Introduction
Barley grain is a widely used animal feed in Northern Europe. It contains ∼65% starch (Waldo, Reference Waldo1973), and is extensively (up to 90%) fermented in the rumen (Larsen et al. Reference Larsen, Lund, Weisbjerg and Hvelplund2009), and the glycogenic precursor propionate is produced in abundance by starch fermentation. Rapidly degradable starch is a good energy source for rumen microbes, and it is fermented into volatile fatty acids (VFA), which are cows’ major source of energy (Huntington, Reference Huntington1997).
VFA proportion in the rumen is associated with diet composition (Murphy et al. Reference Murphy, Baldwin and Koong1982), milk yield and its composition (Thomas et al. Reference Thomas, Pearce, Oldham, Martin and Lindsay1988). Of the VFA produced in the rumen, propionate, isobutyrate and valerate, are used for glucose production in the liver and are thus precursors for gluconeogenesis (Larsen et al. Reference Larsen, Lund, Weisbjerg and Hvelplund2009). Most of the organic acids as well as the precursors for gluconeogenesis, adsorb through the rumen wall and are removed from blood by the liver to be synthesized into glucose. During lactation, over 70% of the synthesized glucose is used for milk production (Elliot, Reference Elliot1976), and is irreversibly lost to the animal with the milk. Therefore there is always requirement for new glucose, and a need for the diet to meet cows’ metabolic requirements. This is particularly important for high-producing cows, especially during the transition period, when the energy output exceeds the input. To ensure energy balance during the transition period glycerol, as a sweet liquid substance and by-product of biodiesel production (Donkin & Doane, Reference Donkin and Doane2007), has been used as a feed additive as an additional glucose precursor (DeFrain et al. Reference DeFrain, Hippen, Kalscheur and Jardon2004). According to DeFrain et al. (Reference DeFrain, Hippen, Kalscheur and Jardon2004) glycerol, fed as a part of a total mixed ration (TMR), is mainly used as an energy substrate by rumen microbes, rather than directly contributing to milk synthesis via gluconeogenesis. Accordingly there should be no direct effect of glycerol feed on milk production and composition, although Khalili et al. (Reference Khalili, Varvikko, Toivonen, Hissa and Suvitie1997) and Donkin et al. (Reference Donkin, Koser, White, Doane and Cecava2009) have stated otherwise. In addition to the amount and composition of milk produced, milk coagulation ability, the basis of cheese making, is also an important trait. Different laboratories have studied the effect of diet on milk coagulation properties (Malossini et al. Reference Malossini, Bovolenta, Piras, DallaRosa and Ventura1996; Guinee et al. Reference Guinee, Mulholland, O'Brien and Murphy2001), and differences in metabolic profiles of raw milk with different coagulation ability have also been noted (Sundekilde et al. Reference Sundekilde, Frederiksen, Clausen, Larsen and Bertram2011; Harzia et al. Reference Harzia, Kilk, Jõudu, Henno, Kärt and Soomets2012). But still, to date, there is a lack of studies describing the effects of changing the glycogenic precursors on milk coagulation properties and metabolic profile.
In this study, the effects of different amounts of reduced barley meal and fed crude glycerol on milk were studied. It was hypothesized that crude glycerol in the diet alters the profile of low molecular weight compounds in milk. The aim was to find out if a change in the glycogenic precursor alters the milk metabolic profile and technological properties (e.g. coagulation) of the milk.
Materials and methods
Cows and dietary treatment
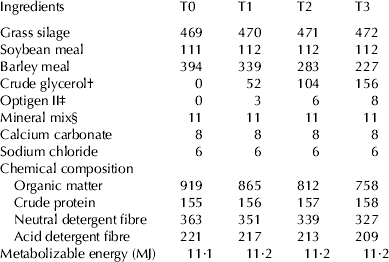
Eight primiparous Estonian Holstein dairy cows (days in milk, DIM 134±5) were used in a replicated 4 × 4 Latin square trial; one of each of the replicates was fitted with a ruminal fistula. Cows were divided into pairs according to milk yield (24·7±1·0 kg/d) and body weight (535±13·5 kg), and each treatment period lasted 21 d (16 d adaptation period and 5 d of data collection). During the experimental period the cows were fed with TMR twice a day, and milked before feeding. Based on Estonian feeding recommendations (Vabariiklik Söötmisalase Uurimistöö Koordineerimise Komisjon, Reference Oll and Tölp1995) the base ration contained grass silage, barley meal, soya meal, limestone, sodium chloride and lactating cow mineral mix (Table 1). Feed samples were analyses according to established methods (AOAC, 2005). Experimental diets consisted of the base diet, containing only barley meal and no crude glycerol (control; T0) or crude glycerol in the following amounts: 1 (T1), 2 (T2) or 3 kg (T3). Liquid crude glycerol with metabolisable energy value of 14 MJ/kg (Mach et al. Reference Mach, Bach and Devant2009), was hand-mixed to TMR. The quantity of crude glycerol increased isoenergetically to the decrease in the quantity of barley meal. Optigen II was used to replace the difference in crude protein in experimental diets. The experiment was carried out according to the Estonian Animal Protection Act at the Eerika Experimental Farm of the Estonian University of Life Sciences (Märja, Estonia).
