were excavated by digging a soil layer (0–15 cm) around five randomly selected chilli plants at each growth stage. Roots were washed gently and fixed in formalin : acetic acid : alcohol (FAA) solution (formalin : glacial acetic acid : 70% ethyl alcohol, 5 : 5 : 90 ml v : v : v) until processing. The soil surrounding and shaken from roots was placed in individual polythene bags, labelled and taken to the laboratory. After air drying in shade, one part of the soil samples belonging to each growth stage was used for extraction of AMF spores and another part was bulked together and used for assessment of soil properties and the establishment of trap cultures.
Analysis of soil properties
Soil physicochemical characteristics were determined using three sub-samples collected at each growth stage of chilli. Soil texture was analysed by the Bouyoucos hydrometer method (Allen et al., Reference Allen, Grimshaw, Parkinson and Quarmby1974), whereas soil pH and electrical conductivity (EC) were determined at room temperature in an aqueous solution of soil : water (1 : 1, v : v) using digital meters (ELICO, India). The OC was assessed by the Walkley and Black rapid titration method (Walkley and Black, Reference Walkley and Black1934). Soil total nitrogen (N) and available P were determined according to Jackson (Reference Jackson1971) and exchangeable potassium (K) was analysed after extraction with ammonium acetate (Jackson, Reference Jackson1971).
Extraction and identification of AMF spores
AMF spores were extracted by wet sieving and decanting methods (Gerdemann and Nicolson, Reference Gerdemann and Nicolson1963): In total, 100 g of each soil sample was dispersed in 1 litre of water and the suspension was decanted through a series of 710 to 37 µm sieves. Residues in the sieves were washed into beakers, whereas the filtrates were dispersed in water and filtered through filter papers with a grid drawn on it. Each filter paper was then spread on glass plates and observed under a light microscope at 40× magnification. All the intact and healthy AMF spores (non-collapsed spores with cytoplasmic contents and free from parasitic attack) were counted and the sporocarps and spore clusters were considered as one unit. Isolated AMF spores were transferred onto glass slides using a wet needle and mounted in polyvinyl alcohol-lacto glycerol with or without Melzer's reagent (Schenck and Perez, Reference Schenck and Perez1990). Identification of AMF spores was carried out based on the original description on the Schüssler's web site (http://www.amf-phylogeny.com/amphylo_species.html). Spore morphology was also compared with the cultural database of the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM; https://invam.wvu.edu/cultures). The AMF infective propagules in the soil were assessed by the most probable number (MPN) technique as proposed by Porter (Reference Porter1979).
Assessment of AMF colonization
Fixed roots were washed with distilled water to remove the FAA solution, cut into 1-cm long segments, processed for clearing in 2.5% potassium hydroxide (KOH) at 90 °C for 1–2 h, depending on the degree of lignification of the roots, in a water bath (Koske and Gemma, Reference Koske and Gemma1989) acidified with 5 N hydrochloric acid (HCl) solution for at least 15–20 min and stained with 0.05% of trypan blue-lacto glycerol overnight at room temperature. The stained root pieces were mounted on glass slides and examined for AMF structures with a compound microscope (Olympus BX 51, Japan). Percentage of root length colonization was estimated according to the magnified intersection method (McGonigle et al., Reference McGonigle, Miller, Evans, Fairchild and Swan1990). Arbuscular mycorrhizal morphology was characterized based on the inter- or intra-cellular nature of fungal structures within the roots (Dickson, Reference Dickson2004).
Establishment of trap cultures
Five trap cultures were established using 2-litre earthenware pots filled with rhizospheric soil samples mixed with sterilized sand (1 : 1 v/v). The trap cultures were placed in the greenhouse and seeded with maize (Zea mays L.) as a host plant. The pots were watered on alternate days. Developed spores of AMF were isolated 120 days after culture initiation and identified as described above.
Bioinoculant potential on growth and yield performance of C. chinense
Experimental site and soil preparation
The study was carried out in the greenhouse of the Life Sciences Department, Manipur University, Imphal, India (24°45′N, 93°55′E, 795 m asl). The mean minimum and maximum temperatures of the experimental site during the study period, i.e. February to June 2016, ranged from 9.6 to 27.5 °C, while the RH varied from 64 to 90.3%. The climate is monsoonal with a dry period between February and March. The soil used in the current experiment was sandy loam in texture, collected during the first week of February 2016 from a natural chilli field situated in Senapati District of Manipur, India. The soil was sieved to remove pebbles and root fragments. One part of the soil was autoclaved (sterilized, SS) three times at 121 °C for 1 h with a 24 h gap between each sterilization process, while the remaining portion was used as collected (non-sterilized, NS). Soil properties were assessed using standard procedures as outlined above, which revealed: 99.7 kg/ha total N, 80.5 kg/ha available P, 163.4 kg/ha exchangeable K and 2.26% of OC. The pH and EC of the test soil was 5.8 and 0.26 dS/m, respectively.
Isolation, pure culture and inoculum preparation of indigenous AMF
Dominant AMF were isolated from the rhizosphere soil of field-grown Naga King chilli by the wet sieving and decanting technique, as described by Gerdemann and Nicolson (Reference Gerdemann and Nicolson1963). Field-collected soil had an indigenous AMF population of 18 propagules/g soil as assessed by MPN (Porter, Reference Porter1979) and the indigenous AM flora consisted of Funneliformis geosporum (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler, Glomus ambisporum G.S. Sm. & N.C. Schenck, Glomus macrocarpum Tul. & C. Tul., Glomus sp. and Racocetra sp., of which F. geosporum was the most dominant AMF.
The starter inoculum of F. geosporum was developed from a single spore by the funnel technique (Menge and Timmer, Reference Menge, Timmer and Schenck1982) and multiplied for 30 days using maize as the host plant. The pure culture of F. geosporum was further mass-multiplied in earthenware pots of 22 cm diameter containing 3 kg of sterilized soil : sand (1 : 1 v/v) mixture as the substrate and C. chinense as the host plant. After 120 days of growth, roots of the host plant were assessed for AM colonization levels and their spore density in inoculum soil as described above. The percentage of root colonization of C. chinense by AMF ranged from 80 to 90% and the AM fungus spore population consisted of 115 ± 4 spores per 10 g fresh soil, which included 65 infective propagules/g of soil as assessed by the MPN technique of Porter (Reference Porter1979). For mycorrhizal treatment, 100 g of fresh inoculum soil along with AMF spores and chopped colonized roots of chilli were prepared for each test pot.
Bioinoculant inoculum preparation
Two pure culture strains of rhizobacteria were used, i.e. Pseudomonas fluorescens (PSB) (LSDCC-21) and Azotobacter chroococcum (PGPR) (LSDCC-34) for this experiment. The bacterial inocula were procured from the Laboratory of Microbiology and Plant Pathology, Department of Life Sciences, Manipur University, Imphal, India. P. fluorescens and A. chroococcum were cultured in liquid King's broth and Mannitol agar medium, respectively and incubated at 28 ± 1.3 °C with orbital agitation (120 rpm) for 48 h. The medium was centrifuged (8000 g units/10 min) and the pellet resuspended in 50 ml sterile distilled water; 1 ml of the solution, adjusted to 1 × 108 colony forming units (CFU), was maintained for each rhizobacterial culture.
Experimental design
The pot experiment consisted of a randomized complete block design (2 × 5 × 5) with two soil conditions, sterilized soil (SS) and non-sterilized (NS) field soil, and five inoculation treatments (C – control, T1 – F. geosporum, T2 – F. geosporum + PSB, T3 – F. geosporum + PGPR, T4 – F. geosporum + PSB + PGPR) each with five replicates. Ripened Naga King chilli fruits were harvested from the plants growing in the natural field, placed in a polythene bag and taken to the laboratory. The fruits were air dried under warm sunny conditions and room temperature for 1 week. The dried seeds were surface-sterilized with 2% sodium hypochlorite solution for 1 min, washed in several changes of sterilized distilled water, air dried and placed in plastic trays containing sterilized soil : sand mixture (1 : 1, v : v). After 20 days of growth, uniform chilli seedlings were transferred (1 seedling/treatment), individually, into pots (25 × 30 cm2, height × diameter) filled with 4 kg of sterilized (SS) or non-sterilized (NS) soil. Before transplanting each seedling, 100 g of AMF inoculum was layered at a depth of about 5 cm. Rhizobacterial (PSB and PGPR) inoculations were made either individually or in combination as per the treatments by adding 3 ml cell suspension of each test inoculant (108 CFU/ml) adjacent to the chilli seedling root system at 5 min intervals. An equal quantity of heat sterilized microbial inocula was added to the control treatments. The pots with chilli seedlings were watered on alternate days to maintain the moisture at ~60% of the water holding capacity of the soil.
