Introduction
The sodium Zr-silicate lovozerite, discovered more than 80 years ago (Gerasimovsky, Reference Gerasimovsky1939) in the Lovozero alkaline massif, Kola Peninsula, Russia, became the forefather of the lovozerite-group minerals (LGM), which currently includes 11 species. Most of these species are also found in the Lovozero massif. The general formula of the LGM is A 3B 3C 2MSi6O12O6–x(OH)x⋅nH2O, where M = Zr, Ti, Fe3+, Ca; C = Ca, Mn, Na, □ (where □ denotes vacancy); A = Na, Ca; B = Na, □; 0 ≤ x ≤ 6; n = 0–1 (Pekov et al., Reference Pekov, Krivovichev, Zolotarev, Yakovenchuk, Armbruster and Pakhomovsky2009). The crystal structure of the LGM is based upon a heteropolyhedral zeolite-like framework consisting of rings of Si-centred tetrahedra and M-centred octahedra forming a three-dimensional system of channels that host A, B and C cations (Ilyukhin and Belov, Reference Ilyukhin and Belov1960; Chernitsova et al., Reference Chernitsova, Pudovkina, Voronkov, Kapustin and Pyatenko1975; Fischer and Tillmanns, Reference Fischer and Tillmanns1987; Tamazyan and Malinovskii, Reference Tamazyan and Malinovskii1990). The structure can also be considered as based upon pseudocubic modules centred by the midpoint of the Si6(O,OH)18 ring of tetrahedra. The M, A and B cations are located at the borders of the module, whereas the C cations are inside the module (Pekov et al., Reference Pekov, Krivovichev, Zolotarev, Yakovenchuk, Armbruster and Pakhomovsky2009).
Lovozerite-group minerals are divided into three subgroups: (1) zirsinalite–lovozerite subgroup including zirsinalite, lovozerite, combeite, kapustinite, kazakovite, litvinskite, townendite and tisinalite; (2) koashvite subgroup with the only member being koashvite and (3) imandrite subgroup with the only member, imandrite. Minerals of the zirsinalite–lovoserite subgroup are divided on the basis of the occupation of the B site into cation-saturated members (with occupied B site) and cation-deficient members (with vacant B site). It has been established that only cation-saturated lovozerite-group minerals may nucleate from melts or hydrothermal solutions, whereas all the cation-deficient lovozerite-group minerals are solely secondary phases (Semenov, Reference Semenov1972; Kapustin et al., Reference Kapustin, Bykova and Pudovkina1973, Reference Kapustin, Pudovkina and Bykova1974a, Reference Kapustin, Pudovkina, Bykova and Lyubomilova1974b, Reference Kapustin, Pudovkina and Bykova1980; Khomyakov et al., Reference Khomyakov, Semenov, Es'kova and Voronkov1974, Reference Khomyakov, Kaptsov, Shchepochkina, Rudnitskaya and Krutetskaya1978; Khomyakov, Reference Khomyakov1977, Reference Khomyakov1995; Pekov et al., Reference Pekov, Ekimenkova, Chukanov, Zadov, Yamnova and Egorov-Tismenko2000, Reference Pekov, Chukanov, Yamnova, Egorov-Tismenko and Zadov2003; Pekov, Reference Pekov2005). These form as a result of partial loss of Na from primary cation-saturated minerals in accordance with the following substitution schemes (Pekov et al., Reference Pekov, Krivovichev, Zolotarev, Yakovenchuk, Armbruster and Pakhomovsky2009):
Lovozerite, litvinskite and tisinalite have been defined as the B-vacant analogues of zirsinalite, kapustinite and kazakovite, respectively:



In the leucocratic nepheline syenites (foyaites) of the Lovozero alkaline massif, we found a new lovozerite-group mineral which has a unique combination of structural sites, not observed previously in any other mineral from this group. The crystal structure has the A site fully occupied by Na, the occupancy of the C site corresponds to 2 Na atoms per formula unit (apfu), and the B site contains a variable amount of H2O, which is higher than one molecule per formula unit in all samples studied. This new mineral species with the ideal formula Na5Zr[Si6O15(ОН)3]⋅3H2O was named zolotarevite after Dr. Andrey A. Zolotarev (b. 1982), a crystallographer from St. Petersburg State University, Russian Federation, in recognition of his remarkable contributions to the crystal chemistry and mineralogy of titano- and zirconosilicates.
