Phenotypic features compared to the normal heart
From the stance of the morphology of the atrioventricular junctions, patients having a common junction differ totally from the majority of patients with either normal or congenitally malformed hearts, who exhibit separate right and left atrioventricular junctions. As is well shown in the four-chamber planes, the division between the right and left junctions, and hence the phenotypic feature of normality, is produced by the atrioventricular separating structures. In the past, we described the postero-inferior area of this separation as a muscular septum.4 We now know that, rather than representing a true septum, the area is a sandwich, made up of a layer of atrial myocardium, which overlaps the base of the ventricular mass, the two muscular layers being separated by a superior continuation of the inferior atrioventricular groove (Fig. 1).

Figure 1. This image obtained in the so-called “four chamber” plane using three-dimensional echocardiography shows well the separate atrioventricular junctions, and the overlapping of the atrial and ventricular walls in the area of the atrioventricular muscular sandwich. Abbreviations: RA: right atrium; LA: left atrium; RV: right ventricle; LV: left ventricle.
Antero-superior to this area of muscular separation, there is a true atrioventricular septal structure, but one that is made of fibrous tissue (Fig. 2).

Figure 2. This section, taken through a normal human heart in simulated “four chamber” plane, shows how the fibrous membranous septum is crossed by the hinge of the septal leaflet of the tricuspid valve, separating it into atrioventricular (white arrow), and interventricular (black and white arrow) components.
This is the atrioventricular component of the membranous septum, which interposes between the subaortic outflow tract and the right atrium by virtue of the fact that, in the normal heart, the outflow tract of the left ventricle is itself interposed between the mitral valvar orifice and the muscular ventricular septum. This relationship is well demonstrated in the short axis sections of the ventricular mass (Fig. 3). The overlap of the inlet and outlet components of the normal left ventricle is also well seen in the parasternal long axis sections, with these sections showing that, in the normal situation, the inlet and outlet dimensions of the ventricular mass are the same (Fig. 4).

Figure 3. This image, produced using three-dimensional echocardiography, is in the short axis of the left ventricle. It shows how the inlet and outlet (LVO) overlap, separated by the aortic leaflet of the mitral valve (AML). Note the location of the membranous part of the septum (M). Abbreviation: RV: right ventricle.

Figure 4. This image, again produced using three-dimensional echocardiography shows how, in the normal heart with separate atrioventricular junctions, the inlet (LVI) and outlet (LVO) dimensions of the left ventricle are virtually identical. Abbreviation: LA: left atrium.
All of these relationships are changed in the setting of a common atrioventricular junction. The atrioventricular separating structures are totally deficient, a feature well demonstrated in the four-chamber plane (Fig. 5). Because of the common junction, and in the absence of the separating structures, it is no longer possible for the left ventricular outflow tract to interpose between the left atrioventricular valve and the septum, this feature being seen best in the short axis plane (Fig. 6).

Figure 5. This image, produced using three-dimensional echocardiography in a patient with deficient atrioventricular septation, shows the common atrioventricular junction which is the hallmark of the malformation. In this example, the bridging leaflets of the common valve float within the atrioventricular septal defect, so that there is the potential for shunting at both atrial (ASD) and ventricular (VSD) levels.

Figure 6. This three-dimensional image, from a patient with deficient atrioventricular septation, is viewed from the apex looking towards the ventricular base, with the right side of the heart (R) to the left side of the panel. Note that the aorta (Ao) is no longer interposed between the atrioventricular valve and the septum, which is bridged by the superior (S) and inferior (I) bridging leaflets of the common atrioventricular valve (See Figure 8). Abbreviation: L: left.
The sections replicating the parasternal long axis plane then show how the unwedged aortic valve, in presence of the common atrioventricular junction, is lifted away from the ventricular base, with concomitant disproportion between the inlet and outlet ventricular dimensions, the outlet dimension being significantly longer than the inlet (Fig. 7).