Harvest and measurement
Naga King chilli plants were carefully uprooted 135 days after sowing and different growth parameters viz., shoot and root length (cm), number of leaves, shoot collar diameter (cm) and shoot and root dry weights (g), were determined. Similarly, days to flowering, flower number, fruit set (%), the number of fruits and fruit fresh weight (g) were also recorded. All tissue samples, i.e. shoots (leaves and stems) and roots were placed in separately labelled paper envelopes and oven-dried at 80 °C for 48 h to determine dry weights. The root/shoot (R/S) ratios were calculated from the dry weights of roots and shoots. Total root length was calculated according to Newman (Reference Newman1966) and fruit set (%) was calculated according to Wubs et al. (Reference Wubs, Ma, Hemerik and Heuvelink2009) as:
Oven-dried shoots and roots were analysed for tissue N, P and K contents. The N content in shoot and root samples were determined by the micro-Kjeldahl digestion method, while total P was determined by the molybdenum blue method after triple acid (concentrated nitric acid [HNO3], sulphuric acid [H2SO4] and 60% perchloric acid [HClO4]) digestion (Jackson, Reference Jackson1971). Total K was estimated by flame photometry (Davis, Reference Davis, Peach and Tracey1962). Spore numbers of AMF in the test soil were estimated as described above. At sampling times, a weighed sub-sample of the fresh feeder roots was taken from each plant to assess the percentage root length AM colonization. Microbial inoculation effect (MIE) was calculated according to Bagyaraj (Reference Bagyaraj, Norris, Read and Verma1992).
Statistical analyses
The AMF spore density, species richness, relative abundance (%RA) and isolation frequency (%IF) were calculated as indicated by Zhao and Zhao (Reference Zhao and Zhao2007). Analysis of variance was used to test the significance of variations between AMF variables in field-collected chilli roots and to compare growth and yield parameters evaluated in bioinoculant treatments in pot experiments (SPSS version 9, SPSS Inc., Chicago, Illinois). Pearson's correlations were carried out to assess the relationship between soil physicochemical properties and mycorrhizal colonization and the relationship between plant growth variables, tissue nutrient contents and AMF colonization. To achieve the normalization prior to statistical analysis, the AMF spore numbers and the percentage values for root colonization by endomycorrhizal fungi were log and arcsine transformed, respectively.
Results
Soil properties
The soils of Naga King chilli during different growth periods had a pH range of 5.7–6.3, an EC of 0.26–0.30 dS/m and an organic C of 2.2–2.7% (Table 1). Total N, available P and exchangeable K of the soils varied from 95 to 113 kg/ha, 8.4 to 9.1 kg/ha and 195 to 211 kg/ha, respectively. Maximum concentrations of organic C, N, P, and K were recorded in the pre-flowering period. However, all the soil characters (except for EC) examined varied significantly (P < 0.05 to P < 0.01) among different growth periods.
Table 1. Soil properties in the upper 15 cm soil profile of Capsicum chinense during different plant growth stages
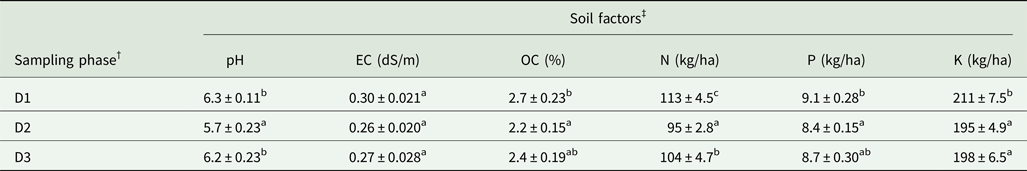
† D1, D2 and D3 indicate pre-flowering (at 40–45 days after sowing), flowering (at 85–90 days after sowing) and fruit ripening (at 130–135 days after sowing), respectively.
‡ pH, EC, OC, N, P and K indicate percentage hydrogen ion concentration, EC, OC, total nitrogen, available phosphorus and exchangeable potassium, respectively.
Means ± standard error in a column followed by different superscript letters are significantly different according to Duncan's multiple range test (P < 0.05).
The extent of AMF colonization
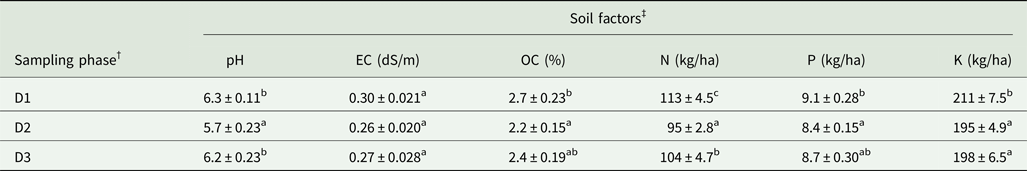
All the root fragments of Naga King chilli examined had an association of AMF (Table 2, Fig. 1). Paris-type AM morphology was observed in Naga King chilli with intracellular hyphal coils, arbusculate coils and vesicles. Significant (P < 0.001) differences were observed in the extent of AMF colonization and root length with AMF structures during different growth periods (Table 2). The percentage of root length with total AM colonization (%RLTC) ranged from 57 to 75%. Similarly, the percentage root length with hyphal coils (%RLHC) varied between 25 and 34%; arbusculate coils (%RLAC) ranged from 28 to 40%, and the vesicles (%RLV) varied between 3.9 and 4.8%. Overall, the percentage root length colonization with hyphal coils and vesicles were highest during the fruit-ripening stage, while that of arbusculate coils and total AM colonization were maximum during the flowering period. Significant (P < 0.001) variations existed among the different growth stages of Naga King chilli for %RLHC, %RLAC and %RLTC. However, there was no significant variation in %RLV among three different growth periods.
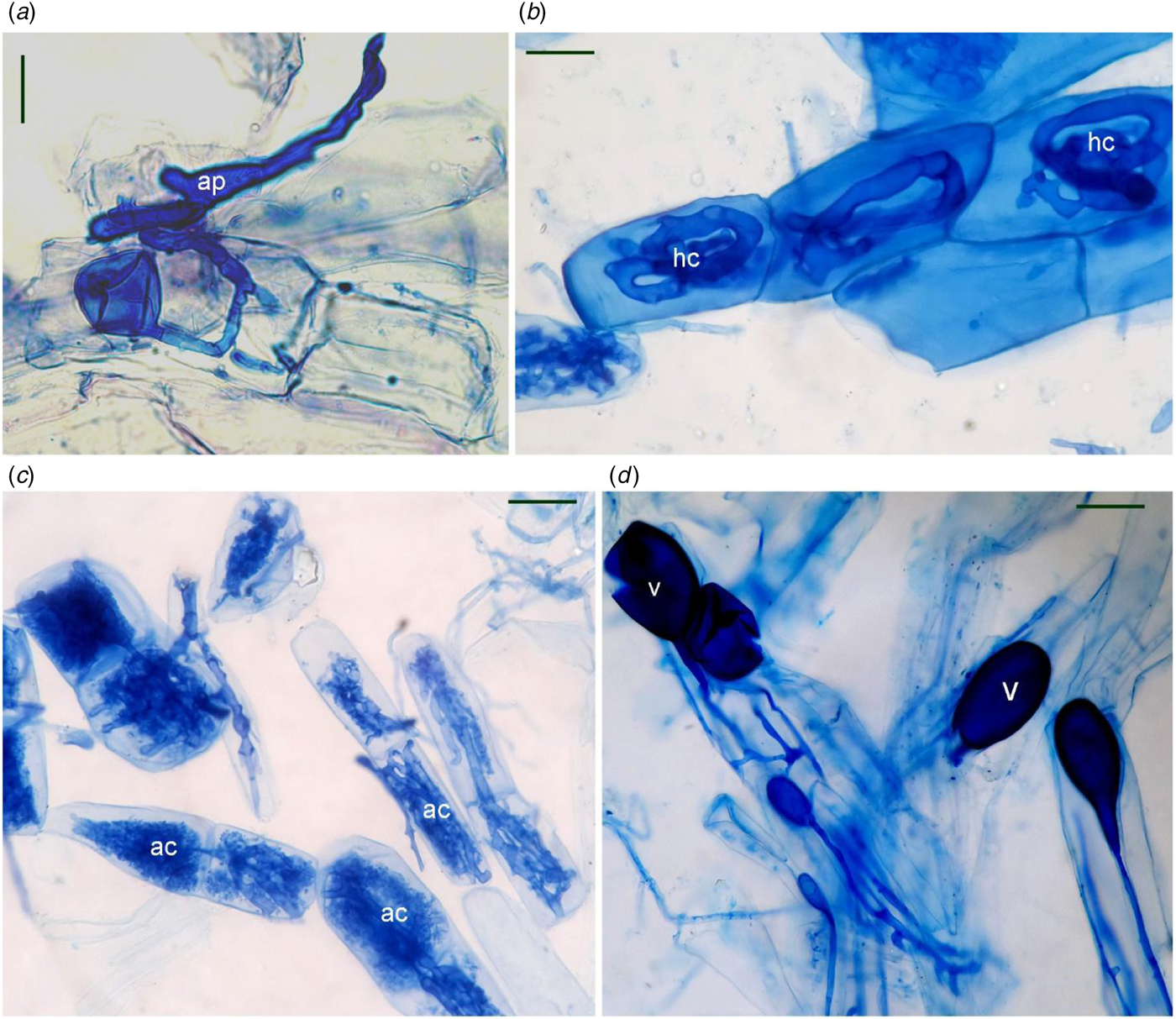
Fig. 1. (Colour online) AMF structures in Capsicum chinense roots. Appressorium (a); hyphal coils (b); arbusculate coils (c); vesicles (d). ap, appressorium; ac, arbusculate coil; hc, hyphal coil; v, vesicle. Scale bars: 20 µm.