In this article we present the structural, chemical and physical characteristics of zolotarevite (symbol Zlo) and discuss its probable genesis. The holotype material is deposited in the collections of the Geological and Mineralogical Museum of the Geological Institute of the Kola Science Centre of the Russian Academy of Sciences, Apatity, Russia, under catalogue number GIM 7910. This new mineral and its name were approved by the Commission on New Minerals, Classification and Nomenclature of the International Mineralogical Association (IMA2020-076, Mikhailova et al., Reference Mikhailova, Selivanova, Krivovichev, Pakhomovsky and Chukanov2021).
Occurrence
The Lovozero layered pluton (370 ±7 Ma; Kramm and Kogarko, Reference Kramm and Kogarko1994) is located in the west of the Kola Peninsula, Russia, and covers an area of 650 km2. It is the second largest alkaline massif in the world after Khibiny. The pluton consists of two main complexes (Gerasimovsky et al., Reference Gerasimovsky, Volkov, Kogarko, Polyakov, Saprykina and Balashov1966; Bussen and Sakharov, Reference Bussen and Sakharov1972): the Layered one located in the lower part and the Eudialyte one in the upper part (Fig. 1a). The Layered complex is more than 1700 metres thick and consists of regularly repeating subhorizontal layers (or rhythms). Each rhythm is a sequence of rocks ranging (from top to bottom): melanocratic nepheline syenite (lujavrite) – leucocratic nepheline syenite (foyaite) – foidolite (urtite). The Layered complex is overlapped by the Eudialyte complex, which mainly consists of lujavrite enriched in eudialyte-group minerals. Within the eudialyte-bearing lujavrite, there are layers or lenses of foyaite, less often urtite. Foyaite is very diverse in texture, including coarse-grained, fine-grained and porphyritic varieties (Mikhailova et al., Reference Mikhailova, Ivanyuk, Kalashnikov, Pakhomovsky, Bazai and Yakovenchuk2019). In particular, it hosts a large amount and variety of the lovozerite-group minerals, including a new member of the group, zolotarevite, described herein. The mineral was found in a drill core (hole 32) of foyaite from Mt. Kedykverpakhk (67°51′48″N, 34°30′16″E) at a depth of 225 metres below the present-day surface (Fig. 1b,c).

Fig. 1. (a) Geological scheme of the Lovozero alkaline massif (after Bussen and Sakharov, Reference Bussen and Sakharov1972, with simplifications); (b) location of wells in a detailed area; (c) cross-section along line 1–2 with the sample location indicated by black arrow (see Fig. 1a,b).
Appearance and physical properties
Zolotarevite was found in coarse-grained foyaite, consisting of euhedral microcline–perthite (up to 0.5 cm across), anhedral nepheline (up to 1 cm across), sodalite (up to 0.8 cm across) and elongate prismatic crystals of aegirine (Fig. 2a,b). The accessory minerals are lamprophyllite, nordite-(Ce), lueshite, umbozerite, lomonosovite, nastrophite, a mineral of the kazakovite–tisinalite series, sphalerite and löllingite.

Fig. 2. (a) Back-scattered electron (BSE) image of anhedral grains of zolotarevite (Zlt) in association with nepheline (Nph), microcline (Mc), albite (Ab), aegirine (Aeg), and a mineral of the kazakovite–tisinalite series (Lov); (b) close-up image (yellow box in Fig. 1a) showing intergrowths of zolotarevite (Zlt) and a mineral of the kazakovite–tisinalite series (Lov) (BSE-image); (c) zolotarevite grain in the foyaite (sample photo); (d) grains of zolotarevite (Zlt) and a mineral of the kazakovite–tisinalite series (Lov) in foyaite (photo of a polished thin section in transmitted light). All images are of sample LV-31/225. Symbols according to Warr (Reference Warr2021).