Figure 7. In this three-dimensional image, it is possible to see the left ventricular aspect of the scooped-out ventricular septum, with the bridging leaflets of the common atrioventricular valve (CAVV) dividing the atrioventricular septal defect into atrial (ASD) and ventricular (VSD) components. Note the disproportion between the inlet (LVI) and outlet (LVO) dimensions of the ventricular mass (Compare with Figure 4).
When making the initial diagnosis of patients with common atrioventricular junction, it should be appreciated that the commonality of the junction, and the degree of disproportion between the inlet and outlet ventricular dimensions, is just as great in the so-called “partial” variants of the lesion as in the presumed “complete” forms (Fig. 8). Indeed, when the leaflets guarding the common atrioventricular junctions are stripped away from the base of the ventricular mass, there is no way, in an individual heart, of determining whether the patient initially possessed a so-called “partial” or “complete” variant.4 It is the relationships between the leaflets which guard the common atrioventricular junction to each other that determine the number of orifices within the valve, whilst it is the relationships between the leaflets and the septal structures which determines the options for shunting between the right and left sides of the heart. Fully to understand these variations, it is necessary to understand the fundamental difference in structure of the common valve itself when compared to the tricuspid and mitral valves, which guard the separate right and left atrioventricular junctions.

Figure 8. The graph shows the lengths of the inlet dimension of the ventricular mass presented as a proportion of the outlet dimension in a large series of hearts from Pittsburgh Children's Hospital.7 Those with exclusively atrial shunting are shown in dark blue, whilst those with the potential for atrial and ventricular shunting are shown in pale blue. There is no difference between the two groups.
Common atrioventricular valves versus mitral and tricuspid valves
The normal mitral valve possesses two leaflets, located antero-superioly and postero-inferiorly when viewed in the short axis, which close along a solitary zone of apposition (Fig. 3). The postero-inferior leaflet, also well described as the mural leaflets, guards two-thirds of the circumference of the normal left atrioventricular junction. The normal tricuspid valve, as its name suggests, possesses three leaflets, positioned septally, inferior or morally, and antero-superiorly, with the leaflets closing in trifoliate fashion (Fig. 3). The common atrioventricular valve, in contrast, possesses five leaflets. Two of these leaflets are positioned exclusively in the right ventricle, and correspond to the antero-superior and inferior leaflets of the tricuspid valve, albeit that the dimensions of the antero-superior leaflet can vary markedly in different variants of atrioventricular septal defect with common atrioventricular junction.6 One of the leaflets of the common valve is positioned exclusively within the left ventricle, and is located murally. Unlike the mural leaflet of the mitral valve, however, the leaflet guarding the mural component of the left half of the common atrioventricular junction occupies no more than one-third of this left half of the junction.7 Delineation of the mural leaflet by the echocardiographer prior to surgical intervention is of particular import, as deficiency of the mural leaflet predisposes valvar regurgitation post-operatively.8 The remaining two leaflets of the common valve have no counterpart in patients having separate right and left atrioventricular junctions. These are the leaflets that bridge the crest of the ventricular septum, and which are located superiorly and inferiorly within the common junction (Fig. 9).

Figure 9. The cartoon shows the arrangement of the leaflets of the common atrioventricular valve as seen from the apex looking towards the ventricular base. The dotted lines show the location of the ventricular septum, and the arrow shows the zone of apposition between the two leaflets which bridge the ventricular septum.
Because of the presence of the bridging leaflets, the left half of the common atrioventricular valve opens and closes in trifoliate fashion, with a zone of apposition between the bridging leaflets themselves, and with further zones of apposition between both of the bridging leaflets and the left mural leaflet. There is, therefore, a fundamental difference in the pattern of closure between the left component of a common atrioventricular valve, and the two leaflets of the mitral valve which guards a separate left atrioventricular junction. Because of this, irrespective of any surgical manoeuvre, it is impossible to convert surgically the left component of a common valve into a mitral valve. It is also incorrect to describe the left component of the common valve as a mitral valve with a cleft in its anterior leaflet.
“Ostium primum” defect versus the “complete” defect
In the past, distinguished investigators have shown the so-called “partial” defect with “pinched-in” atrioventricular junctional morphology, intermediate between that seen with a presumed “complete” defect and the normal heart.9 This is incorrect. The junction is just as common in the patients with exclusively atrial shunting as in those with shunting at both atrial and ventricular levels. The difference between the two variants within the group is that, in the majority of patients with shunting confined exclusively at atrial level, there is a bridge of valvar tissue joining together the facing surfaces of the superior and inferior bridging leaflets, whereas these two leaflets are separate and discrete structures in the majority of patients with the potential for shunting at both atrial and ventricular levels. Those with shunting confined at atrial level, for the most part, have dual orifices within the common atrioventricular junction, with the two orifices being committed to the right and left ventricles, respectively (Fig. 10). Indeed, the concept of bridging tongues joining together the leaflets can then be extended to account for two or more orifices within the right or left components of the common valve, such dual orifices being most frequent in the left component of the valve10 (Fig. 10), but also existing on occasion within the right component.11