Table 1. Feed ingredients and chemical composition of experimental diet g/kg of DM during different treatments (T0, T1, T2 and T3)
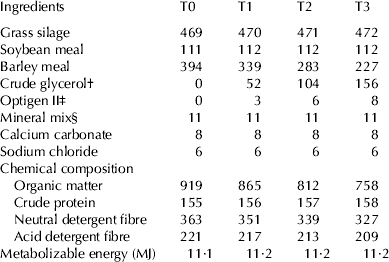
† BioOil Ltd, Estonia (82·6% glycerol, 9·3% salts, 7·1% water, 0·6 ether extract and 0·4% methanol)
‡ Alltech, USA (41·0% nitrogen and 11·4% crude fat)
§ Veskimeister Ltd, Estonia (contained CaCO3 30%, NaCI 20%, Ca(H2PO4)2 20%, Mg3(PO4)2 19·5%; Fe – 2000 mg/kg, Zn – 6000 mg/kg, Mn – 3000 mg/kg, Cu – 750 mg/kg, Se – 30 mg/kg, I – 150 mg/kg, Co – 50 mg/kg, 450 000 IU/kg of vitamin A, 100 000 IU/kg of vitamin D, and 3000 IU/kg of vitamin E)
Data collection and analysis
Rumen liquid samples were taken at the end of each treatment period (d 20 and 21) to analyse VFA composition (propionic, valeric and isobutyric acids). Acid proportion was measured using the method described by (Cottyn & Boucque, Reference Cottyn and Boucque1968), and was analysed by an Agilent 7890A gas chromatograph (Agilent Technologies Inc, USA) using 4% Carbowax 20 m, matrix 80/120 Carbopack B-DA column (Sigma-Aldrich, St. Louis, USA).
Blood samples were collected from the coccygeal vein on the 20th and 21st experimental day using 10-ml heparin Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes NJ, USA), and analysed for insulin and glucose concentrations. Plasma samples for insulin concentrations were analysed radio-immunologically (Wallac 1470 Wizard Gamma Counter; Perkin Elmer Life and Analytical Sciences, Inc., USA) using 125I radioimmunoassay test kits (Coat-A-Count Insulin, Siemens Medical Solutions Diagnostics, USA). Spectrophotometrical analysis (Helios β; Unicam Ltd., PO Box 206, York St., Cambridge, CB1 2ST, UK) of plasma glucose concentrations with Randox reagents (Ranbut, Randox Laboratories Ltd, United Kingdom) was performed.
Milk yield was recorded on the last 5 d of the experimental period, and samples were collected at the end of each treatment period (d 20 and 21). Milk samples were stabilised with bronopol (Broad Spectrum Microtabs, D & F Control Systems Inc., Norwood, USA). Concentrations of milk fat, protein, urea and somatic cell count (SCC) were measured in each milk sample using an automated infrared milk analyser (System 4000, Foss Electric, Hillerød, Denmark). Milk pH was measured using a pH-meter (MP 220; Mettler Toledo GmbH, Greifensee, Switzerland) before the coagulation ability was analysed, at 20 °C. Curd firmness (E30) and coagulation time (RCT) were obtained by Optigraph (Alliance Instruments, France) using the method described previously by Kübarsepp et al. (Reference Kübarsepp, Henno, Kärt and Tupasela2005).