Zolotarevite occurs as anhedral grains (up to 1 mm across) in the interstices of microcline–perthite, nepheline and aegirine. Close intergrowths of zolotarevite and a mineral of the kazakovite–tisinalite series are commonly observed (Fig. 2b). No euhedral grains of zolotarevite have been found. Zolotarevite is transparent, cherry red in colour and appears cherry red in thin section (Fig. 2c,d). The mineral is non-fluorescent. Zolotarevite is anomalously biaxial (–), with the refractive indices α = 1.580(2), β = 1.600(2) and γ = 1.602(2) (for λ = 589 nm); 2Vmeas < 10° and 2Vcalc = 35.1°. Pleochroism and dispersion were not observed. The streak is white and lustre is vitreous. Mohs hardness is ~5. The mineral is brittle and has an uneven fracture. Cleavage and parting were not observed. The density determined by the float-sink method in Clerici solution is 2.75(5) g⋅cm–3, whereas the density calculated using the empirical formula and single-crystal unit-cell parameters is 2.85 g⋅cm–3. When the grains of zolotarevite were in the Clerici solution for more than 20 minutes, they began to slowly float to the surface. A Gladstone-Dale calculation provides a compatibility index of 0.031, which is regarded as excellent.
Chemical composition
The chemical analyses of zolotarevite was carried-out by means of an electron microprobe (WDS mode, 22 kV, 20–30 nA and 5–20 μm beam diameter). The amounts of the analysed chemical elements, in particular sodium, were measured at an accelerating voltage of 20 kV and a current of 20 nA. The elimination of mineral damage and analytical data distortion was achieved by the rastering of the electron beam to an area with a diameter of 20–30 μm and constant moving of the mineral sample under the electron probe for 10 s while simultaneously measuring the intensity of the corresponding characteristic X-ray radiation of the three chemical elements. To study the uniformity of the mineral within individual grains, a scanning electron microscope LEO-1450 equipped with a Bruker QUANTAX 200 energy dispersion system was used.
The chemical composition of holotype specimen of zolotarevite is given in Table 1. We did not have sufficient material for the direct determination of H2O, but the presence of water molecules was confirmed by Fourier-transform infrared spectroscopy (FTIR) spectroscopy, and the content of H2O was determined from structural data.
Table 1. Chemical composition of holotype specimen of zolotarevite.

* Calculated from the crystal-chemical formula: ANa2.85Mn0.15C(Na1.46Ca0.22□0.22Mn0.10)M(Zr0.72Mn0.15Ti0.08Fe0.05)B((H2O)2.67Na0.33)[Si6O15.22(OH)2.78]
The empirical formula calculated on the basis of 6 Si apfu, taking into account the charge-balance requirement is Na4.53Zr0.63Mn0.34Ti0.11Ca0.05Fe3+ 0.05Si6O14.43(ОН)3.56(H2О)2.11.
The ideal formula is Na5Zr[Si6O15(ОН)3]⋅2–3H2O, which requires (wt. %): Na2O 21.53–22.08, ZrO2 17.12–17.56, SiO2 50.09–51.37, H2O 8.98–11.26.
FTIR spectroscopy
In order to obtain the infrared (IR) absorption spectrum (Fig. 3), powdered zolotarevite grains were mixed with dried KBr, pelletised, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) in the range 360–4000 cm–1 with a resolution of 4 cm–1. A total of 16 scans were collected. The IR spectrum of an analogous pellet of pure KBr was used as a reference. IR spectra of other lovozerite-group minerals (zirsinalite and litvinskite) are given in Fig. 3 for comparison.