Table 2. Extent of AMF colonization in roots of C. chinense during different plant growth stages

† D1, D2 and D3 indicate pre-flowering (at 40–45 days after sowing), flowering (at 85–90 days after sowing) and fruit ripening (at 130–135 days after sowing), respectively.
‡ %RLHC, %RLAC, %RLV, %RLTC and SPN indicate percentage root length with AMF hyphae, hyphal coils, arbuscules/arbusculate coils, total colonization and spore number (per 100 g soil), respectively.
Means ± standard error in a column followed by different superscript letters are significantly different according to Duncan's multiple range test (P < 0.05).
AMF spore density
The highest spore population of AMF was recorded in root-zone soils of Naga King chilli during the pre-flowering stage, while that of the flowering stage revealed the lowest spore counts (Table 2). Moreover, AMF spore density varied significantly (P < 0.01) among the different growth periods and was significantly (P < 0.05) and negatively correlated with the AMF root colonizing variables (Table 3).
Table 3. Pearson's correlation coefficient values for root colonizing structures of AMF and soil variables (n = 45)
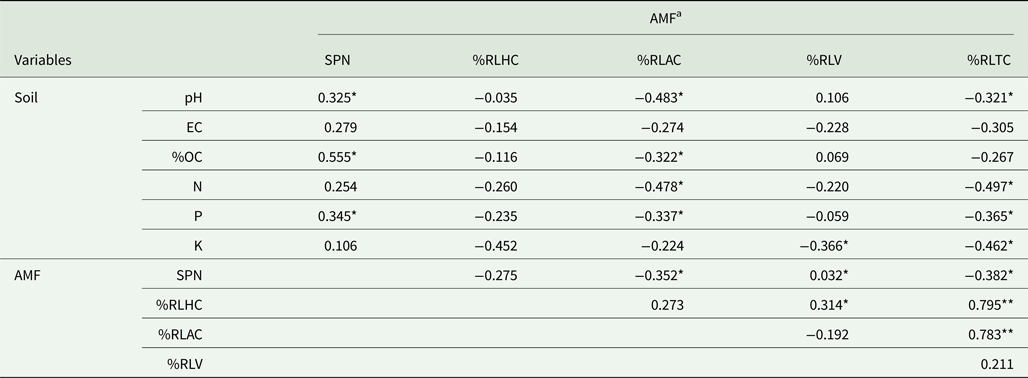
a SPN, %RLHC, %RLAC, %RLV and %RLTC indicate AMF spore numbers, percentage root length with AMF hyphal coils, arbusculate coils, vesicles and total colonization, respectively.
* and **: Significant at P < 0.05 and P < 0.01, respectively.
The relationship between endomycorrhizal fungal and soil variables
Pearson's correlation analysis revealed the existence of significant (P < 0.05) positive correlations between SN and soil pH, organic C and P (Table 3). The %RLAC and %RLTC were significantly (P < 0.05) and negatively correlated with soil pH and total N and available P. A similar correlation also existed for %RLHC and soil exchangeable K, and that of %RLV as well as %RLTC with K and P concentrations. Likewise, AMF spore numbers were significantly (P < 0.05) and negatively correlated with %RLAC and %RLTC, while %RLAC and %RLHC were significantly (P < 0.05) and positively correlated with %RLTC.
AMF species distribution
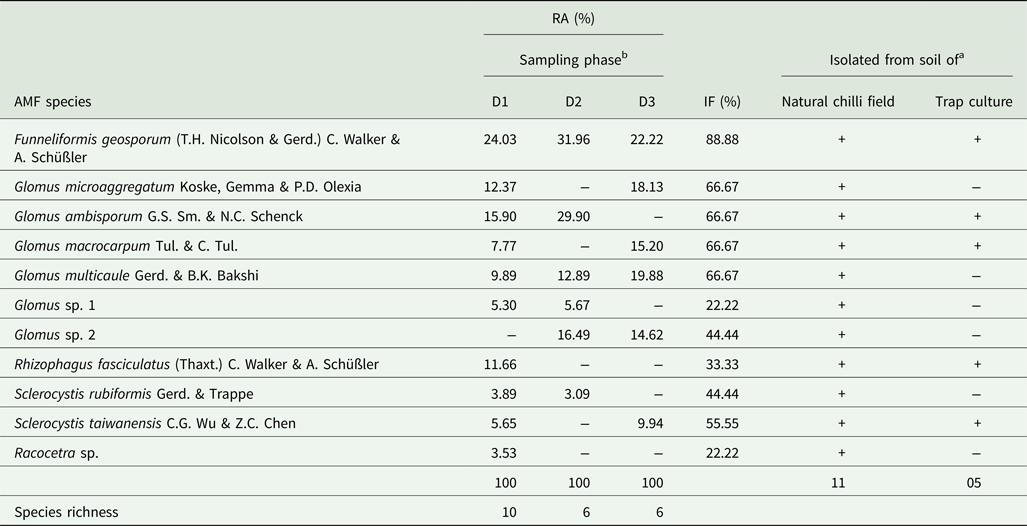
A total of 11 AMF spore morphotypes corresponding to five genera, i.e. Funneliformis, Glomus, Rhizophagus, Sclerocystis and Racocetra, were extracted from the natural field soil of Naga King chilli (Table 4, Fig. 2). Six of these AMF spore morphotypes belonging to four genera were also isolated from trap cultures of C. chinense (Table 4). Glomus was the dominant genus that was represented by six different species, out of which, G. multicaule was recovered from the soil samples collected during all three growth periods, while G. microaggregatum and G. macrocarpum were extracted from the soils belonging to pre-flowering and fruit ripening periods of crop growth. Spore morphotypes of genera such as Sclerocystis were represented by two species, whereas a single species each of Funneliformis, Rhizophagus and Racocetra were isolated from Naga King chilli rhizosphere. Of these, F. geosporum and G. multicaule were recovered from all the three growth periods with highest percentages of RA and IF (Table 4). Rhizophagus fasciculatus and Racocetra sp. were exclusively found only during the pre-flowering stage, while Sclerocystis rubiformis and S. taiwanensis were specifically isolated during the flowering and fruit ripening stages.

Fig. 2. (Colour online) AMF spores isolated from rhizosphere of C. chinense. (a) Funneliformis geosporum, (b) Glomus microaggregatum, (c) Glomus ambisporum, (d) Glomus macrocarpum, (e) Glomus multicaule, (f) Rhizophagus fasciculatus, (g) Sclerocystis rubiformis, (h) Racocetra sp., (i) Sclerocystis taiwanensis. Scale bars: 20 µm (b, c, e, g, h, i) and 40 µm (a, d, f).