The milk samples for mass spectrometry analyses were prepared and analysed as previously described by Harzia et al. (Reference Harzia, Kilk, Jõudu, Henno, Kärt and Soomets2012). The milk samples for mass spectrometry analyses were prepared and analysed as previously described by Harzia et al. (Reference Harzia, Kilk, Jõudu, Henno, Kärt and Soomets2012). For preliminary citric-acid cycle compound quantification HILIC (Luna 5 μm HILIC 200A, 150 × 3·00 mm, Phenomenox, Torrance, USA) column and standard solutions were used. The gradient for retention time studies was as follows: 5 min isocratic at 95% ammoniumformate in methanol, gradual decline to 5% ammoniumformate in methanol within 15 min, 5 min at 5% acetonitrile in water. Negative ions formed in the TurboIonSpray source were scanned in the Multiple Reaction Monitoring mode (MRM). The scan rate was 1000 amu/s. Curtain and nebulizer gas had settings at 10 and 5, respectively. Ionization was performed at a temperature of 200 °C. The Ion Spray voltage was set at 4500 V. The entrance and declustering potential, and the collision energy, were set at 20, 10 and 2 V, respectively.
Statistical analysis
The statistical significance of treatment effect was tested following the model y ijkl=μ+D i+P j+C k+e ijkl, where y ijkl is the dependent variable, μ is the model intercept, D i is the diet effect (i = 1,…,4), P j is the period effect (j = 1,…,4), C k is the random cow effect (k = 1,…,8), and e ijkl is the model error. Single treatments were compared according to their least square means. To study the associations between different VFA, blood and milk metabolites, and milk metabolic profile, Spearman partial correlation coefficients, adjusted for the period effect, were estimated. All statistical analyses were performed with SAS software (version 9.1; SAS Institute Inc., Cary, NC, USA).
Results and discussion
Effect of glucogenic precursors on rumen VFA and milk composition
A change in the proportions of glycogenic VFAs was observed when starch supplementation was partially replaced with crude glycerol (Fig. 1). The proportion of propionic acid produced changed as an effect of treatment, being significantly different from the control at T2 and T3 (P = 0·03, and P < 0·001, respectively). A strong positive correlation was observed between propionic acid and glycerol addition (r = 0·71, P=0·0002), and the change in the valeric acid proportion was significantly different between the control and T3 (P = 0·03).

Fig. 1. Least square means (± standard error) of isobutyric (○), valeric (●) and propionic (Δ) acid depending on the diet. abc Least square means with different letters are statistically significantly different (P < 0·05).
As stated previously, the proportion of propionic acid increased during this study. According to Huhtanen et al. (Reference Huhtanen, Miettinen and Ylinen1993) the milk yield is limited by propionate. This was not the case in the current study. Huhtanen et al. (Reference Huhtanen, Miettinen and Ylinen1993) also noted that the use of propionate for gluconeogenesis in the liver may be inhibited by butyrate, leading to decreased glucose production, and increasing the utilization of amino acids for gluconeogenesis, thereby reducing the availability of amino acids (AA) to the mammary gland. In the current study the proportion of butyrate produced changed (P < 0·001) in the same direction as propionic acid, indicating that the equilibrium between these two acids remained during the whole treatment diet, and change in AA metabolism should not have been affected. According to Ørskov (Reference Ørskov1986) there can be problems utilizing a large volume of propionate; as the concentration of propionate increases in blood, insulin production also increases. When insulin production is stimulated, the uptake of glucose in different tissues is increased and lipolysis is reduced, and milk production and milk fat is decreased (Ørskov, Reference Ørskov1986; Hayirli, Reference Hayirli2006). The change in the proportion of propionate produced indicated that glucose availability, through gluconeogenesis in liver and in blood, should have increased. Contrary to Wang et al. (Reference Wang, Liu, Yang, Huo, Dong, Huang, Yang and He2009), who observed an increase in plasma glucose when feeding glycerol, in this study there was no significant increase in either glucose (from 85·97 to 88·47 mg/dl, P = 0·5) or insulin concentrations (from 14·13 to 14·71 μIU/ml, P = 0·8). The changes in blood metabolites were not significant nor had a definite trend curve with increasing glycerol in the diet. But a strong negative correlation between insulin level and milk yield was observed (r = −0·40, P = 0·003). The change in insulin concentration was probably too small to have an affect on either liver or peripheral tissues, as there was no change in BW, this being consistent with results reported by Boyd et al. (Reference Boyd, West and Bernard2011).