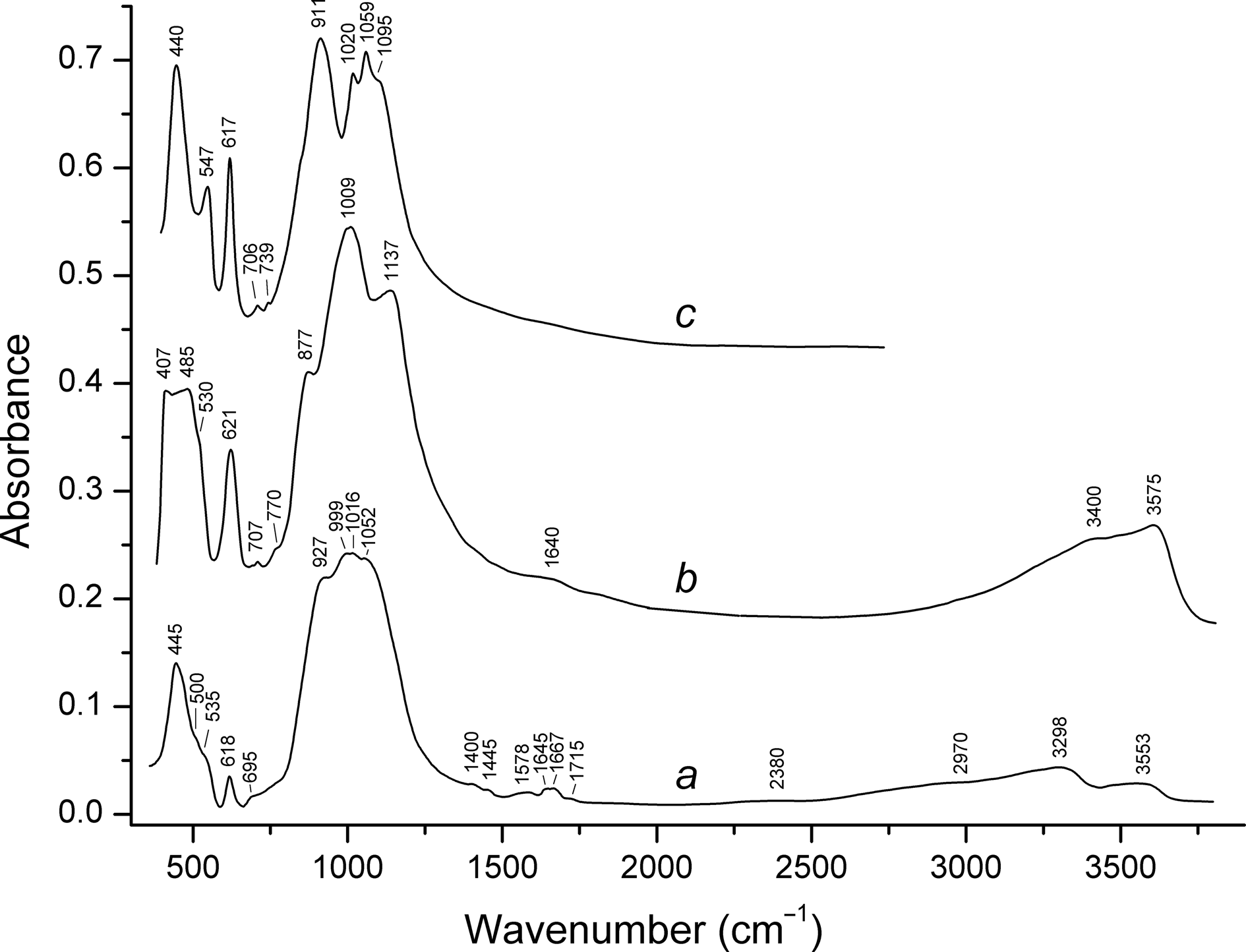
Fig. 3. Powder infrared absorption spectra of (a) zolotarevite; (b) litvinskite; and (c) zirsinalite (Chukanov, Reference Chukanov2014).
The IR spectra of hydrogen-free and hydrogen-bearing (with Si–OH groups) lovozerite-group minerals differ in the 850–1150 cm–1range of Si–O stretching vibrations. In the former, two groups of bands are observed in the regions 850–940 and 1020–1150 cm–1, whereas the strongest band of the latter is observed between 1000 and 1020 cm–1 (Chukanov, Reference Chukanov2014). The zolotarevite spectrum belongs to the latter type.