Figure 10. The cartoon shows the arrangement of the leaflets in the setting of the so-called “ostium primum” defect. A tongue of valvar tissue joins together the apposing surfaces of the bridging leaflets, producing separate valvar orifices for the right and left ventricles, but within the common atrioventricular junction. The so-called cleft, shown by the yellow arrow, is the zone of apposition of the left ventricular components of the bridging leaflets.
Shunting across the atrioventricular septal defect
When patients possessing a common atrioventricular junction are imaged in four chamber or parasternal long axis planes, then it can be seen that the atrioventricular septal defect is the space between the leading edge of the atrial septum and the scooped-out crest of the muscular ventricular septum (Figs. 5 and 6). It follows that, if the bridging leaflets of the common atrioventricular valve are bound down firmly to the crest of the ventricular septum, then shunting will be possible only at atrial level, albeit that much of this shunting will take place below the level of the atrioventricular junction (Figs. 11 and 12).

Figure 11. The cartoon shows how the potential for shunting is dependent on the relationship between the septal structures and the leaflets of the common atrioventricular valve that bridge the ventricular septum.

Figure 12. The cross-sectional echocardiogram, with colour Doppler imaging, shows the shunting through the atrioventricular septal defect exclusively at atrial level when the bridging leaflets are firmly bound to the crest of the ventricular septum. This is the so-called “ostium primum” defect.
Binding of the leaflets to the ventricular septal crest is seen most frequently when the bridging leaflets are also joined to each other by a connecting tongue of leaflet tissue, but this is not always the case. Sometimes, the bridging leaflets, and the connecting tongue, have fenestrations on their undersurface. This means that there is the potential for limited shunting at ventricular level, in addition to the major shunting which takes place at atrial level. On rare occasions, the leaflets and their connecting tongue can float freely within the atrioventricular septal defect. Much more frequently, however, the leaflets float to permit both atrial and ventricular shunting when they are also discrete and separate one from the other, in other words when there is a common atrioventricular orifice (Figs. 11 and 13).

Figure 13. This cross-sectional echocardiogram, in four chamber section with colour flow Doppler, shows how there is the potential for shunting at both atrial (white arrow) and ventricular (dotted white arrow) levels when the leaflets float within the atrioventricular septal defect. In this instance, the ventricular shunting is restrictive.
On occasions, however, the leaflets can be firmly attached to the undersurface of the atrial septum. In this situation, shunting will be possible only at ventricular level, producing a ventricular septal defect of “atrioventricular canal type”. In such settings, the left atrioventricular valve will close in trifoliate fashion. Patients with such defects should not be confused with those having perimembranous defects opening primarily to the inlet of the right ventricle. On very rare occasions, the bridging leaflets may be attached to both atrial and ventricular septal structures, such that there is no potential for shunting across the atrioventricular septal defect (Fig. 14). These patients, without the potential for shunting, will likely be recognized because of the trifoliate arrangement of their left atrioventricular valves (Fig. 15).12, 13
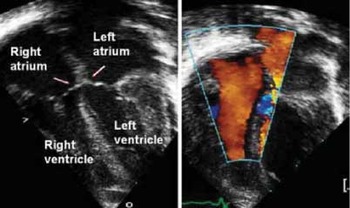
Figure 14. This cross-sectional echocardiogram, in four chamber section (left panel) shows a patient with a common atrioventricular junction, the bridging leaflets being attached to the leading edge of the ventricular septum (arrows). The colour flow Doppler signals (right hand panel) show there is no shunting at ventricular level, due to closure of the ventricular component by valvar tissue.