Table 4. Occurrence, %RA and %IF of AMF during different growth stages of C. chinense
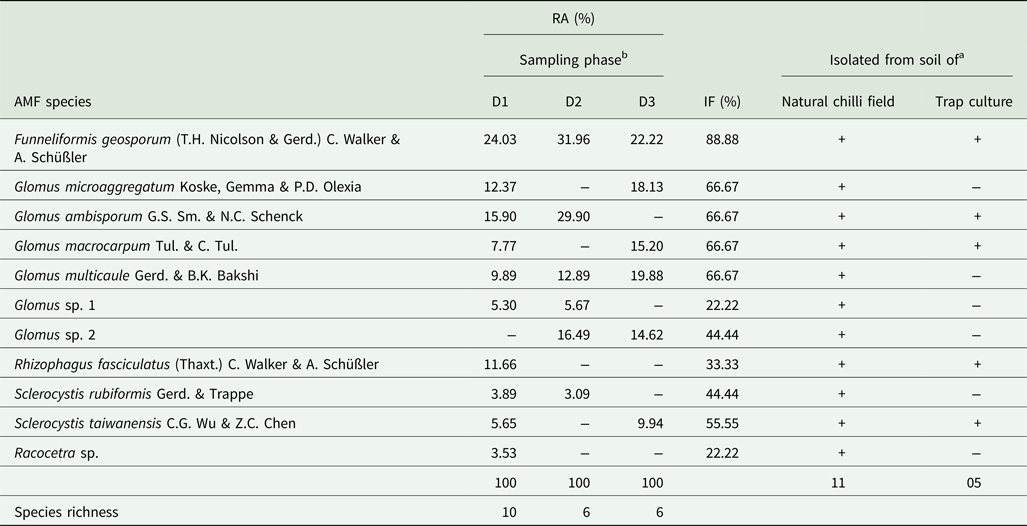
a + and − indicate presence and absence of a particular AMF species.
b D1, D2 and D3 and indicate pre-flowering (at 40–45 days after sowing), flowering (at 85–90 days after sowing) and fruit ripening (at 130–135 days after sowing), respectively.
Effect of bioinoculants on growth performance of Naga King chilli
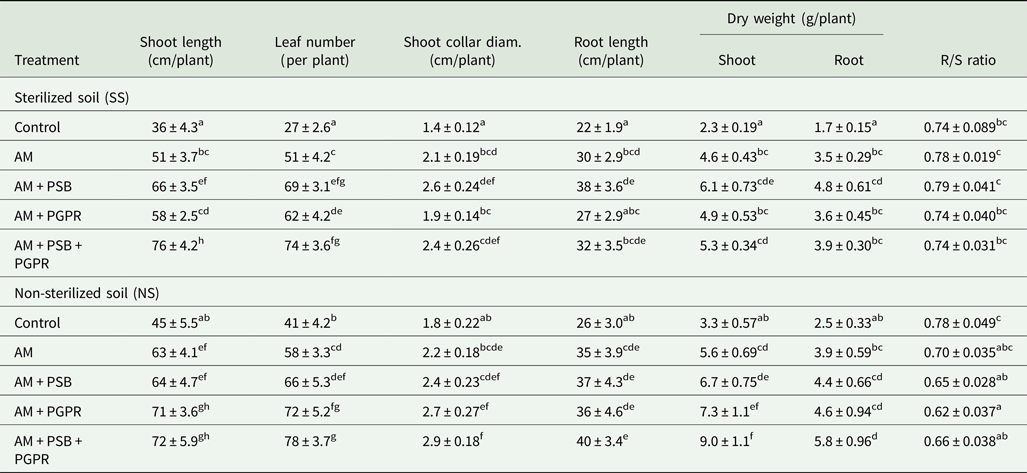
The current findings revealed significant (P < 0.05 to P < 0.001) enhancement in different evaluated growth parameters and fruit yield compared with the control chilli plants. At final harvest (130–135 days), microbial inoculated Naga King chilli seedlings had greater shoot and root lengths, collar diameter, biomass and leaf number, compared with uninoculated controls under SS and NS soil conditions (Table 5). Maximum shoot length of chilli plants was recorded when all three microbes i.e. AM + PSB + PGPR were combined under the SS soil conditions, whereas other growth parameters viz., leaf number, root length, shoot collar diameter and shoot and root dry biomass were higher under NS soil conditions. The most effective treatment in SS soil was AM + PSB, which revealed significantly (P < 0.001) higher root length, shoot collar diameter, shoot and root dry weights compared with uninoculated controls. The interaction between soil × treatments was not significant in the case of root length and root dry weight, whereas Naga King chilli seedlings inoculated with AMF individually or together with PSB and PGPR had lower R/S ratios under NS soil conditions compared with controls.
Table 5. Growth response of C. chinense plants inoculated with native arbuscular mycorrhizal fungus, phosphate solubilizing and growth promoting rhizobacteria under pot conditions
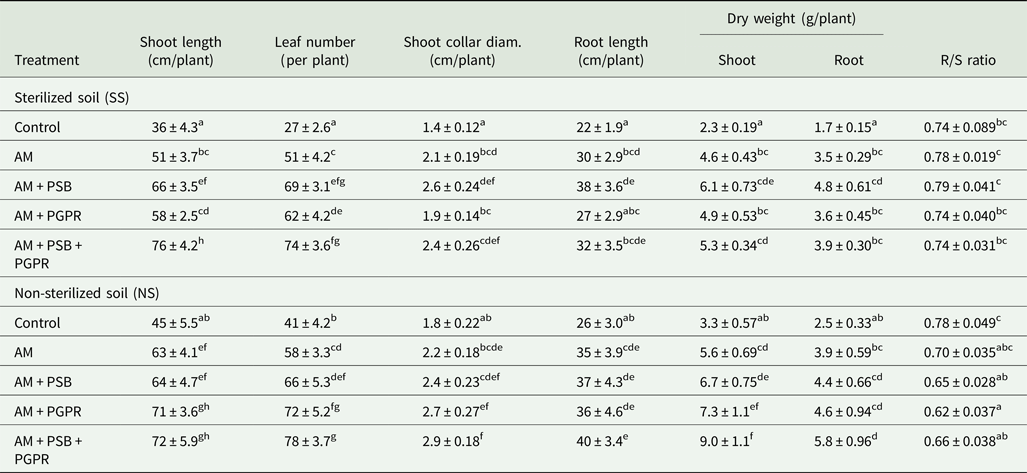
AM, Funneliformis geosporum; PSB, Pseudomonas fluorescens; PGPR, Azotobacter chroococcum.
Means ± standard error in a column followed by different letter(s) are significantly different according to Duncan's multiple range test (P < 0.05).
Flowering and fruit yield
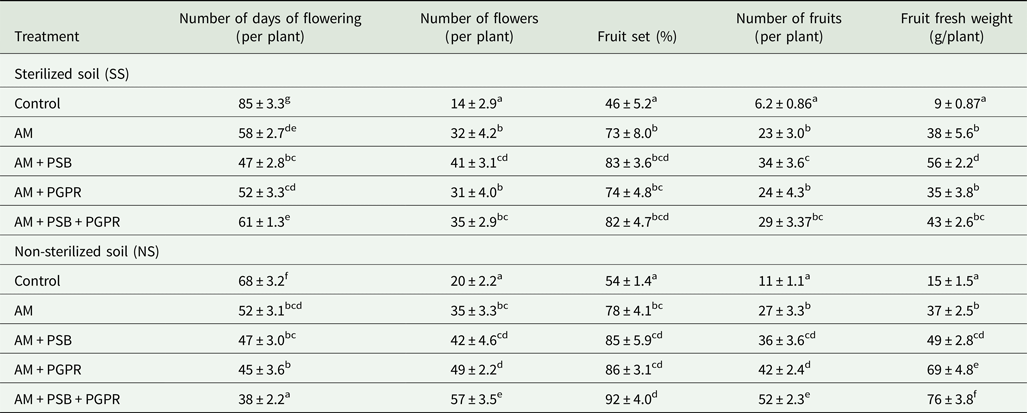
The current results showed that Naga King chilli flowering and fruit yield parameters under SS and NS soil conditions were highly influenced by different microbial inoculations (Table 6). Early flowering was recorded in the inoculated ones compared with that in the controls in which flowering was delayed. The flowers were first seen in AM + PSB + PGPR-treated chilli plants under NS soil, followed by AM + PSB under SS soil conditions compared with control seedlings, in which flowers appeared at 85 days. Similarly, the number of flowers and fruits along with fresh weight (g) of fruit yield varied significantly (P < 0.001) among the two soil conditions, treatments and soil × treatments. Maximum numbers of flowers, fruits and the fruit yield were recorded in all three microbial consortia, i.e. AM + PSB + PGPR-treated Naga King chilli plants under NS soil conditions. However, AM + PSB had more influence on fruit yield parameters under SS soil conditions. In contrast, there was no significant difference in fruit set (%) between soil × treatments (P > 0.05).
Table 6. Yield of C. chinense inoculated with native arbuscular mycorrhizal fungus, phosphate solubilizing and growth promoting rhizobacteria under pot conditions

AM, F. geosporum; PSB, P. fluorescens; PGPR, A. chroococcum.
Means ± standard error in a column followed by different superscript letters are significantly different according to Duncan's multiple range test (P < 0.05).