Bands in the ranges 615–625, 500–550 and 400–490 cm–1 correspond to mixed vibrations of the rings of tetrahedral (so-called ‘ring band’: Sitarza et al., Reference Sitarza, Handke, Mozgawa, Galuskin and Galuskina2000), Zr–O stretching vibrations and mixed modes involving Si–O–Si bending vibrations, respectively (Chukanov and Chervonnyi, Reference Chukanov and Chervonnyi2016). In the IR spectra of H2O-bearing lovozerite-group minerals, the band of Zr–O stretching vibrations is observed as a shoulder (at 535 cm–1 for zolotarevite, at 530 cm–1 for lovozerite and at 540 cm–1 for kapustinite) (Chukanov, Reference Chukanov2014).
Bands of vibrations involving H atoms are observed in the range 1400–3600 cm–1. In particular, bands in the range 3550–3580 cm–1 correspond to O–H stretching vibrations of silanol Si–OH groups. In the IR spectrum of zolotarevite, this band is observed at 3553 cm–1. Bands in the ranges 1570–1670 and 3280–3450 cm–1 correspond to bending and stretching vibrations of H2O molecules, respectively.
Additional weak peaks and shoulders observed in the IR spectrum of zolotarevite at 1400, 1445, 1715, 2380 and 2970 cm–1 correspond to very strong hydrogen bonds formed by acid groups (Chukanov and Chervonnyi, Reference Chukanov and Chervonnyi2016). Taking into account that silanol groups belonging to a ring of tetrahedra are not acidic, corresponding modes have been assigned to a minor admixture of hydronium groups, H3O+ (the bands at 1715, 2380 and 2970 cm–1) and H+ cation formed as a result of dissociation of H3O+ (the bands at 1400 and 1445 cm–1) (for the band assignment see Yukhnevich, Reference Yukhnevich1973; Wilkins et al., Reference Wilkins, Mateen and West1974; Kaposta, Reference Kaposta2005; Park et al., Reference Park, Shin, Singh and Kim2007; Chukanov, Reference Chukanov2014; Kulig and Agmon, Reference Kulig and Agmon2014; Chukanov and Chervonnyi, Reference Chukanov and Chervonnyi2016).
Powder X-ray diffraction
The powder X-ray diffraction pattern of zolotarevite (Table 2) was measured from a powdered microsample using a Rigaku R-AXIS RAPID II diffractometer equipped with a cylindrical image plate detector using Debye-Scherrer geometry (d = 127.4 mm; CoKa radiation). The unit-cell parameters determined from the powder pattern are: a = 10.298(4) Å, c = 13.098(8) Å, V = 1203.0(10) Å3, and Z = 3, which are in good agreement with the single-crystal data. The strongest lines of the powder X-ray diffraction [listed as d in Å (I) (hkl)] are: 7.37 (69) (101); 5.26 (56) (012); 3.686 (64) (202); 3.330 (79) (113); 3.265 (99) (211); 2.640 (100) (024); and 2.576 (60) (220).
Table 2. Powder X-ray diffraction data (d in Å) for zolotarevite*.

* The strongest lines are given in bold
Crystal structure
The single-crystal X-ray diffraction study of zolotarevite was performed at the X-ray Diffraction Resource Centre of St. Petersburg State University. The crystal of zolotarevite selected for the X-ray diffraction experiment was mounted on a Bruker Kappa APEX DUO diffractometer operated at 45 kV and 0.6 mA and equipped with a CCD area detector. The study was done by means of a monochromatic MoKα radiation (λ = 0.71073 Å), frame widths of 0.5° in ω and 10 s counting time for each frame. The intensity data were reduced and corrected for Lorentz, polarisation and background effects using the Bruker software APEX2 (Bruker, 2014). A semi-empirical absorption-correction based upon the intensities of equivalent reflections was applied (Sheldrick, Reference Sheldrick2007). The structure was solved by direct methods and refined in the space group R ${\bar 3}$![]() m to R 1 = 0.049 (wR 2 = 0.143) for 578 unique observed reflections with I ≥ 2σ(I) using the ShelX program package (Sheldrick, Reference Sheldrick2015) within the Olex2 shell (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). The basic refinement parameters are given in Table 3. The final atom coordinates, site-occupancies and displacement parameters are listed in Tables 4 and 5, while selected interatomic distances are in Table 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material.
m to R 1 = 0.049 (wR 2 = 0.143) for 578 unique observed reflections with I ≥ 2σ(I) using the ShelX program package (Sheldrick, Reference Sheldrick2015) within the Olex2 shell (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). The basic refinement parameters are given in Table 3. The final atom coordinates, site-occupancies and displacement parameters are listed in Tables 4 and 5, while selected interatomic distances are in Table 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material.