Figure 15. This cross-sectional echocardiogram, in short axis across the left ventricle, shows that the left atrioventricular valve from the patient shown in Figure 14 closes in trifoliate fashion, with an obvious zone of apposition (arrow) between the left ventricular components of the bridging leaflets of the valve guarding the common atrioventricular junction.
Are there “intermediate” or “transitional” variants?
We should reiterate at this point that, in terms of junctional morphology, there is no basic difference between the so-called “partial” and “complete” variants within the overall group. In terms of the arrangement of the junction, therefore, there will be no intermediate variants. Those who chose to describe such variants in the past based their categorisation either because patients with separate valvar orifices for the right and left ventricles also possessed the potential for shunting at ventricular level,14 or else because those with the bridging leaflets bound down firmly to the crest of the ventricular septum also had a gap between the edges of the leaflets themselves such that there was a common valvar orifice.15 Attempts to combine these two separate systems for categorisation of presumed intermediate forms produced a complicated alphanumeric system.16 It is possible to avoid such complications, and achieve simplicity in description, by stating first whether there is a common valvar orifice, or separate orifices for the right and left ventricles, and then accounting separately for the level of shunting. Those who choose to continue to use the terms “partial” or “complete” when describing patients within the overall group should then remember that there is no difference in either junctional or basic left ventricular morphology between the two variants, the differences instead reflecting the morphology of the bridging leaflets guarding the common atrioventricular junction, and their relationships to the scooped-out ventricular septum.
What about the Rastelli classification?
The categorisation of hearts with common atrioventricular junction on the basis of the structure of the superior bridging leaflet6 was a crucially important advance in our understanding of the morphology of the overall group. The concept, however, was less than perfect, since it accounted only for the arrangement of the superior bridging leaflet, and argued, incorrectly, that the superior leaflet was itself cleft in the so-called “A” variant. Those wishing to understand the classification should appreciate that the variation depends on the extend of bridging of the superior leaflet into the right ventricle, with the greatest bridging seen in the so-called “Type C” variant, and minimal, if any, bridging found in the “Type A” variant. Concomitant with the variation in bridging of the superior leaflet, there is a reciprocal reduction in size of the antero-superior leaflet of the right ventricle (Fig. 16).