Tissue nutrient concentrations of Naga King chilli plants
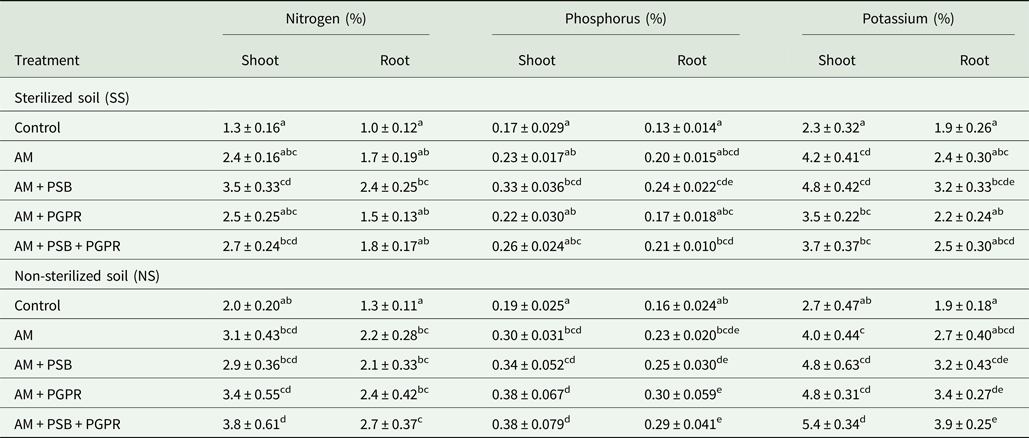
Significant differences in N, P and K concentrations of shoots and roots were found between soil (P < 0.05 to P < 0.01) and treatments (P < 0.01 to P < 0.001) (Table 7). Seedlings inoculated with AM + PSB + PGPR had a significantly (P < 0.01) higher accumulation of nutrients in their shoots and roots compared with other treatments under NS soil conditions. However, AM + PSB inoculated chilli seedlings had maximum N, P and K concentrations in SS soil compared with the control. Root P and K concentrations revealed significant (P < 0.05 to P < 0.01) variation between soil conditions, treatment and soil × treatments (Table 7).
Table 7. Nutrient concentrations in C. chinense tissues as influenced by inoculation of arbuscular mycorrhizal fungus, phosphate solubilizing and growth promoting rhizobacteria
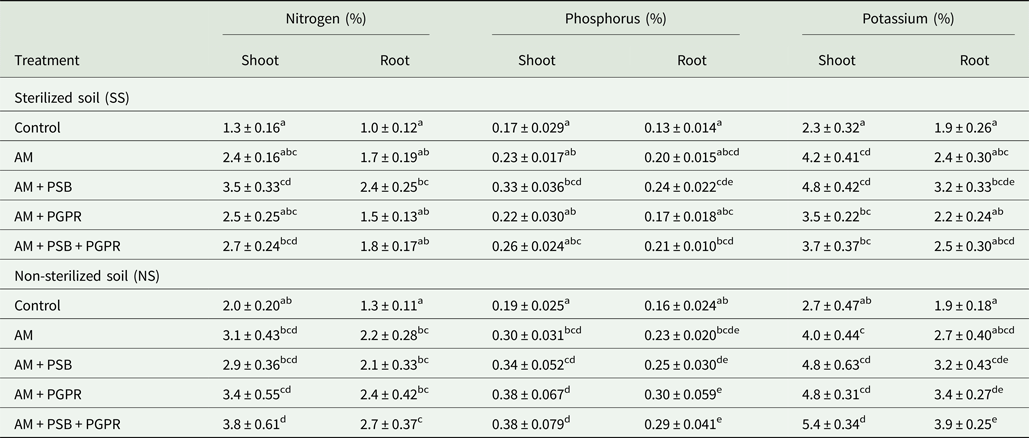
AM, F. geosporum; PSB, P. fluorescens; PGPR, A. chroococcum.
Means ± standard error in a column followed by different letter(s) are significantly different according to Duncan's multiple range test (P < 0.05).
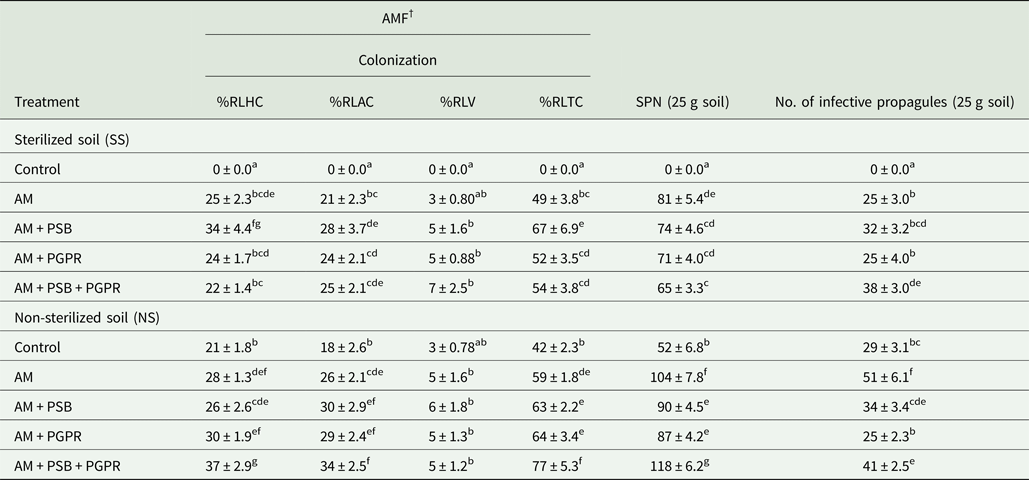
AMF colonization and spore density in treated Naga King chilli plants
The percentage of root length colonization in C. chinense plants increased significantly (P < 0.001) with inoculation of AMF when compared with the control treatments (Table 8). The %RLTC in Naga King chilli varied between 42 and 77% in different treatments. Among all, the highest %RLTC by AMF was observed in the plants inoculated with AM + PSB + PGPR under NS soil conditions. PSB or PGPR along with AMF and the triple microbial combinations increased mycorrhizal colonization significantly (P < 0.001) over AM alone and uninoculated controls. However, AMF spore density was higher in AM alone in SS soil and AM + PSB + PGPR for NS soil conditions, as compared with other treatments (Table 8).
Table 8. AMF root colonization, spore numbers (SPN) and infective propagules of AMF in the rhizosphere of C. chinense as influenced by microbial inoculations under pot conditions
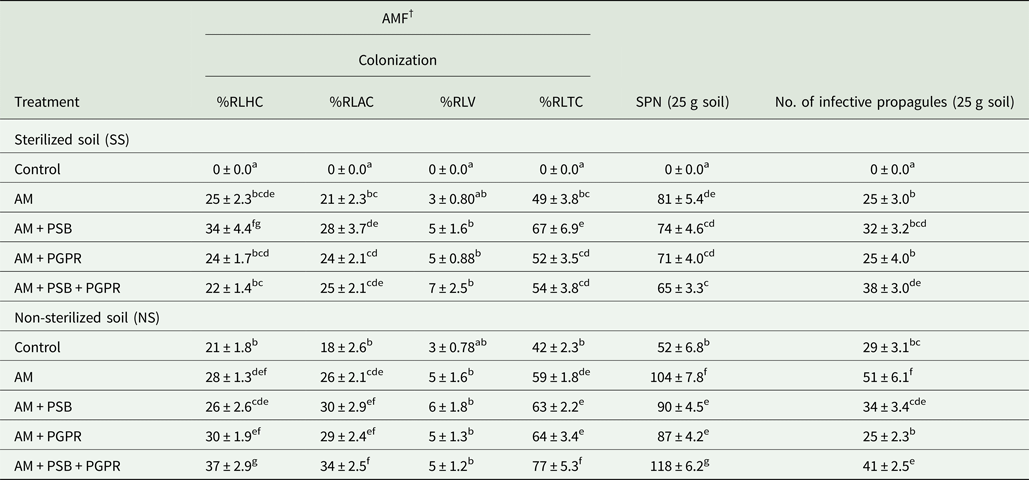
† %RLHC, %RLAC, %RLV, %RLTC and SPN indicate percentage root length with hyphal coils, arbusculate coils, vesicles, total AMF colonization and spore number of AMF, respectively.
AM, F. geosporum; PSB, P. fluorescens; PGPR, A. chroococcum.
Means ± standard error in a column followed by different letter(s) are significantly different according to Duncan's multiple range test (P < 0.05).