Table 3. Crystal data and structure refinement parameters for zolotarevite.

Weighting scheme: w = 1/[σ2(F o 2)+(0.0693P)2+9.9697P] where P = (F o 2+2F c 2)/3
Table 4. Atomic fractional coordinates, site-occupancies, experimental (SSFexp) and calculated (SSFcalc) site-scattering factors (e –), and equivalent displacement parameters (Å2) for zolotarevite*.

* The crystal-chemical formula of this crystal is ANa2.85Mn0.15C(Na1.46Ca0.22□0.22Mn0.10)M(Zr0.72Mn0.15Ti0.08Fe0.05)B(Na0.33)[Si6O15.22(OH)2.78]⋅2.67H2OB
Table 5. Anisotropic displacement parameters of atoms (Å2) for zolotarevite.

Table 6. Selected bond lengths (Å) in the crystal structures of zolotarevite

The crystal-chemical formula of the crystal of zolotarevite studied by single-crystal X-ray diffraction is ANa2.85Mn0.15 C(Na1.46Ca0.22□0.22Mn0.10)M(Zr0.72Mn0.15Ti0.08Fe0.05)B((H2O)2.67Na0.33)[Si6O15.22(OH)2.78]. Under the assumption of complete occupation of the A and M sites, the empirical formula can be rewritten as follows: A(Na3)B[(H2O)2.11□0.89]C(Na1.53□0.29Mn0.13Ca0.05)M(Zr0.63Mn0.21Ti0.11Fe3+0.05)[Si6O14.43(ОН)3.56].
The structure of trigonal lovozerite-group minerals has been described previously (Malinovsky et al., Reference Malinovsky, Burzlaff and Rothammel1993; Yamnova et al., Reference Yamnova, Egorov-Tismenko, Pekov and Ekimenkova2001; Zolotarev et al., Reference Zolotarev, Krivovichev, Yakovenchuk, Armbruster and Pakhomovsky2008; Chernitsova et al., Reference Chernitsova, Pudovkina, Voronkov, Kapustin and Pyatenko1975; Shevchenko et al., Reference Shevchenko, Krivovichev and Mackay2010; Krivovichev, Reference Krivovichev2015). The minerals are cyclosilicates with chair-like conformation of the Si6(O,OH)18 rings and the general formula A 3B 3C 2MSi6O12O6–x(OH)x⋅nH2O, where 0 ≤ x ≤ 6, n = 0–1; M = Zr, Ti and Fe3+, Ca; A = Na and Ca; B = Na and H2O, □; C = Ca, Mn2+, Na and □. The mean orientation and location of the rings is parallel to (001) of the hexagonal cell setting and centred on the threefold axes. MO6 octahedra share all six vertices with tetrahedra from six different rings, forming a three-dimensional framework with cavities occupied by Na+ cations and H2O molecules.
Discussion
Compared to the other members of the lovozerite group (Table 7), zolotarevite has a unique combination of structural sites. It is also unique in that it is a highly hydrated lovozerite-group mineral with the B site occupied by variable amounts of H2O. The assignment of H2O to the B site is justified by its local geometrical characteristics: the B–O distances are longer than 2.6 Å (Table 6), which is acceptable for the H2O–O contacts. The A and C sites cannot accommodate H2O molecules due to the presence of the A–O and C–O contacts of 2.2–2.3 Å. In the chemical composition of zolotarevite, the amount of H2O per formula unit exceeds 1. This is a novelty for the lovozerite group, where the number of H2O molecules apfu was never unambiguously determined to be higher than 1.
Table 7. Coefficients in the structural formulae of end members of lovozerite-group minerals (Z = 3).