Figure 16. The cartoon shows the spectrum of bridging of the superior leaflet of the common atrioventricular valve that underscores the classification proposed by Rastelli and colleagues for categorisation of patients with common atrioventricular valvar orifice. In the extreme form (left hand upper panel), the superior bridging leaflet (star) extends well into the right ventricle, and the antero-superior leaflet of the right ventricle (double headed arrow) is correspondingly small. With less bridging, as seen in the upper right and lower left panels, there is increasing size of the antero-superior leaflet. The spectrum can then be extended to include the “ostium primum” variant, with dual valvar orifices within the common atrioventricular junction (lower right panel).
The concept, when understood in this fashion, nonetheless, can be extended to include the variant with separate valvar orifices for the right and left ventricle, since in this variant the superior bridging leaflet is fused to the septal crest, rather than being separated from the crest by intercordal spaces as in the “Type A” variant. This is then important, since the adjacency of the superior bridging leaflet to the septal crest increases the length of the left ventricular outflow tract,17 and also increases the chance of development of significant obstruction, seen most frequently in the so-called “Type A” variant and the “ostium primum” defect. In addition, detailed imaging of the “cleft” of the superior bridging leaflet plays an important role in the surgical repair in some centres in which direct closure of the ventricular element of the defect is undertaken.18 Though this technique has been applied in various sub-types of atrioventricular septal defect, some prefer to employ this approach in those subjects with a “Type A” anatomy of the superior bridging leaflet.
Ventricular dominance
The advent of sophisticated echocardiographic techniques has also served to clarify the variable arrangement of the common atrioventricular junction itself to the ventricular septum. In the majority of patients undergoing uncomplicated surgical correction of their atrioventricular septal defects, the common atrioventricular junction is equally shared between the right and left ventricles. This is the so-called “balanced” arrangement (Fig. 17). In more complex patients, there is often ventricular imbalance. This can be because the ventricular septum, relative to the junction, is shifted in either rightward or leftward direction. Leftward shift of the ventricular septum will produce right ventricular dominance, whilst rightward shift will give left ventricular dominance, the complimentary ventricle becoming increasingly hypoplastic with a greater degree of ventricular dominance. The end-points of these spectrums are double inlet right and double inlet left ventricle, both existing in the setting of a common atrioventricular valve (Fig. 17). It is arbitrary as to when the line is drawn to describing an atrioventricular septal defect with ventricular dominance as opposed to double inlet ventricle. One approach is to make the cut-off point when more than three-quarters of the common valve is committed to the dominant ventricle. The significant point is that, when a patient is deemed to have double inlet ventricle, there will be a functionally univentricular arrangement. Decisions as to the ideal surgical approach will depend not only the proportion of common valve “committed” to the dominant ventricle but also the anatomy of the subvalvar apparatus such as the number and anatomy of papillary muscles and chordal attachments.

Figure 17. The cartoon shows the concept of ventricular dominance, with shift of the common atrioventricular junction relative to the ventricular septum. The balanced arrangement is shown in the middle panel. Depending on the direction of shift, there is a spectrum either through left ventricular dominance to double inlet left ventricle with common atrioventricular valve (upper panels), or right ventricular dominance to double inlet right ventricle with common atrioventricular valve (lower panels).
What of double outlet atrium?
The basis of ventricular dominance is unequal sharing of the common atrioventricular junction between the ventricular chambers. In most of such situations, there will also be malalignment between the atrial and ventricular septums. Malalignment of the septums, however, can also be found when the ventricles retain their balance, with the common junction equally shared between them. This arrangement produces one variant of so-called “double outlet atrium”, with malalignment of the atrial septum to the right producing double outlet left atrium, and leftward malalignment giving double outlet right atrium (Fig. 18). Significantly, the patients with these variants of double outlet atrium retain the phenotypic feature of the common atrioventricular junction. They should be distinguished from the other variant of double outlet atrium produced by overriding and straddling of a solitary atrioventricular valve when there is absence of one or other atrioventricular connection.19 The latter variants will require repair in functionally univentricular fashion, whereas those with common atrioventricular junctions can be repaired in biventricular fashion having resected the malaligned atrial septum.20

Figure 18. The cartoon shows how it is shift of the atrial septum relative to the ventricular septum that produces double outlet from either the right or the left atrium in the setting of common atrioventricular junction. With double outlet, the other atrium opens either through the oval fossa, or else through the “ostium primum” defect (double headed black arrow). The middle panel shows the balanced arrangement.
Conclusions
In a brief review of this type, it is impossible to do justice to all the morphologic features of patients having deficient atrioventricular septation and a common atrioventricular junction. Nor is it possible to discuss all the different echocardiographic techniques now available to aid diagnosis, and in particular to determine features such as ventricular function or patterns of shunting. Instead, we have chosen to describe the variations seen in arrangement of the leaflets of the common atrioventricular valve relative to each other, the septal structures, and the atrioventricular junction. Suffice it to say that, by working from the basic arrangement described in our review, modern-day echocardiographic techniques will readily reveal all these other aspects of patients having an atrioventricular septal defect in the setting of a common atrioventricular junction.
Acknowledgements
Robert H. Anderson is supported by grants from the British Heart Foundation together with the Joseph Levy Foundation. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.




