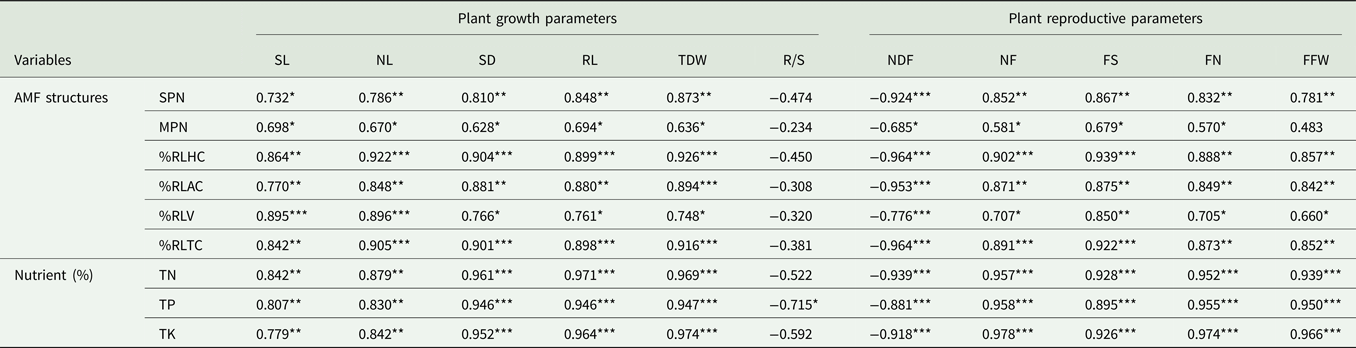
The relationship between Naga King chilli growth parameters and AMF structures
Pearson's correlation coefficient analysis revealed a significant (P < 0.05 to P < 0.001) positive correlation between chilli plant growth variables, tissue nutrient concentrations and the AMF structures (Table 9). In case of tissue P concentrations, it had a significant (P < 0.05) negative correlation with a plant R/S ratio. Similarly, the days of flowering (NDF) were significantly (P < 0.05, P < 0.001) and negatively correlated with AMF structures and the tissue nutrients. Significant (P < 0.05 to P < 0.001) and positive correlations also existed among the different yield variables of Naga King chilli and AM structures together with tissue nutrient concentrations.
Table 9. Pearson's correlation coefficients for plant growth, reproductive parameters, AMF structures and tissue nutrient concentrations (n = 10)
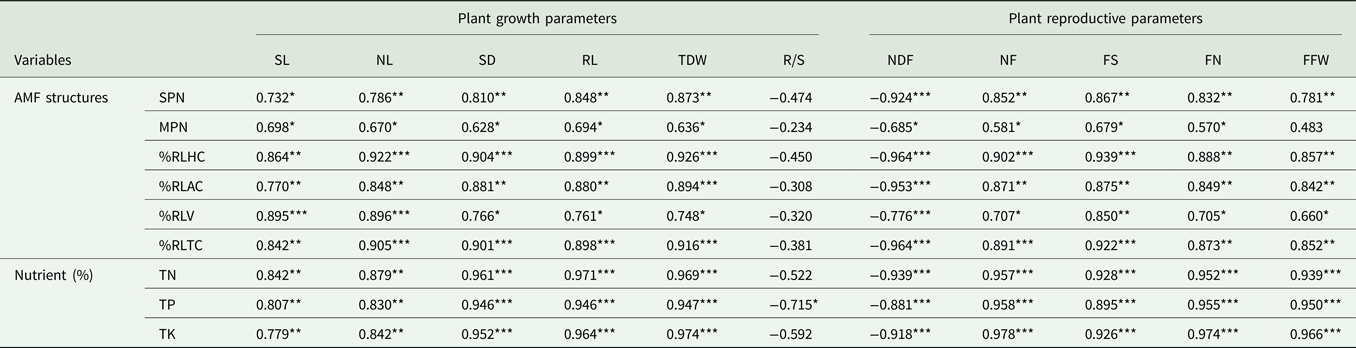
SPN, spore number; MPN, most probable number; %RL HC, root length with hyphal coils; %RLAC, root length with arbusculate coil, %RLV, root length with vesicles; %RLTC, total root length colonization; TN, total plant nitrogen; TP, total plant phosphorus and TK, total plant potassium; SL, shoot length; NL, number of leaves; SD, shoot diameter; RL, root length; TDW, total dry weight; NDF, date of flowering; NF, number of flowers; FS, fruit set; FN, fruit number; FFW, fruit fresh weight.
*, **, ***: Significant at P < 0.05, P < 0.01 and P < 0.001, respectively.
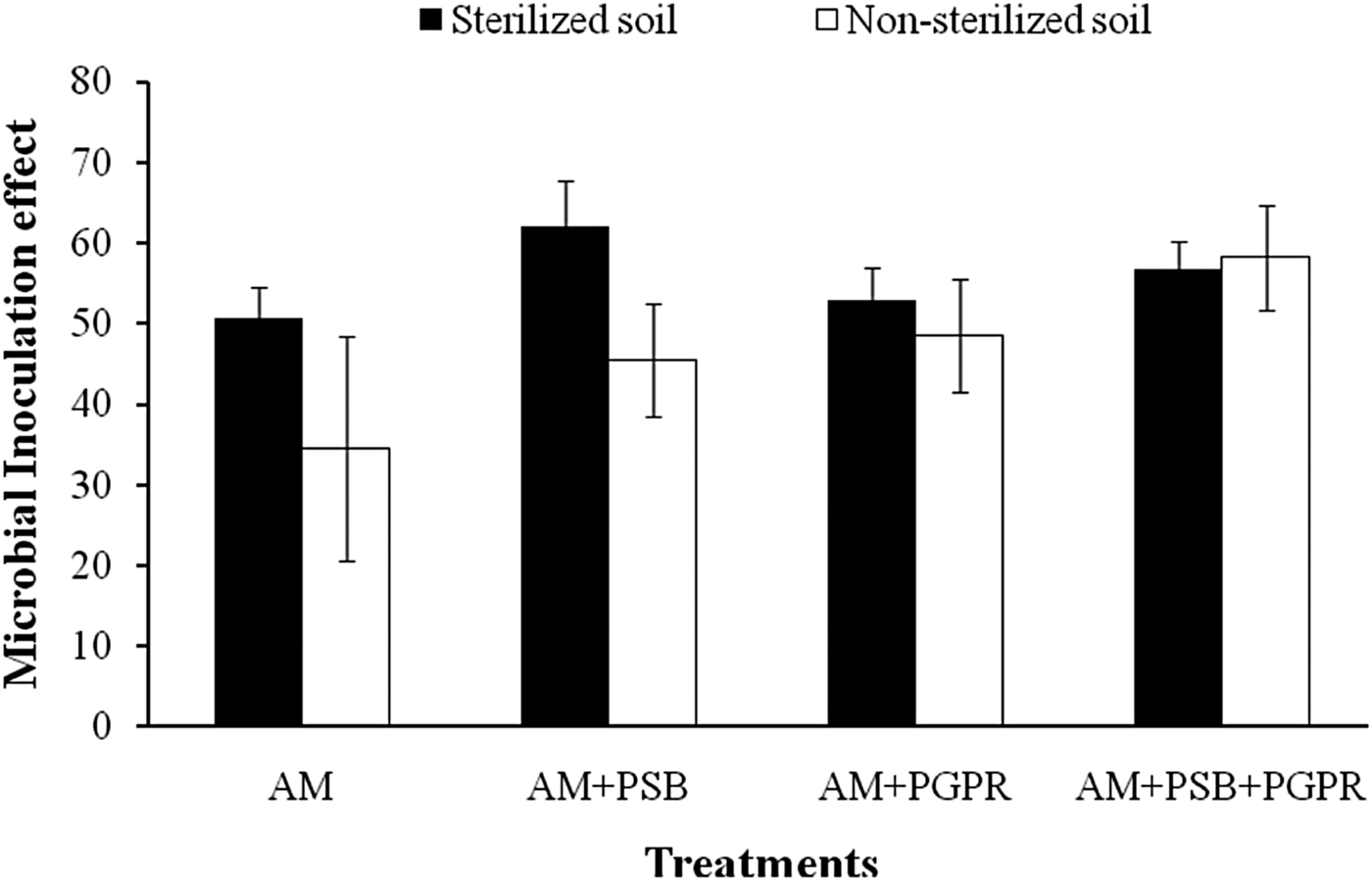
Microbial inoculation effect
MIEs of Naga King chilli under different treatments and soils did not vary significantly with each other. The higher MIE was observed in the chilli seedlings colonized by AM + PSB (62%) under SS soil conditions (Fig. 3).