In intergrowths with zolotarevite, there is a mineral belonging to the kazakovite–tisinalite series. Its grains are very heterogeneous in composition, with some areas enriched in sodium (kazakovite, analysis 1 in Table 8, point 1 in Fig. 4) and areas in which the sodium content is significantly lower (tisinalite, analysis 2 in Table 8, point 2 in Fig. 4). The sodium-depleted tisinalite is located at the edges of grains and along cracks and thus probably formed as a result of decationisation of kazakovite according to the following schemes: Na+ + O2– → □ + (OH)– and/or Na+ + O2– → H2O0 + (OH)–.
Table 8. Composition of some kazakovite–tisinalite series minerals intergrown with zolotarevite.

n.d. – not detected
Zolotarevite grains found in intergrowths with the minerals of the kazakovite–tisinalite series are homogeneous in composition and contain up to 4.67 Na pfu and up to 2.83 H2O pfu. We suggest that H2O is most probably a necessary component that defines the stability field of zolotarevite, and that the variability of the H2O content is an intrinsic feature of this mineral.
In accordance with Pekov et al. (Reference Pekov, Krivovichev, Zolotarev, Yakovenchuk, Armbruster and Pakhomovsky2009), only cation-saturated lovozerite-type compounds may nucleate from liquids (melt or solution) or fluid phases under high-temperature conditions and high chemical potential of Na. Generally, the microporous lovozerite-type framework, by analogy with other zeolite-like structures, can be formed only if its cavities are fully or almost fully occupied by alkali metal cations or H2O molecules (Belov, Reference Belov1976; Barrer, Reference Barrer1982). For the crystallisation of zolotarevite, the latter case is most likely, which indicates that the mineral is of primary origin and forms at the late magmatic/hydrothermal stage under conditions of high content of H2O. Indeed, zolotarevite, similar to other minerals of the lovozerite group, is characteristic of the highly evolved rocks of the Lovozero massif (as well as pegmatites). For alkaline melts, a gradual transition from magmatic melt to hydrothermal solution has been established (Kogarko, Reference Kogarko1977), i.e. foyaite was formed as a result of crystallisation of a water-saturated low-temperature melt–solution. At the same time, anhydrous minerals of the lovozerite group, such as kazakovite, saturated with Na, could also have formed. Subsequently, as a result of intensive autometasomatic changes in the foyaite, kazakovite was decationised with the formation of tisinalite.
Zolotarevite is related to townendite, Na8ZrSi6O18 (Grey et al., Reference Grey, Macrae, Mumme and Pring2010), and litvinskite, Na3ZrSi6O13(OH)5 (Pekov et al., Reference Pekov, Ekimenkova, Chukanov, Zadov, Yamnova and Egorov-Tismenko2000; Yamnova et al., Reference Yamnova, Egorov-Tismenko, Pekov and Ekimenkova2001), and can be formally considered an intermediate hydrated species between these two end-members. In townendite, the A, B and C sites are occupied predominantly by Na. In contrast, in litvinskite, Na is dominant at the A site only, whereas the B and C sites are predominantly vacant. The crystal structure of zolotarevite has the A site fully occupied by Na, the occupancy of the C site corresponds to 2 Na pfu, and the B site contains variable amounts of H2O.
In accordance with the nomenclature of minerals of the lovozerite group (Pekov et al., Reference Pekov, Krivovichev, Zolotarev, Yakovenchuk, Armbruster and Pakhomovsky2009), zolotarevite should be considered as a cation-deficient member of the zirsinalite–lovozerite subgroup. It should be noted that, according to this nomenclature, in lovozerite-group minerals, the amount of H2O is not considered as a species-defining criterion, similar to the nomenclature of zeolite-group minerals.
Another feature of zolotarevite is that it has a notable lack of cations typically occurring at the M site of LGM (namely, Zr + Ti + Fe3+), which allowed us to suggest the admixture of Mn2+ at this site, confirmed indirectly by the crystal-structure analysis.
Acknowledgements
The authors thank Dr I.V. Pekov for comprehensive advice, and the reviewers and editors who helped us improve the presentation of our results. Investigations were funded by the Ministry of Science and Higher Education of the Russian Federation, project no. 0226-2019-0051. The IR spectroscopic investigation was carried-out in accordance with the state task, state registration number ААAА-А19-119092390076-7.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.13