Overall, variations between different milk traits were not significantly different, except for protein concentration between all treatments (P < 0·001), and for pH between the control and T3 (P = 0·01). The increase in milk protein concentration may have been due to a faster outflow of microbial protein from the rumen (Pathak, Reference Pathak2008), thus increasing amino acid production, which may be converted into milk protein. A significant change in lactose concentration was observed between the control and T1 (P = 0·03), and also between T1 and T3 (P = 0·02). Circulating glucose, which is taken up by the udder, is used to synthesize lactose, and to provide enough energy for fat synthesis (Rigout et al. Reference Rigout, Hurtaud, Lemosquet, Bach and Rulquin2003). The blood glucose level had weak correlation with milk lactose concentration (r = 0·18, P = 0·19), and was negatively correlated with milk fat (r = −0·36, P = 0·01). A negative correlation was also observed between milk lactose and fat concentrations (r = −0·34, P = 0·01), indicating that the glucose taken up by the udder ensured a sustainable level for lactose synthesis, as fat synthesis depends on glucose availability and lactose synthesis.
Effect of glycogenic precursors on milk coagulation properties and metabolites
Least square mean values of milk yield, somatic cell score (SCS; SCS=ln (SCC)), fat, urea, lactose and protein concentration, pH, RCT and E30, of all four rations are shown in Table 2.
Table 2. Least square means of concentrations of different milk traits and milk metabolites with different treatments (T0, T1, T2, and T3). Values are least square means for n = 64. Standard errors of mean of all least square mean values are given as sem

a, b, c Means within a row with different superscripts are statistically different (P < 0·05)
† SCS – ln(somatic cell count). E30 – curd firmness after 30 min. RCT – coagulation time
Mean milk coagulation ability, measured as E30, improved linearly as the barley concentration decreased and glycerol supplementation increased (P < 0·001). According to Jõudu et al. (Reference Jõudu, Henno, Kaart, Püssa and Kärt2008) protein concentration has a positive effect on milk coagulation ability. In the current study there was a strong positive correlation between curd firmness and protein concentration (r = 0·58, P < 0·001).
There was a negative correlation between isobutyric acid and curd firmness (r = −0·56, P = 0·005), and a trend for a positive correlation between propionic acid and curd firmness (r = 0·38 and P = 0·07), but none for valeric acid (r = 0·32 and P = 0·13). These findings indicate that a change in rumen VFA proportion is related to milk coagulation ability, which may be a result of a change in the microbial population during the period of the experimental diet.
The hypothesis of this study was that the change of glycogenic precursor alters the milk metabolic profile. Therefore mass spectrometry analysis and identification of low molecular weight milk compounds was carried out to gain more knowledge about reasons behind the change in coagulation ability. A change in the metabolic profile for milk samples with different curd firmness was observed with treatment. In the positive ion mode there were 20 statistically different signals representing differences between coagulation ability, and 17 of these were negatively correlated with coagulation. In addition, mass to charge ratios (m/z) describing relationships between coagulation ability and change in the glycogenic precursor in the diet were investigated (Fig. 2). Signals located in the upper right corners of Fig. 2A, B were positively correlated with both glycerol addition and curd firmness, indicating that the higher amount of crude glycerol corresponded to the stronger mass spectrometry signals of these masses, which is further reflected in the higher values for curd firmness. From the recorded signals, only one was significantly different for both variables. Signals m/z = 293 represented good coagulation and glycerol addition to the diet (Fig. 2A). Fragment analysis was conducted on this signal, and a database search resulted in the finding that it had fragments identical to histidine, His–His and lysine as the [2M-H]+ ion. The abundance of both of these amino acids in the well coagulating milk samples is explained by increased protein concentrations. Histidine, and lysine, and also methionine are the limiting amino acids for milk protein production (Kim et al. Reference Kim, Choung and Chamberlain2001).

Fig. 2. Spearman partial correlations (adjusted for period effect) of masses measured in positive ion mode (A) and negative ion mode (B) with glycerol addition and curd firmness. Larger labelled dots correspond to correlations with P < 0·01 in a horizontal or a vertical direction or indicate masses significantly (P < 0·05) correlated with both glycerol addition and curd firmness. Dashed lines denote the cut-off for statistical significance of correlation coefficients (P = 0·05).