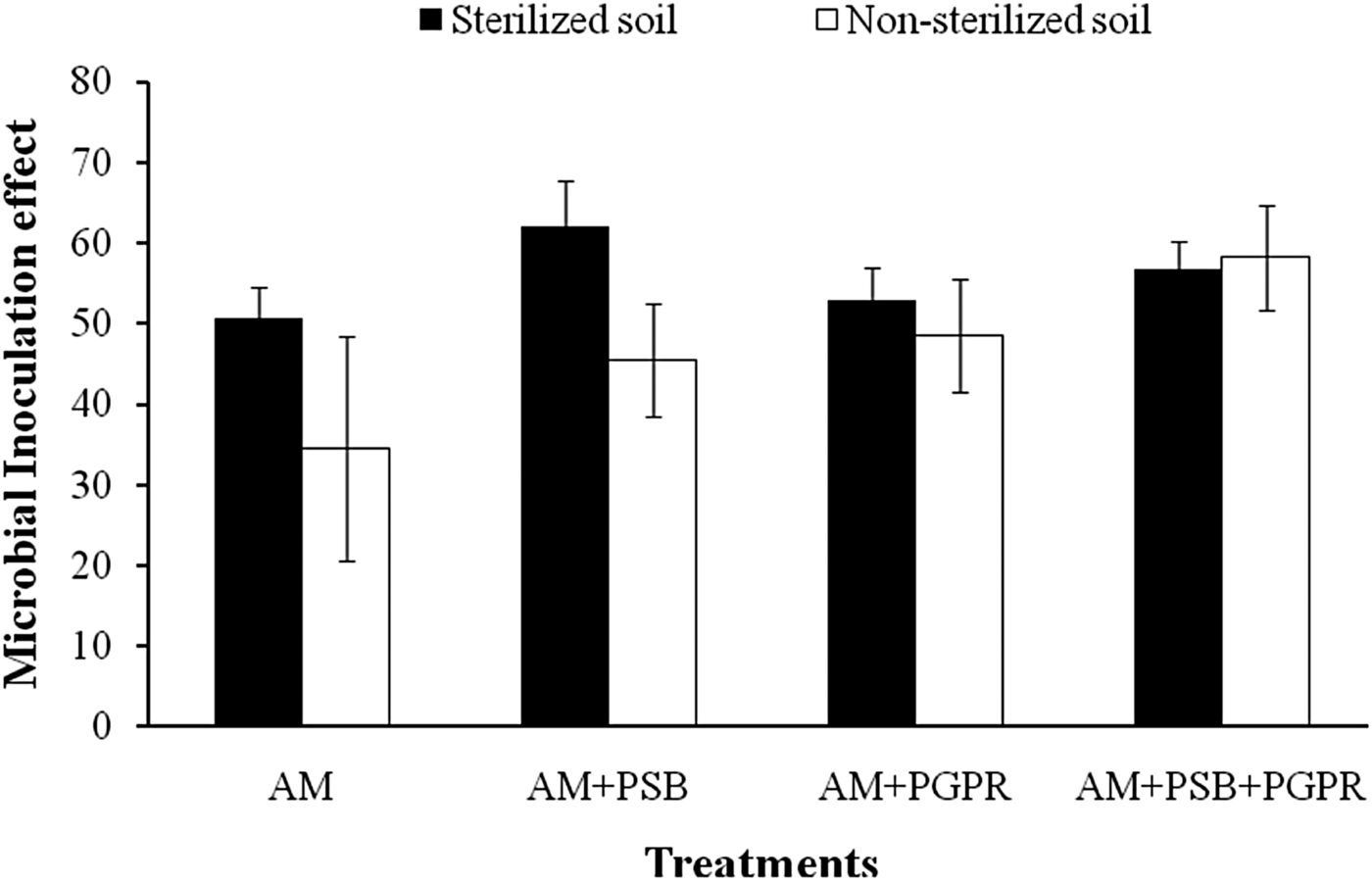
Fig. 3. MIE of C. chinense plants inoculated with native AMF, phosphate solubilizing and growth promoting rhizobacteria under pot conditions using sterilized and non-sterilized soils. Error bars indicate ± standard error.
Discussion
The current study provides the first comprehensive report on AMF species diversity in rhizosphere soil, as well as the morphology and colonization patterns of AMF in the roots of C. chinense during three different stages of crop growth in the sub-tropical habitat of NE India. Total spore density of AMF (232–455 spores per 100 g air-dried soils) recorded in the current study was much higher than those reported in C. annuum rhizosphere (16–24 spores per 100 g dried soil) by Muthukumar and Tamilselvi (Reference Muthukumar and Tamilselvi2010). In contrast, Boonlue et al. (Reference Boonlue, Surapat, Pukahuta, Suwanarit, Suwanarit and Morinaga2012) reported a range from 270 to 790 of AMF spores per 100 g dried soil of C. frutescens cultivated in organic farms of Thailand. The findings of the current study indicate that traditional shifting cultivation by slash and burn practice does not adversely affect AMF communities in the long run, as observed by Aguilar-Fernández et al. (Reference Aguilar-Fernández, Jaramillo, Varela-Fregoso and Gavito2009). However, crop rotation and tillage generally influence the composition of AMF communities as well as the spore population and mycelial density in tropical and temperate agro-ecosystems (Priyadharsini et al., Reference Priyadharsini, Pandey and Muthukumar2012), indicating that agricultural practices are important factors that affect AMF diversity (Li et al., Reference Li, Zhang and Zhao2007). Soil properties such as texture, pH and nutrient availability therein are also determining variables for the development of AMF. In the current study, soil samples collected during the pre-flowering stage of Naga King chilli revealed maximum AMF spore density followed by the fruit ripening stage. Rajeshkumar et al. (Reference Rajeshkumar, Hosagoudar and Gopakumar2013) reported that soil pH influences the status of AM spores in crop rhizosphere soil, and slightly acidic soils (pH 6.0–6.3) generally harbour a significantly greater number of AM propagules than soils with more acidic pH (5.3–5.7). Spore population of AMF was significantly and positively correlated with soil pH and %OC, which is in accordance with Singh et al. (Reference Singh, Tiwari and Dkhar2003) who reported a similar relationship between these variables from a natural forest of NE India. However, an array of environmental, host and fungal growth factors influence AMF diversity under natural field soils (Smith and Read, Reference Smith and Read2008).
Spores of 11 AMF species belonging to five genera associated with soils of C. chinense were isolated. A similar range of AM morphospecies (9–17 species) and dominance of Glomus species as that observed in the current study have recently been reported from rhizosphere of C. annuum and C. frutescens cultivated in different geographical areas with varied soil conditions of China (Chen et al., Reference Chen, Liu, Guo, Liu and M2012), Thailand (Boonlue et al., Reference Boonlue, Surapat, Pukahuta, Suwanarit, Suwanarit and Morinaga2012), India (Vyas and Vyas, Reference Vyas and Vyas2012) and Vietnam (Vo et al., Reference Vo, Magurno and Posta2015), whereas Carballar-Hernández et al. (Reference Carballar-Hernández, Hernández-Cuevas, Montaño, Larsen, Ferrera-Cerrato, Taboada-Gaytán, Montiel-González and Alarcón2017) extracted a higher number of AMF morphotypes (33 species) from C. annuum rhizosphere in Puebla State, Mexico. Such variations in the occurrence of AMF species diversity in different habitats is in agreement with reports for a variety of agroecosystems, land use types and crop production systems (Oehl et al., Reference Oehl, Laczko, Bogenrieder, Stahr, Bösch, van der Heijden and Sieverding2010; Verbruggen et al., Reference Verbruggen, van der Hijden, Weedon, Kowalchuk and Röling2012). Glomus species possess the smallest spores among AMF taxa, which enables them to disperse easily and sporulate in large numbers within a short period. The high RA and IF of F. geosporum in the shifting cultivated field soil of NE India was interesting and agreed with the findings of Zhao and Zhao (Reference Zhao and Zhao2007), Oehl et al. (Reference Oehl, Laczko, Bogenrieder, Stahr, Bösch, van der Heijden and Sieverding2010), Pandey et al. (Reference Pandey, Chongtham and Muthukumar2016) and Carballar-Hernández et al. (Reference Carballar-Hernández, Hernández-Cuevas, Montaño, Larsen, Ferrera-Cerrato, Taboada-Gaytán, Montiel-González and Alarcón2017), suggesting that a large number of spore-producing AMF species can be considered as generalists showing ability to adopt to a wide range of habitats.
In the current study, C. chinense had typical Paris-type of AM morphology. In contrast, Muthukumar and Tamilselvi (Reference Muthukumar and Tamilselvi2010) reported an intermediate- type of morphology in C. annuum. However, the ability of the AMF to form different morphologies in species of the same plant genera could be due to the influence of plant growth rates and environmental factors such as light intensity, temperature and soil moisture (Yamato and Iwasaki, Reference Yamato and Iwasaki2002). Dickson et al. (Reference Dickson, Smith and Smith2007) also indicated that AM morphology is influenced strongly by the identity of the host plant and may vary with ecosystems. The roots of Naga King chilli were colonized heavily by AMF throughout the crop growth period, which reveals the ubiquity of mycorrhizal association in tropical and sub-tropical agroecosystems. Nevertheless, the incidence of percentage root colonization, i.e. 57–75% in the current study, was lower compared with that of 90% in other Capsicum spp. reported by Muthukumar and Tamilselvi (Reference Muthukumar and Tamilselvi2010). Boonlue et al. (Reference Boonlue, Surapat, Pukahuta, Suwanarit, Suwanarit and Morinaga2012) reported the AMF root colonization ranging from 46 to 60% in C. frutescens growing in organic farms of Thailand. Such variation in AMF colonization usually occurs due to the influence of edaphic and climatic factors such as elevation, temperature, rainfall, soil nutrient level and host plant specificity in different ecosystems, which may be altered by a variety of potential mechanisms including biological characteristics of the rhizosphere under host species, mycorrhizal dependency and host plant-mediated changes in soil microenvironments, all of which are known to affect the mycorrhization patterns (Smith and Smith, Reference Smith and Read2008; Wang et al., Reference Wang, Shu, Wang, Zhang, Liu and Xia2013). At higher altitudes, extreme environments such as cool dry winter and late rainy season with heavy rainfall followed by soil erosion can also affect AMF sporulation and subsequently reduce root colonization by AMF. Differences in AMF communities and their root colonization patterns have been observed among plants, ecosystems, locations and seasons, and it seems inherent, therefore, that the coexisting AMF have distinct functional capabilities (Öpik et al., Reference Öpik, Moora, Liira and Zobel2006; Oehl et al., Reference Oehl, Laczko, Bogenrieder, Stahr, Bösch, van der Heijden and Sieverding2010). Bhattarai and Mishra (Reference Bhattarai and Mishra1984) reported that percentage AM colonization increases with the age of the plant, as found in the current study.
The current results showed that AM colonization had a negative correlation with soil pH, N, P and K which is in accordance with the finding of Li et al. (Reference Li, Zhang and Zhao2007). AMF could enhance uptake of P and other mineral elements by the plant, especially in a nutrient-deficient environment. Increasing soil P is known to suppress AMF formation, which may either be due to the direct effect of P on the external hyphal growth or an indirect effect associated with the P status of the plant (Muthukumar and Udaiyan, Reference Muthukumar and Udaiyan2002). The AMF spore population was significantly and negatively correlated with total AM root colonization. This indicates clearly that the spore density of AMF community does not reflect exactly their root colonizing ability because of the possible existence of some non-sporulating AMF species (Daniell et al., Reference Daniell, Husband, Fitter and Young2001). Furthermore, AM spore density may also be affected by the uneven spatial distribution of fungal spores and the complex structure of the underground root component.
Naga King chilli seedlings inoculated with biofertilizers revealed better growth compared with uninoculated controls under both sterilized and non-sterilized soil conditions. Okon (Reference Okon2014) also recorded a similar result in okra [Abelmoschus esculentus (L.) Moench] plants inoculated with AM fungus in sterile and non-sterile soils. Higher biomass and fruit yield as observed in bio-inoculated Naga King chilli plants corroborates the findings of earlier studies related with C. chinense and red bell pepper (Constantino et al., Reference Constantino, Gómez-Álvarez, Álvarez-Solís, Geissen, Huerta and Barba2008; Tanwar and Aggarwal, Reference Tanwar and Aggarwal2014). In the current study, inoculation with the AMF species i.e. F. geosporum alone was also found to improve chilli growth and yield significantly compared with uninoculated ones. Similar results were also observed in other studies with C. annuum (Davies et al., Reference Davies, Olalde-Portugal, Aguilera-Gómez, Alvarado, Ferrera-Cerrato and Boutton2002) and C. frutescens (Elahi et al., Reference Elahi, Mridha and Aminuzzaman2012), even when AM were applied along with P. fluorescens (PSB) and A. chroococcum (PGPR), thus exhibiting high biological specificity between AMF strain and the host plant species (Constantino et al., Reference Constantino, Gómez-Álvarez, Álvarez-Solís, Geissen, Huerta and Barba2008). Free-living soil bacteria such as P. fluorescens and Azotobacter sp. can increase microbial populations in the rhizosphere of mycorrhizal plants and are known to affect the growth of the AMF hyphae through their influence on spore germination (Barea and Jeffries, Reference Barea, Jeffries, Hock and Varma1995). The current study also confirmed a similar interaction between AM fungus and P. fluorescens or A. chroococcum. P. fluorescens was more compatible with the AM fungus compared with the consortium involving A. chroococcum in colonizing host roots. The dual inoculation with AM and P. fluorescens showed stimulatory effects on chilli plant growth and biomass compared with uninoculated control, which may be due to the acquisition of more P from the soil and its accumulation resulting in increased plant root length and shoot and root dry biomass in sterile soil (SS) treatments. However, higher shoot length was recorded in the treatments with combined inoculation of AM + PSB + PGPR, which is in accordance with the findings of Okon (Reference Okon2014). Further, the low soil P might have increased the plant's responsiveness to AM colonization.
The current findings revealed that chilli plant height was higher in SS than in NS soil. Due to the mobilization of ammonium and nitrate-like soil nutrients, in particular, autoclaved soils reduced competition between soil-borne microorganisms and the plant roots to obtain more nutrients (Ortas, Reference Ortas2003). Whereas inoculation with all three bioinoculants under NS soil conditions showed a maximum increase in all studied plant growth parameters compared with the control. These results differ from those of Okon (Reference Okon2014) and Ortas (Reference Ortas2015), who reported that plant growth response including shoot and root dry biomass decreased randomly in NS treatments compared with SS conditions. The presence of either PSB or PGPR along with mycorrhizal inoculum can synergistically improve plant growth (Gamalero et al., Reference Gamalero, Trotta, Massa, Copetta, Martinotti and Berta2004). Such improved plant growth and yield parameters could be possible due to the establishment of higher root colonization by native AMF species that increased the water and nutrient uptakes from soil and decreased the transplanting shock of chilli pepper (Castillo et al., Reference Castillo, Morales, Rubio, Barea and Borie2013).
In the current study, combined inoculation of PSB and PGPR along with AMF reduced the number of days for flowering and increased the number of flowers, fruit set and fruit yield of Naga King chilli. Indeed, fruits appeared in the uninoculated control also but were less in number and weight. Tanwar and Aggarwal (Reference Tanwar and Aggarwal2014) also found a better result in plant reproductive parameters such as increased number of flowers and fruit yields in red bell pepper (F1 hybrid, Indam Mamatha) under pot experiments. AMF might have stimulated the population of bioinoculants such as P. fluorescens and A. chroococcum in the soil rhizosphere by directly providing energy-rich carbon compounds derived from the host assimilation and transported through fungal hyphae to the mycosphere, changes in suitable pH and also by secretion of some stimulatory substances (Johansson et al., Reference Johansson, Paul and Finlay2004). Plant nutritional status of AM inoculated plants was significantly different under various treatments and soil conditions. In SS, AM + P. fluorescens inoculated plants had better shoot and root P, N and K concentrations compared with uninoculated control. This could be due to the secretion of phosphatases by AMF and P. fluorescens, which is also considered a common mode of conversion of insoluble P to soluble forms, thus enhances the nutrient uptake efficiency of plant roots from the soil and directly influences plant growth and yield (Alori et al., Reference Alori, Glick and Babalola2017). Further, solubilization of inorganic P sources by synthesis of organic acids is possible by P. fluorescens inoculation. The presence of either PSB or PGPR or both along with mycorrhizal inoculum can synergistically improve plant growth.
In conclusion, the current findings suggest that the studied C. chinense rhizosphere soil harbours a diverse AMF community relative to other tropical and sub-tropical habitats. Glomus was the dominant genus which was found during all three growth stages of Naga King chilli grown under shifting cultivated lands. Roots of Naga King chilli were found to be heavily colonized by AMF throughout the crop growth period which reveals the ubiquity of mycorrhizal association in this region of the agroecosystem. The studied chilli cultivar treated with different bioinoculants revealed enhanced growth and yields, which could be attributed to improvement in the physiological and reproductive status of plants, as well as the nutrient contents in shoots and roots under sterilized and non-sterilized soil conditions. Hence, the application of indigenous AMF along with efficient bioinoculants during seedling transplantation can be considered as potential bioagents that increase the production of chillies in NE India.
Financial support
The authors gratefully acknowledge the financial support received from the Indian Council of Agricultural Research, Government of India, Network Project on ‘Application of Microorganisms in Agriculture and Allied Sectors’ (AMAAS), to carry out this research work.
Conflict of interest
None.
Ethical standards
Not applicable.