In negative ion mode the number of statistically different (P < 0·05) signals was higher (53), and five of these represented both coagulation and change in glycogenic precursor in the diet (Fig. 2B). These five correlations corresponded to the signals m/z = 218, 422, 421, 333 and 292, and fragmentation analyses was also carried out. No defined matches were found for the signals m/z = 421, 422, and 333. Although the fragment spectra of signal m/z = 421 had fragments common to cortisol, such as the [M-H]− ion, and signal m/z = 333 had a fragment common to decanoic acid [M-H]−. Signal m/z = 292 had fragment spectra matching glycerophosphocholine. According to Klein et al. (Reference Klein, Buttchereit, Miemczyk, Immervoll, Louis, Wiedemann, Junge, Thaller, Oefner and Gronwald2012) the ratio of glycerophosphocholine to phosphocholine is a good biomarker for ketosis, where high values of glycerophosphocholine indicate a low risk of ketosis. Fragment spectra of signal m/z = 218 indicated pantothenic acid, the [M-H]− ion. Ruminants either obtain their pantothenic acid from feed, or it is synthesized by the rumen microbes (Bechdel et al. Reference Bechdel, Honeywell, Dutcher and Knutsen1928; Bender, Reference Bender and Bender2009). The occurrence of free pantothenic acid in milk is rare; usually it is bound to Coenzyme A, and therefore involved in energy metabolism in cells (Bender, Reference Bender and Bender2009). According to previous studies (Lardinois et al. Reference Lardinois, Mills, Elvehjem and Hart1944; Hollis et al. Reference Hollis, Chappel, MacVicar and Whitehair1954; Hayes et al. Reference Hayes, Mitchell and Little1967) the synthesis of pantothenic acid depends on the diet, and increases as the supplementation of rapidly degradable carbohydrates is increased. Although Bonomi (Reference Bonomi2000) observed that pantothenic acid has no effect on milk coagulation, it was found in the current study that it was positively correlated with E30, and also with a change in the glycogenic precursor in the diet.
To analyse impacts of changes in the diet on the milk energy profile, milk organic acid composition was analysed, and changes in citric-acid cycle components’ (e.g. citrate, pyruvate, cis-aconitate, α-ketoglutarate, malonate, and oxaloacetate; Table 2) concentrations between treatment periods were measured. Of the organic acids in milk, oxaloacetic acid was positively correlated with curd firmness (r = 0·33, P = 0·013) while there was a trend for malonic acid and curd firmness (r = 0·25, P = 0·06). No correlations were observed between citrate and cis-aconitate with curd firmness (r = −0·01, P = 0·94, and r = 0·02, P = 0·88, respectively). No correlations were found between curd firmness and pyruvic, and α-ketoglutaric acid (r = 0·13, P = 0·34, and r = 0·11, P = 0·42, respectively).
As the main buffer system of milk, citrate is a common milk component affecting milk-processing quality (e.g. coagulation) by interacting with other milk constituents (Rosenthal, Reference Rosenthal1991), as the citrate ions can improve calcium and phosphate ions binding to casein micelles (Visser et al. Reference Visser, Schaier and van Gorkom1979). As an intermediate in the citric-acid cycle, citric acid takes a role in cellular energy metabolism, and therefore it has been reported that citric acid is an indicator of energy status in the cow (Baticz et al. Reference Baticz, Tomoskozi, Vida and Gaal2002). Linzell et al. (Reference Linzell, Mepham and Peaker1976) indicated that mammary epithelium is impermeable to citrate, it is formed in mammary secretory cells and its abundance in milk depends on season (Holt & Muir, Reference Holt and Muir1979; Mitchel, Reference Mitchel1979; Keogh et al. Reference Keogh, Kelly, O'Keeffe and Phelan1982), lactation (Garnsworthy et al. Reference Garnsworthy, Masson, Lock and Mottram2006), and can be altered by feeding (Faulkner & Peaker, Reference Faulkner and Peaker1982). However the data in the literature about the effect of feeding are inconsistent. As mentioned by Gransworthy et al. (Reference Garnsworthy, Masson, Lock and Mottram2006), bovine milk citrate concentration is not affected by either milk yield or diet. In the current study the concentrations of milk citric-acid cycle components changed insignificantly with treatment.
In conclusion, this study has shown that a change in the glycogenic precursor in the diet can alter the milk metabolic profile, and may improve its coagulation ability through altered proportions of rumen VFA, milk protein and concentrations of energy metabolites (e.g. citric-acid cycle components, pantothenic acid) in milk. As was hypothesized, the crude glycerol in the diet alters the profile of low molecular weight compounds, and technological properties (e.g. coagulation) of the milk. Therefore using glycerol as a dietary source for glucogenesis appears to be of potential value in the feeding of the dairy cow.
This research was carried out by the Bio-Competence Centre of Healthy Dairy Products and was co-financed by the European Community's Regional Development Fund within the framework of the Competence Centre Programme of the Enterprise Estonia, project no. EU30002, targeted finance project SF0170165s08, and Estonian Science Foundation grants 7494 and 7856.